Photochemical preparation of the ternary composite CdS/Au/g-C3N4 with enhanced visible light photocatalytic performance and its microstructure
Daluo Pengab,
Huihu Wang*ab,
Kun Yuab,
Ying Changab,
Xinguo Mac and
Shijie Dongab
aSchool of Materials and Chemical Engineering, Hubei University of Technology, Wuhan, P. R. China. E-mail: wanghuihu@mail.hbut.edu.cn; Fax: +86-027-59750460; Tel: +86-027-59750460
bHubei Provincial Key Laboratory of Green Materials for Light Industry, Hubei University of Technology, Wuhan, P. R. China
cSchool of Science, Hubei University of Technology, Wuhan, P. R. China
First published on 10th August 2016
Abstract
A ternary composite photocatalyst CdS/Au/g-C3N4 was synthesized by a facile two-step photoreduction method. In this hybrid structure, CdS nanoparticles and CdS@Au core–shell nanoparticles are homogeneously deposited on the surface of g-C3N4 to produce two different structures CdS–g-C3N4 and CdS@Au–g-C3N4. The microstructure analysis results of the composition, chemical states, and optical properties of as-prepared ternary hybrid reveal that a strong interaction exists between CdS nanoparticles, CdS@Au nanoparticles and the g-C3N4 bulk. The ternary composite CdS/Au/g-C3N4 exhibits significantly enhanced photocatalytic activity for RhB degradation under visible light irradiation compared with the binary composites CdS/g-C3N4, Au/g-C3N4 and pure g-C3N4. Meanwhile, CdS/Au/g-C3N4 also demonstrates good photostability in the recycling test of RhB degradation. The active species in the photocatalytic reaction over CdS/Au/g-C3N4 are detected as O2˙− and h+. The superior photocatalytic performance of CdS/Au/g-C3N4 should be attributed to the enhanced separation efficiency of photogenerated electrons–holes induced by the formed heterojunctions CdS–g-C3N4 and Z-scheme structure CdS@Au–g-C3N4 where Au nanoparticles serve as electrons mediator.
1. Introduction
In recent years, carbon nitride with a graphitic-like structure (g-C3N4) as a photocatalyst has been widely researched for water splitting,1–3 CO2 reduction4,5 and environment remediation6,7 due to its unique two-dimensional structure, excellent chemical stability and tunable electronic structure. As g-C3N4 is a polymer semiconductor with a bandgap of 2.7 eV, it can effectively absorb visible light photons. Sunlight can be used as energy source in g-C3N4-based photocatalysis, which makes it an ideal visible light driven photocatalyst candidate. However, the visible light photocatalytic activity of g-C3N4 is seriously limited by its low specific surface area and quick recombination of photogenerated electron–hole pairs. The way to improve its photocatalytic activity under visible light irradiation is quite needed.8Several efforts have been developed to overcome this drawback of g-C3N4. Surface modification of noble metals, for instance Ag,9,10 Pt,11,12 and particularly Au,13,14 to improve the photocatalytic activity of g-C3N4 is one of the frequently used methods. It is believed that noble metal nanoparticles can serve as traps and enhance the transfer efficiency of photogenerated charges.15 Furthermore, the inherent surface plasmon resonance (SPR) of noble metal nanoparticles can strengthen the visible light absorption of bulk g-C3N4 and simultaneously provide the thermal redox active centre during photocatalytic reaction.16 Interestingly, Au nanoparticles loaded g-C3N4 nanosheets were also prepared. It is worth noting that the specific surface area of g-C3N4 nanosheets is increased by ultrasonication-assisted liquid exfoliation of bulk g-C3N4. The synergistic role of high surface area of g-C3N4 nanosheets and photogenerated charges transfer efficiency due to Au nanoparticles surface modification makes the nanohybrids show superior photocatalytic performance for methyl orange degradation under visible light irradiation compared to bulk g-C3N4, g-C3N4 nanosheets, and Au/bulk g-C3N4 hybrids.17
Another frequently used method for improving the photocatalytic activity of g-C3N4 is coupling it with the other semiconductors, such as TiO2,18–20 ZnO,21,22 Bi2WO6,23–25 CdS26,27 and so on. Among various semiconductors, CdS has received much attention because it is one of the most attractive visible light active semiconductor photocatalysts for its desired bandgap width and suitable band edge position. Till now, CdS nanoparticles,28,29 CdS nanorods,30,31 CdS nanowires,32 and CdS quantum dots33 have been used to modify bulk g-C3N4 in order to improve the visible light photocatalytic activity of g-C3N4. Besides this, some special nanostructures, such as CdS@g-C3N4 core–shell nanorods,34 CdS@g-C3N4 core–shell nanowires,35 and CdS nanoparticles modified g-C3N4 nanosheets36 were also prepared. All the CdS/g-C3N4 structures have shown activity due to the well-matched band structure and intimate contact interfaces between g-C3N4 and CdS. In addition, the photocorrosion of CdS can be effectively inhibited in CdS/g-C3N4 hybrids by forming interfacial transfer of photogenerated charges between g-C3N4 and CdS, which leads to effective charge separation on both parts and inhibits the photocorrosion of CdS.
