Synthesis and characterization of an injectable and self-curing poly(methyl methacrylate) cement functionalized with a biomimetic chitosan–poly(vinyl alcohol)/nano-sized hydroxyapatite/silver hydrogel
Abstract
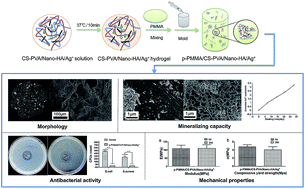
Injected bone substitutes (IBSs) have become increasingly attractive in the field of tissue engineering due to their patient convenience, easy administration as well as minimally invasive procedure for tissue repair. To develop a novel and smart IBS with desirable properties for future in vivo bone regenerative, nano-sized hydroxyapatite (Nano-HA) or antibacterial Ag+, or a combination of them, was initially loaded into a chitosan–poly(vinyl alcohol) (CS–PVA) thermo-sensitive hydrogel. Then the functionalized hydrogel was mixed with PMMA to optimize the final bulk behaviors of the PMMA cement. Finally, the physicochemical properties, anti-bacterial activity, biomineralization ability, and mechanical property changes under simulated physiological conditions of the cements were tested by a type K thermocouple, scanning electron microscopy (SEM), X-ray diffraction (XRD), micro-computed tomography (μ-CT), X-ray photoelectron spectroscopy (XPS), calcium ion test kit and mechanical compression tests. The results showed that the CS–PVA thermo-sensitive hydrogel decreased the Tmax, prolonged the working time, created irregular pores and led to appropriate mechanical properties of the cements. Nano-HA particles induced better mineralization capacity of the cements without acting negatively on the mechanical properties. Ag+ incorporation remarkably enhanced the anti-bacterial activity of the cements for prevention of post-operative infection. Ultimately, such results suggested the injectable and multi-functional cement with p-PMMA/CS–PVA/Nano-HA/Ag+ combination would hold strong promise for future bone reconstruction applications.

 Please wait while we load your content...
Please wait while we load your content...