Very recently, a sandwich-structured sulfur-doped CdS/Au/g-C3N4 ternary photocatalyst were prepared by bath deposition method.37 The composite showed an enhanced photocatalytic activity for water splitting and dye degradation compared to the binary heterojunctions CdS/g-C3N4 and Au/g-C3N4. The Z-scheme charges transfer mechanism in which Au nanoparticles serve as electron transfer mediator was proposed to explain the experimental results. Besides this, Au@CdS core–shell structure was also deposited on the surface of g-C3N4 to prepare a novel heterojunction structured composite photocatalyst CdS/Au/g-C3N4. The photocatalytic activity of the as-prepared composite photocatalyst for hydrogen production is about 125.8 times higher than that of pure g-C3N4 under visible light irradiation.38 From these reports, it is seen that preparing a ternary photocatalyst that takes advantage of both noble metal nanoparticles and semiconductor is an effective method for further improving the photocatalytic activity of g-C3N4. However, the effects of Au and CdS co-modification on the microstructure and photodegradation mechanism for pollutants over CdS/Au/g-C3N4 photocatalysts have not been discussed in detail. Furthermore, it is supposed that the total all-solid-state Z structure of CdS@Au–g-C3N4 can be constructed by the photoreduction or deposition method due to the affinity of Au surface atoms to sulfur atoms.39 However, this kind of interaction role is very weak. The binary heterojunction CdS–g-C3N4 may still exist in the ternary composite, which in turn influences its photocatalytic mechanism. In this work, a ternary photocatalyst CdS/Au/g-C3N4 was synthesized via a facile two-step photoreduction method which was different from the previous literatures.37,38 The microstructures of CdS/Au/g-C3N4 were characterized in detail. Both CdS–g-C3N4 heterojunction and Z-scheme structure CdS@Au–g-C3N4 were observed in the composite. Furthermore, the main reactive species for RhB photodegradation over CdS/Au/g-C3N4 were verified by using various scavengers. Based on these results, the photodegradation mechanism was proposed and discussed. This work is instructive for the future researches on the construction of Z-scheme structure.
2. Experimental
2.1 Materials
Carbamide, dicyandiamide, nitric acid, chloroauric acid, cadmium nitrate, sulfur precipitated, potassium iodide (KI) and isopropyl alcohol (IPA) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai city, P. R. China). All reagents in this work are AR grade and used without further purification. The purity of argon and nitrogen gas is 99.9%. Deionized water is used throughout this work.2.2 Synthesis of photocatalysts
In this work, g-C3N4 was prepared by thermal polymerization of precursor. In a typical process, 14 g of carbamide powder and 6 g of dicyandiamide powder were mixed and put into the milling tanks in a planetary ball mill. Grinding balls were added in the mixtures. The milling speed and milling time were set as 360 rpm and 1 hour. The obtained mixtures after mechanical milling were grinded to powder. 5 g of this powder was put in a alumina crucible and heated in a muffle furnace at 550 °C for 4 h with a ramp of 2 °C min−1. After cooling down to room temperature, the light yellow products were obtained and then washed in 0.1 mol L−1 nitric acid solution by continuous stirring for 1 h in order to remove the impurities in g-C3N4 powder. Finally, the as-prepared g-C3N4 was washed several times with deionized water and then dried in an oven at 80 °C for 12 h.Au/g-C3N4 composites were synthesized by photoreduction method.17 In detail, 1.28 mL of chloroauric acid with concentration of 0.01 mol L−1 and 8 mL of methanol was added in 100 mL deionized water. 0.5 g of g-C3N4 powder was added to the above solution and then ultrasonic oscillation for 15 min and stirring in dark for 30 min to achieve adsorption balance. The obtained suspension was purged with argon for 30 min, and then irradiated with a 300 W Xe lamp for 1 h. The as-prepared Au/g-C3N4 was washed several times with deionized water and then dried in an oven at 60 °C for 12 h.
CdS/Au/g-C3N4 composites were also synthesized by photoreduction method.40 Firstly, 0.5 g of Au/g-C3N4 powder was dispersed in a mixed solution consisting of 60 mL ethanol and 40 mL deionized water by ultrasonic oscillation for 15 min and stirring in dark for 30 min. After that, 26.8 mg of cadmium nitrate and 17.8 mg of S8 were added to the above solution and stirred for another 30 min. The obtained suspension was purged with argon for 30 min and then irradiated under UV-Vis light for 3 h provided with a 300 W Xe lamp. The as-prepared CdS/Au/g-C3N4 was washed several times with deionized water and then dried in an oven at 60 °C for 12 h. CdS/g-C3N4 composites were also synthesized with the same method through using g-C3N4 instead of Au/g-C3N4 in the first step.
2.3 Characterization
X-ray diffraction patterns were collected using a XD-2 diffractometer with Cukα radiation at a scan rate of 2° min−1. Morphology of photocatalysts was analyzed with a FEI Tecnai G20 transmission electron microscopy with an acceleration voltage of 200 kV. The elementary composition and information on binding energies of samples were measured with a VG Multilab 2000X X-ray photoelectron spectroscopy. All binding energies were referenced to the C1s peak of surface adventitious carbon at 284.6 eV. UV-Vis diffuse reflectance spectra were conducted to study the optical absorption properties of different photocatalysts with BaSO4 as a reflectance standard using a U-3900 spectrophotometer. The surface functional groups and chemical bands of the samples were identified by a Nicolet NEXUS-6700 Fourier transform infrared spectrometer. Photoluminescence spectra were recorded on an F-7000 spectrophotometer in order to characterize the photoluminescence intensity of the samples with an excitation wavelength of 370 nm.Photoelectrochemical measurements were carried out on an electrochemical analyzer (CHI600E) in a standard three-electrode system. Pt flake and Ag/AgCl were provided as the counter electrode and the reference electrode, respectively. A 300 W Xe lamp with a 400 nm UV cut-off filter was used as the light source. The electrolyte was 0.1 M Na2SO4 solution. The preparation process of working electrode was similar as the other literature.27 Typically, 50 mg of samples were mixed with 150 mg of ethyl cellulose firstly, and then stirred in 3 mL ethanol solution to obtain the homogeneous slurry. The slurry was coated onto an indium-tin oxide glass (ITO glass) by doctor blade method. The as-prepared sample films with the similar thickness were dried at 120 °C for 1 h to get working electrodes.
2.4 Photocatalytic activity tests
The photocatalytic activity of the as-prepared samples was evaluated by the photodegradation of RhB under visible light irradiation. Externally illumination was supplied by a 300 W Xe lamp with a 400 nm UV cut-off filter. Before illumination, 50 mg of the photocatalyst was dispersed in 100 mL RhB solution with a concentration of 10 mg L−1 by ultrasonic oscillation for 15 min and continuous stirring for 30 min in dark to achieve adsorption–desorption equilibrium. Stirring was kept during the photocatalytic reaction to maintain the photocatalyst homogeneously dispersed in solution. 4 mL of the suspensions were collected from the reactor at different irradiation time interval and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to remove photocatalyst completely. The concentration changes of RhB were analyzed by a 2102PC UV-Vis spectrometer.
000 rpm for 5 min to remove photocatalyst completely. The concentration changes of RhB were analyzed by a 2102PC UV-Vis spectrometer.
Five repeated experiments were conducted using the same testing conditions above for stability test. After each reaction, the photocatalysts were filtered and washed several times with deionized water.
The reaction species detection process is similar to the photodegradation experiments. Various scavengers were introduced to the RhB dye solution prior to the illumination.
3. Results and discussion
Fig. 1 shows the XRD patterns of g-C3N4, Au/g-C3N4, CdS/g-C3N4 and CdS/Au/g-C3N4 samples. Two distinct diffraction peaks of g-C3N4 at 27.4° and 13.1° are clearly observed in all samples. The intensity of both diffraction peaks decreases with the coupling of CdS in CdS/g-C3N4 and CdS/Au/g-C3N4 samples. It may be attributed to the strong interaction between g-C3N4 and CdS, suggesting that CdS has been successfully deposited on the surface of g-C3N4.41 However, Au surface modification doesn't influence the peak intensity of g-C3N4, which can be seen in the XRD pattern of Au/g-C3N4 sample.42 Three additional diffraction peaks at 27.1°, 44.1° and 52.1° attributed to the (111), (220) and (311) crystal plane of CdS are found in the XRD patterns of CdS/g-C3N4 and CdS/Au/g-C3N4 samples. The peaks at 38.2°, 44.3° and 64.6° corresponding to the (111), (200) and (220) crystal plane of Au are also seen in Au/g-C3N4 sample.Fig. 2 shows the TEM images of the different samples. It is clearly seen that g-C3N4 is composed of nanosheets with a laminar structure, as shown in Fig. 2a. Fig. 2b and c reveal that Au nanoparticles with diameter in the range of 5–20 nm as black dots are homogeneously dispersed on the surface of g-C3N4 in Au/g-C3N4 sample. Fig. 2d and e show the TEM image of CdS/g-C3N4. CdS nanoparticles with approximately size of 20 nm are deposited on the surface of g-C3N4 as gray dots. Some CdS nanoparticles are aggregated together. Fig. 2f and g show the TEM images of CdS/Au/g-C3N4 sample. It can be observed that the size of CdS nanoparticles is smaller than that of CdS/g-C3N4. Moreover, the CdS nanoparticles are dispersed more uniform than that of binary composite CdS/g-C3N4. Besides the individual CdS nanoparticles directly contacted with g-C3N4, the CdS coated Au nanoparticles (CdS@Au) are also seen in the sample, as shown in Fig. 2g. Fig. 2h shows the HRTEM image of CdS/Au/g-C3N4 sample. The (111) crystal plane of CdS crystal with a crystal plane space of about 0.34 nm is observed. It can be concluded that there are two different heterojunctions CdS–g-C3N4 and CdS@Au–g-C3N4 formed in the ternary composite CdS/Au/g-C3N4, which may definitely affect its photocatalytic activity. It has been reported that S8 molecules are inclined to adsorbing on the surface of Au nanoparticles due to the affinity of Au surface atoms to sulfur atoms.39 After that, the sulfur atoms are reduced to S2− ions by the collected electrons in Au nanoparticles during the photochemical preparation process of Au/CdS/g-C3N4. The as-obtained S2− ions are bonded to Cd2+ to form CdS shells around Au cores. As a result, Z-scheme structure CdS@Au–g-C3N4 is constructed. However, CdS–g-C3N4 structure is also observed in this work, indicating the interaction between Au and CdS nanoparticles is weak. The formation of total Z-scheme structure CdS@Au–g-C3N4 may be very difficult using photoreduction method.
 | ||
| Fig. 2 TEM images of as-prepared samples: (a) g-C3N4; (b) and (c) Au/g-C3N4; (d) and (e) CdS/g-C3N4; (f) and (g) CdS/Au/g-C3N4; (h) HRTEM image of CdS/Au/g-C3N4. | ||
XPS measurements were conducted to investigate the chemical states of C, N, O, Cd, S, and Au in the as-prepared samples. Fig. 3a shows the XPS survey scans of g-C3N4, CdS/g-C3N4, Au/g-C3N4 and CdS/Au/g-C3N4. The elements of C, N, O, Cd, S, and Au have been detected in these samples. High resolution XPS spectra of C1s are shown in Fig. 3b. Three peaks at 284.6 eV, 285.6 eV and 288.0 eV have been deconvoluted, respectively. The peak at 284.6 eV can be attributed to adventitious carbon adsorbed on the surface. The peak at 288.0 eV is related to the sp2 hybridized carbon in N-containing aromatic rings (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) of g-C3N4, and the peak at 285.6 eV is mainly assigned to C
N) of g-C3N4, and the peak at 285.6 eV is mainly assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N from the defect of g-C3N4.11 With the incorporation of CdS nanoparticles, the peak at 285.6 eV for C1s in CdS/g-C3N4 and CdS/Au/g-C3N4 samples shifts to the higher binding energy. However, no obvious change is observed on the C1s electronic structure with the addition of Au nanoparticles.
N from the defect of g-C3N4.11 With the incorporation of CdS nanoparticles, the peak at 285.6 eV for C1s in CdS/g-C3N4 and CdS/Au/g-C3N4 samples shifts to the higher binding energy. However, no obvious change is observed on the C1s electronic structure with the addition of Au nanoparticles.
 | ||
| Fig. 3 (a) XPS survey scans of g-C3N4, Au/g-C3N4, CdS/g-C3N4, and CdS/Au/g-C3N4; high resolution XPS scan of as-prepared samples: (b) C1s; (c) N1s; (d) Cd3d; (e) S2p; (f) Au4f. | ||
Fig. 3c exhibits the high resolution XPS scan of N1s in g-C3N4, which can be deconvoluted into four peaks at 398.4 eV, 399.1 eV, 400.4 eV and 403.7 eV, respectively. The main peaks at 398.4 eV and 399.1 eV are assigned to the sp2 hybridized aromatic N to carbon atoms in the triazine units (C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and the tertiary N bonded to carbon atoms (N–(C)3), respectively. The peak at 400.4 eV belongs to the amino groups (C–N–H) from the defect of g-C3N4. The weak peak at 404.4 eV is attributed to π-excitations, which comes from charging effects or positive charge localization in the heterocycles.11 Similarly, the peak intensity at 403.7 eV for N1s in g-C3N4 is enhanced and the peak position shifts to a high binding energy. Both the high resolution XPS spectra of C1s and N1s reveal that an electronic interaction occurs between g-C3N4 bulk and CdS nanoparticles, indicating the intimate contact interfaces are produced in the sample.
C) and the tertiary N bonded to carbon atoms (N–(C)3), respectively. The peak at 400.4 eV belongs to the amino groups (C–N–H) from the defect of g-C3N4. The weak peak at 404.4 eV is attributed to π-excitations, which comes from charging effects or positive charge localization in the heterocycles.11 Similarly, the peak intensity at 403.7 eV for N1s in g-C3N4 is enhanced and the peak position shifts to a high binding energy. Both the high resolution XPS spectra of C1s and N1s reveal that an electronic interaction occurs between g-C3N4 bulk and CdS nanoparticles, indicating the intimate contact interfaces are produced in the sample.
In Fig. 3d, the high resolution XPS spectra of Cd3d are observed at 404.6 eV and 411.4 eV, corresponding to Cd2+ ions in the CdS nanoparticles. In Fig. 3e, the XPS peaks at 161.0 eV and 162.4 eV are assigned to the S2p in the CdS nanoparticles.27 The analysis results of Fig. 3d and e confirm the presence of CdS nanoparticles in CdS/g-C3N4 and CdS/Au/g-C3N4 samples.
In Fig. 3f, the high resolution XPS peaks at 83.6 eV and 88.2 eV corresponding to the Au4f5/2 and Au4f7/2 in metallic gold nanoparticles have been observed, indicating Au nanoparticles was successfully deposited on the surface of g-C3N4.16 It is worth noting that the Au4f peak intensity of CdS/Au/g-C3N4 is weaker than that of Au/g-C3N4, which may be due to the existence of CdS shell coated on the surface of Au nanoparticles.38
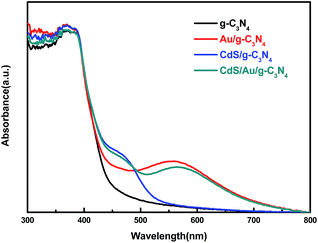
The UV-Vis diffuse reflectance spectra of as-prepared samples are shown in Fig. 4. For each sample, a strong absorption at wavelength around 460 nm, corresponding to the optical bandgap of g-C3N4 at 2.7 eV is observed. Compared with pure g-C3N4 sample, Au/g-C3N4 and CdS/Au/g-C3N4 samples show a more intensive absorption at 560 nm in the visible light region. This broad absorption peak may arise from the SPR effect of Au nanoparticles.13 Furthermore, the absorption intensity at 560 nm of ternary composite CdS/Au/g-C3N4 is weaker than that of Au/g-C3N4 sample, which may be ascribed to the wrapping of CdS shell on the surface of Au nanoparticles.38 Owing to the surface modification of CdS nanoparticles, an additional absorption at 520 nm is also observed in CdS/g-C3N4 and CdS/Au/g-C3N4 samples.33
Fig. 5a depicts the FTIR spectra of g-C3N4, Au/g-C3N4, CdS/g-C3N4 and CdS/Au/g-C3N4 samples. In all samples, the sharp peak around 810 cm−1 can be assigned to the characteristic breathing mode of triazine units in g-C3N4. Compared with g-C3N4 and CdS/g-C3N4, the Au/g-C3N4 and CdS/Au/g-C3N4 samples show enhanced absorption intensity in this region, as shown in Fig. 5b. This may be ascribed to the overlapping of Au–O band at 818 cm−1 with the CN heterocycles of triazine units in g-C3N4.16 Several strong bands from 1200 cm−1 to 1700 cm−1 can be ascribed to the typical stretching modes of CN heterocyclic compounds, and the broad bands at 3100–3300 cm−1 correspond to the stretching vibration of NH2 or N–H groups in g-C3N4. No characteristic peaks between 1050 cm−1 and 1660 cm−1 related to the Cd–S bond of CdS nanoparticles appear in the curves of CdS/g-C3N4 and CdS/Au/g-C3N4 samples due to the low content of CdS.30
 | ||
| Fig. 5 (a) FTIR spectra of g-C3N4, Au/g-C3N4, CdS/g-C3N4 and CdS/Au/g-C3N4 samples and (b) the magnified spectra ranged from 750 cm−1 to 850 cm−1. | ||
Photoluminescence measurements of as-prepared samples have been carried out at an excitation wavelength of 370 nm, as shown in Fig. 6. The pure g-C3N4 sample exhibits a strong emission peak in the region from 400 nm to 600 nm. In comparison, the fluorescence peak intensity of Au/g-C3N4 and CdS/g-C3N4 decreases clearly. Particularly, the ternary composite CdS/Au/g-C3N4 presents the most significant diminished fluorescence peak intensity among all samples. For semiconductors, the photogenerated holes and electrons can bind after the excitation by incident light, which results in partial energy transferring to the fluorescence. The decrease of emission peak intensity for CdS/Au/g-C3N4 sample indicates its low binding efficiency between electrons and holes, which may be ascribed to the inhibiting role of Au nanoparticles.37
Fig. 7 shows the transient photocurrent response of g-C3N4, CdS/g-C3N4, Au/g-C3N4 and CdS/Au/g-C3N4 electrodes under intermittent visible light irradiation. It is observed that the pure g-C3N4 sample demonstrates a low photocurrent density and an evident photocurrent response delay. In contrast, the composite photocatalysts Au/g-C3N4, CdS/g-C3N4 and CdS/Au/g-C3N4 exhibit the increased photocurrent intensity and a fast photocurrent response. Particularly, CdS/Au/g-C3N4 has the highest photocurrent density among all the samples. Because the generation of photocurrent is the result of photo-generated electrons transferring from electrodes to ITO glass, the enhanced photocurrent density implies that more effective photogenerated electrons separation and transferring are achieved when the CdS@Au–g-C3N4 and CdS–g-C3N4 heterojunctions are constructed in CdS/Au/g-C3N4.
The photocatalytic activities of as-prepared samples were determined by the degradation of organic dye under visible light irradiation. RhB as a colored dye was chosen as the model in this work, and results are shown in Fig. 8. RhB photodegradation without photocatalyst was carried out, and result exhibits that RhB is rarely degraded with the absence of photocatalysts. When pure g-C3N4 is used as photocatalyst, RhB has been completely degraded in 30 min. In contrast, the degradation time is notably reduced to 20 min by using Au/g-C3N4, CdS/g-C3N4 and CdS/Au/g-C3N4 photocatalysts, indicating that Au and CdS nanoparticles surface modification can effectively enhance the photocatalytic activity of pure g-C3N4. Among the three different composites, the slope of degradation curves using CdS/Au/g-C3N4 photocatalyst is the sharpest, indicating its highest photocatalytic activity. The RhB photodegradation efficiency in 10 min over CdS/Au/g-C3N4 photocatalyst reaches 90.1%, while it reduces to 84.1%, 77.5%, and 43.0% for CdS/g-C3N4, Au/g-C3N4 and pure g-C3N4 photocatalysts. This result reveals that there is a synergistic role of Au and CdS nanoparticles surface modification.
 | ||
| Fig. 8 Photodegradation curves of RhB over g-C3N4, Au/g-C3N4, CdS/g-C3N4 and CdS/Au/g-C3N4 photocatalysts. | ||
The photocatalytic stability of ternary composite CdS/Au/g-C3N4 was characterized by a recycling test of RhB degradation under visible light irradiation. As shown in Fig. 9, no significant decrease of photocatalytic activity is observed after five repeated experiments, indicating that CdS/Au/g-C3N4 is stable during the photocatalytic reaction.
 | ||
| Fig. 9 Cycling test in photocatalytic degradation of RhB by CdS/Au/g-C3N4 under visible light irradiation. | ||
During photocatalytic process, a large number of active species including O2˙−, ˙OH, h+ are involved in the degradation procedure of pollutant molecules.42 To investigate the influence of different active species on the photocatalytic reaction, KI and IPA were adopted as scavengers to quench h+ and ˙OH, respectively. The concentration of KI and IPA was 1 mmol L−1. As the active species O2˙− mainly originates from the oxygen that dissolved in solution, N2 was continuously purged to exhaust oxygen in solution in order to study the effect of O2˙− on the photocatalytic activity of CdS/Au/g-C3N4. As shown in Fig. 10, the photocatalytic activity of CdS/Au/g-C3N4 is greatly suppressed by N2 purging as O2˙− scavenger, indicating that O2˙− is the main active species in the RhB photodegradation process. Similarly, a significant decrease in the photocatalytic activity is also observed when KI is used as h+ scavenger, suggesting h+ is another important active species in the reaction. However, a slightly decrease in the photocatalytic activity is obtained by the addition of IPA as ˙OH scavenger. This result reveals that ˙OH active species are not the determinant factor in the photocatalytic reaction.
On the basis of above experiments and discussion, the photocatalytic mechanism over CdS/Au/g-C3N4 is illustrated in Fig. 11. As the ternary composite CdS/Au/g-C3N4 prepared in this experiment mainly consists of CdS/g-C3N4 and CdS@Au/g-C3N4 structures, two different mechanisms are proposed.
 | ||
| Fig. 11 Proposed photocatalytic mechanism of CdS/Au/g-C3N4: (a) CdS–g-C3N4 heterojunction; (b) Z-scheme structure CdS@Au–g-C3N4. | ||
As shown in Fig. 11a, both CdS and g-C3N4 can generate electrons and holes under visible light irradiation. The electrons in valence band are photoexcited to the conduction band and leave the holes in valence band. It is reported that the conduction band (−0.5 V) and valence band (+1.9 V) of CdS are lower than that of g-C3N4.43,44 Therefore, the generated electrons on the conduction band (−1.1 V) of g-C3N4 transfer to the conduction band of CdS directly, while the holes in the valance band of CdS migrate to the valence band (+1.6 V) of g-C3N4.36 The intimate interfaces between CdS nanoparticles and g-C3N4 bulk greatly promotes this charges transfer process.
As observed in the TEM images of CdS/Au/g-C3N4, CdS nanoparticles with small size are homogeneously dispersed on the surface of g-C3N4. The heterojunctions between CdS and g-C3N4 are formed, thus enhancing the separation efficiency of photogenerated electron–hole pairs. The electrons can be involved in the O2 to O2˙− reduction process because the conduction edge potential of CdS (−0.5 V) is more negative than the standard redox potential for Eo(O2/O2˙−) (−0.33 V vs. NHE). However, the valence edge potential of g-C3N4 (+1.6 V) is more negative than the standard redox potential Eo(˙OH/H2O) (+2.68 V vs. NHE), H2O/˙OH oxidation reaction cannot be proceeded in this process.13 The holes might be involved in the oxidation reaction of RhB directly, which is accordance with the experimental results of scavenger.
Fig. 11b shows the other possible photocatalytic mechanism over the ternary composite CdS/Au/g-C3N4, which has been reported in previous works.37,38 In this experiment, the Au@CdS–g-C3N4 structure in the ternary composite has been confirmed by the high resolution TEM results. Under visible light irradiation, the electrons and holes transfer mode may be consistent with the Z-scheme mechanism, in which Au nanoparticles serve as the electrons mediator. The photogenerated electrons on the conduction band of CdS and the excited holes on the valance band of g-C3N4 may combine at Au nanoparticles and annihilate. Meanwhile, the photogenerated electrons with strong reducibility would be left on the conduction band of CdS, and the holes with strong oxidability would be left on the valence band of g-C3N4. These photogenerated electrons and holes would be participated in the photocatalytic process.37,38 As the valence edge potential of CdS (+1.9 V) is more negative than the standard redox potential Eo(˙OH/H2O) (+2.68 V vs. NHE), the active species ˙OH are also not involved in the photocatalytic reaction. However, the self-sensitized mechanism of RhB could not be excluded in this experiment, which may also be contributed to its de-coloration.
Compared with the binary composite CdS/g-C3N4 and Au/g-C3N4, the ternary composite CdS/Au/g-C3N4 exhibits the higher photocatalytic activity. This would be ascribed to two reasons: one is that the size of CdS nanoparticles in ternary composite is much smaller than the binary composite. Furthermore, the highly dispersion of CdS nanoparticles may form more heterojunctions in CdS/Au/g-C3N4 than that in CdS/g-C3N4 sample. Thus, the separation efficiency of photogenerated electron–hole pairs in CdS/Au/g-C3N4 is enhanced. It is also believed the high separation and transfer efficiency in photocatalyst may improve its photostability.35 The other reason is that the Z-scheme structure in CdS/Au/g-C3N4 provides the higher redox ability of photogenerated charges than CdS/g-C3N4 and Au/g-C3N4 samples. Therefore, the ternary composite CdS/Au/g-C3N4 shows the high photocatalytic activity and stability in this experiment.
4. Conclusions
In summary, a facile two-step photo-reduction method was applied to synthesize ternary composite photocatalyst CdS/Au/g-C3N4 in this study. Both CdS–g-C3N4 and CdS@Au–g-C3N4 heterojunctions are produced and observed in the hybrid structure. Compared with the binary photocatalyst CdS/g-C3N4, the size of CdS nanoparticles in ternary composite is much smaller. Moreover, the CdS nanoparticles in ternary composite distribute more uniform on the surface of g-C3N4 bulk. The RhB degradation results exhibit that the photocatalytic activity of ternary composite is significantly enhanced relative to the binary photocatalysts CdS/g-C3N4, Au/g-C3N4 and pure g-C3N4. The enhancement of photocatalytic activity may be due to the intimate interfaces formed between CdS and g-C3N4, and the Z-scheme charges transfer mode, which consequently enhance the separation efficiency of photogenerated electrons–holes and strengthen the redox ability of the photoexcited charges. Meanwhile, O2˙− and h+ are the main active species in the photocatalytic reaction over CdS/Au/g-C3N4.Acknowledgements
The authors gratefully acknowledge the financial support of Natural Science Foundation of Hubei Province of China (2013CFA085), the National Natural Science Foundation of China (51202064, 51472081), Research Foundation for Talented Scholars of Hubei University of Technology (BSQD12119), and Open Foundation of Hubei Provincial Key Laboratory of Green Materials for Light Industry ([2013]2-22).Notes and references
- Q. Xiang, J. Yu and M. Jaroniec, J. Phys. Chem. C, 2011, 115, 7355–7363 CAS.
- S. Yang, Y. Gong, J. Zhang, L. Zhan, L. Ma, Z. Fang, R. Vajtai, X. Wang and P. M. Ajayan, Adv. Mater., 2013, 25, 2452–2456 CrossRef CAS PubMed.
- X. Fan, L. Zhang, M. Wang, W. Huang, Y. Zhou, M. Li, R. Cheng and J. Shi, Appl. Catal., B, 2016, 182, 68–73 CrossRef CAS.
- J. Mao, T. Peng, X. Zhang, K. Li, L. Ye and L. Zan, Catal. Sci. Technol., 2013, 3, 1253–1260 CAS.
- Q. Su, J. Sun, J. Wang, Z. Yang, W. Cheng and S. Zhang, Catal. Sci. Technol., 2014, 4, 1556–1562 CAS.
- F. Liang and Y. Zhu, Appl. Catal., B, 2016, 180, 324–329 CrossRef CAS.
- Y. Cui, Z. Ding, P. Liu, M. Antonietti, X. Fu and X. Wang, Phys. Chem. Chem. Phys., 2012, 14, 1455–1462 RSC.
- S. Cao, J. Low, J. Yu and M. Jaroniec, Adv. Mater., 2015, 27, 2150–2176 CrossRef CAS PubMed.
- L. Ge, C. Han, J. Liu and Y. Li, Appl. Catal., A, 2011, 409, 215–222 CrossRef.
- Y. Yang, Y. Guo, F. Liu, X. Yuan, Y. Guo, S. Zhang, W. Guo and M. Huo, Appl. Catal., B, 2013, 142, 828–837 CrossRef.
- W. J. Ong, L. L. Tan, S. P. Chai and S. T. Yong, Dalton Trans., 2015, 44, 1249–1257 RSC.
- J. Yu, K. Wang, W. Xiao and B. Cheng, Phys. Chem. Chem. Phys., 2014, 16, 11492–11501 RSC.
- J. Xue, S. Ma, Y. Zhou and Q. Wang, RSC Adv., 2015, 5, 88249–88257 RSC.
- R. C. Pawar, S. Kang, S. H. Ahn and C. S. Lee, RSC Adv., 2015, 5, 24281–24292 RSC.
- S. Chang, A. Xie, S. Chen and J. Xiang, Electroanal. Chem., 2014, 719, 86–91 CrossRef CAS.
- S. Samanta, S. Martha and K. Parida, ChemCatChem, 2014, 6, 1453–1462 CAS.
- N. Cheng, J. Tian, Q. Liu, C. Ge, A. H. Qusti, A. M. Asiri, A. O. Al-Youbi and X. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 6815–6819 CAS.
- X. Song, Y. Hu, M. Zheng and C. Wei, Appl. Catal., B, 2016, 182, 587–597 CrossRef CAS.
- Y. Li, J. Wang, Y. Yang, Y. Zhang, D. He, Q. An and G. Cao, J. Hazard. Mater., 2015, 292, 79–89 CrossRef CAS PubMed.
- D. Lu, G. Zhang and Z. Wan, Appl. Surf. Sci., 2015, 358, 223–230 CrossRef CAS.
- S. P. Adhikari, H. R. Pant, H. J. Kim, C. H. Park and C. S. Kim, Ceram. Interfaces, 2015, 41, 12923–12929 CrossRef.
- Y. He, Y. Wang, L. Zhang, B. Teng and M. Fan, Appl. Catal., B, 2015, 168, 1–8 Search PubMed.
- M. Li, L. Zhang, X. Fan, Y. Zhou, M. Wu and J. Shi, J. Mater. Chem. A, 2015, 3, 5189–5196 CAS.
- L. Ge, C. Han and J. Liu, Appl. Catal., B, 2011, 108, 100–107 CrossRef.
- H. Wang, J. Lu, F. Wang, W. Wei, Y. Chang and S. Dong, Ceram. Interfaces, 2014, 40, 9077–9086 CrossRef CAS.
- Y. Cui, Chin. J. Catal., 2015, 36, 372–379 CrossRef CAS.
- M. Lu, Z. Pei, S. Weng, W. Feng, Z. Fang, Z. Zheng, M. Huang and P. Liu, Phys. Chem. Chem. Phys., 2014, 16, 21280–21288 RSC.
- F. Jiang, T. Yan, H. Chen, A. Sun, C. Xu and X. Wang, Appl. Surf. Sci., 2014, 295, 164–172 CrossRef CAS.
- J. Fu, B. Chang, Y. Tian, F. Xi and X. Dong, J. Mater. Chem. A, 2013, 1, 3083–3090 CAS.
- J. Yuan, J. Wen, Y. Zhong, X. Li, Y. Fang, S. Zhang and W. Liu, J. Mater. Chem. A, 2015, 3, 18244–18255 CAS.
- Z. L. Fang, H. F. Rong, L. Y. Zhou and P. Qi, J. Mater. Sci., 2015, 50, 3057–3064 CAS.
- Y. Xu and W. D. Zhang, Eur. J. Inorg. Chem., 2015, 2015, 1744–1751 CrossRef CAS.
- L. Ge, F. Zuo, J. Liu, Q. Ma, C. Wang, D. Sun, L. Bartels and P. Feng, J. Phys. Chem. C, 2012, 116, 13708–13714 CAS.
- Y. Li, X. Wei, H. Li, R. Wang, J. Feng, H. Yun and A. Zhou, RSC Adv., 2015, 5, 14074–14080 RSC.
- J. Zhang, Y. Wang, J. Jin, J. Zhang, Z. Lin, F. Huang and J. Yu, ACS Appl. Mater. Interfaces, 2013, 5, 10317–10324 CAS.
- S. W. Cao, Y. P. Yuan, J. Fang, M. M. Shahjamali, F. Y. Boey, J. Barber, S. C. J. Loo and C. Xue, Int. J. Hydrogen Energy, 2013, 38, 1258–1266 CrossRef CAS.
- W. Li, C. Feng, S. Dai, J. Yue, F. Hua and H. Hou, Appl. Catal., B, 2015, 168, 465–471 CrossRef.
- X. Ding, Y. Li, J. Zhao, Y. Zhu, Y. Li, W. Deng and C. Wang, APL Mater., 2015, 3, 104410 CrossRef.
- P. Zhou, J. G. Yu and M. Jaroniec, Adv. Mater., 2014, 26, 4920–4935 CrossRef CAS PubMed.
- H. Tada, T. Mitsui, T. Kiyonaga, T. Akita and K. Tanaka, Nat. Mater., 2006, 5, 782–786 CrossRef CAS PubMed.
- X. Dai, M. Xie, S. Meng, X. Fu and S. Chen, Appl. Catal., B, 2014, 158, 382–390 CrossRef.
- R. C. Pawar, Y. Pyo, S. H. Ahn and C. S. Lee, Appl. Catal., B, 2015, 176, 654–666 CrossRef.
- J. X. Sun, Y. P. Yuan, L. G. Qiu, X. Jiang, A. J. Shen and J. F. Zhu, Dalton Trans., 2012, 41, 6756–6763 RSC.
- A. Sobczynski, A. J. Bard, A. Campion, M. A. Fox, T. Mallouk, S. E. Webber and J. M. White, J. Phys. Chem., 1987, 91, 3316–3320 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |