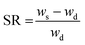What determines the volume transition temperature of UCST acrylamide–acrylonitrile hydrogels?
Amélie Augé and
Yue Zhao*
Département de chimie, Université de Sherbrooke, Sherbrooke, Québec J1K 2R1, Canada. E-mail: yue.zhao@usherbrooke.ca
First published on 20th July 2016
Abstract
A series of covalently crosslinked hydrogels based on acrylamide and acrylonitrile were synthesized by means of free radical copolymerization using methylene bis(acrylamide) as crosslinker and ammonium persulfate–N,N,N′,N′-tetramethylethylenediamine (TEMED) as the redox-initiator system. These hydrogels possess a positive thermosensitivity and their volume expansion in aqueous solution upon temperature increase was monitored by measuring the swelling ratio between 7 and 90 °C. By changing a number of synthesis parameters such as molar ratio between acrylamide and acrylonitrile, crosslinker concentration, polymerization time and presence or absence of TEMED accelerator, the results point out that the apparent volume transition temperature of the hydrogels is mainly determined by the crosslinker concentration, i.e., the crosslinking density. Surprisingly, the composition-tunable UCST of uncrosslinked copolymers of acrylamide and acrylonitrile was not observed with the hydrogels. While the volume transition temperature appears to be insensitive to the composition, the actual change in swelling ratio going through UCST is larger for a hydrogel containing more acrylonitrile units, as a result of being more dehydrated below and more hydrated above UCST, respectively. Furthermore, without TEMED the polymerization time influences the swelling ratio and the transition temperature, while with TEMED it has little effect.
1 Introduction
Hydrogels are three-dimensional networks capable of absorbing a large amount of water.1,2 When crosslinking is carried out by covalent bonding (chemical in nature), an important volume transition is observed between the dry state and swollen state of the hydrogel.3 Among the hydrogels, some are able to respond to one or several stimuli such as pH,4,5 temperature,6,7 magnetic field,8 light,9 and glucose,10 to name a few. Thus, for such hydrogels, the volume transition appears under the effect of one or more of these stimuli.Concerning thermosensitive hydrogels, two main volume transition types are identified (Fig. 1).11 The first is that hydrogels exhibit a negative thermosensitivity.12 This behaviour is similar to thermoresponsive polymer solutions that display a lower critical solution temperature (LCST) in aqueous solution. By analogy with these polymers soluble below and insoluble above the critical temperature, respectively, the hydrogels with negative thermosensitivity are in swollen state at low temperature and their volume decreases when the temperature increases above their LCST. This is explained by temperature-dependent intermolecular interaction strength. At low temperature, the water–polymer interactions are stronger than polymer–polymer interactions, leading to polymer solubility and swollen state of hydrogel. However, when temperature increases above LCST (or phase separation temperature), the strength of polymer–polymer interactions become stronger than water–polymer interactions, resulting in the polymer demixing and collapse of hydrogel. The second type of thermosensitive hydrogels refers to those with positive thermosensitivity.13 In this case, the behaviour is similar to thermoresponsive polymer solutions that exhibit an upper critical solution temperature (UCST) in their phase diagram. The hydrogel is in collapse state at low temperature and swells when temperature increases above UCST.
The volume transition of hydrogels that exhibit a positive thermosensitivity (UCST behaviour) has not been much studied with respect to hydrogels with negative thermosensitivity. This is consistent with the fewer number of publications dealing with polymers that display a UCST in aqueous solution, especially non-ionic polymers.13 This situation is explained by two reasons. Firstly, most polymers possess their UCST outside the 0 to 100 °C range, such as poly(ethylene oxide),14 poly(vinyl alcohol)15 or poly(hydroxyethylmethacrylate).16 Secondly, many polymers (zwitterionic and carboxylic polymers) are very sensitive to concentration of ions or ionic strength in solution and only some specific conditions allow for observation of the phase transition within the 0 to 100 °C temperature range.17–19 Nevertheless, many studies were realized on hydrogels based on acrylamide, acrylic acid and eventually a third monomer.20–22 The positive thermosensitivity of such hydrogels is due to formation and dissociation of hydrogen bonding between amide and acid groups, the so-called “zipper effect”. Although the volume transition temperature is observed inside of the 0–100 °C range, these hydrogels are still sensitive to pH and ionic strength.
In this regard, some uncharged polymers possess desired properties: a sharp phase transition within 0 to 100 °C range and insensitive to pH and ionic strength. Among them, the copolymers based on acrylamide and acrylonitrile are good candidates for UCST hydrogels and may have several advantages compared to LCST hydrogels in such applications as temperature-triggered drug release, bioseparation or catalyst systems, where a positive thermosensitivity is more suited. This is due to the ability of hydrogels based on acrylamide to form hydrogen bonding. Nevertheless, only few studies were reported regarding hydrogels based on these monomers. Most notable is the latest report by Liu et al. who made fibers and thin films through RAFT copolymerization of acrylamide, acrylonitrile and N-(4-benzoylphenyl)acrylamide or 4-acryloyloxybenzophenone monomers.23 The networks were obtained by photocrosslinking. Although interesting, the photocrosslinking method is suitable for synthesis of thin layers but not adapted for synthesis of massive hydrogels.
In the present study, using free radical copolymerization of acrylamide (AAm), acrylonitrile (AN) and methylene bis(acrylamide) as crosslinker, we synthesized and characterized a number of massive hydrogels. Seuring et al. previously studied the thermoresponsive behaviour of poly(AAm-co-AN) in aqueous solution and demonstrated that the AAm/AN molar ratio could be used to tune the phase transition temperature.24 Thus in this work we investigated the influence of AAm/AN molar ratio on swelling degree and volume transition temperature. We also studied the effect of crosslinker concentration. This parameter may strongly affect the average chain molecular weight between two crosslinking points and consequently the swelling ratio. Moreover, the effect of polymerization kinetics was also examined by comparison of swelling ratio and volume transition temperature of the hydrogels prepared with several polymerization times with and without the accelerator of N,N,N′,N′-tetramethylethylenediamine (TEMED). TEMED is frequently used as accelerator for hydrogel synthesis and particularly for synthesis of electrophoresis gels based on acrylamide. It has been shown that the first step of polymerization (initiation step) in the presence of TEMED is about five times faster than in its absence.25 Thus the amount of free radicals and the polymerization time both can affect the crosslinking density and consequently the hydrogel properties. As shown below, the present systematic investigation has yielded results, some are surprising, that provide new insight into what determines the UCST-type volume transition of the hydrogels.
2 Experimental
2.1. Materials
Acrylamide (AAm), acrylonitrile (AN), methylene bis(acrylamide) (MBA), N,N,N′,N′-tetramethylethylenediamine (TEMED) and ammonium persulfate (APS) were purchased from Sigma-Aldrich. Acrylamide was purified by recrystallization in chloroform. Acrylonitrile was purified by passing through a column filled with basic alumina. MBA, APS and TEMED were used as received. Water was filtered with Millipore apparatus before use.2.2. Hydrogels synthesis
Fig. 2 shows the synthesis of the UCST hydrogels based on chemically crosslinking a random copolymer of AAm and AN by using the crosslinker MBA, with or without an accelerator. Unless otherwise stated, the accelerator TEMED was not used in the hydrogel synthesis. More details are given below. | ||
| Fig. 2 Scheme of hydrogel synthesis by free radical copolymerization of AAm and AN with MBA as crosslinker and APS-TEMED as redox-initiator system. | ||
| Sample number | Sample acronym | AAm/AN molar ratio | Monomers AAm + AN (mol L−1) | MBA (mmol L−1) | MBA (mol%) | Appearance | Cloud point (°C) | Volume transition (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | AAm/AN-1.5 | 1.5 | 2.62 | 20 | 0.76 | Opaque | — | 58 |
| 2 | AAm/AN-2 | 2.0 | 2.62 | 20 | 0.76 | Translucent | — | 59 |
| 3 | AAm/AN-2.5 | 2.5 | 2.62 | 20 | 0.76 | Transparent | — | 59 |
| 4 | AAm/AN-5 | 5.0 | 2.62 | 20 | 0.76 | Transparent | — | 58 |
| 5 | MBA-1 | 2.0 | 2.62 | 4 | 0.15 | Translucent | — | 38 |
| 6 | MBA-2 | 2.0 | 2.62 | 12 | 0.46 | Translucent | — | 59 |
| 7 | MBA-3 | 2.0 | 2.62 | 41 | 1.50 | Translucent | — | 77 |
| 8 | MBA-4 | 2.0 | 2.62 | 208 | 7.90 | Translucent | — | 80 |
| 9 | Poly(AAm-co-AN)-1.5 | 1.5 | 2.62 | 0 | 0 | Turbid | 78 | — |
| 10 | Poly(AAm-co-AN)-2 | 2.0 | 2.62 | 0 | 0 | Turbid | 38 | — |
| 11 | Poly(AAm-co-AN)-2.5 | 2.5 | 2.62 | 0 | 0 | Limpid | Soluble | — |
| 12 | Poly(AAm-co-AN)-5 | 5.0 | 2.62 | 0 | 0 | Limpid | Soluble | — |
2.2.2.1 Without TEMED accelerator. The used protocol was similar to the previous section. The monomer concentrations used were similar to the synthesis of AAm/AN-2 (Table 1). Only polymerization time varied between 10 and 60 minutes.
2.2.2.2 With TEMED accelerator. AAm, AN, MBA and APS were dissolved in MilliQ water at concentrations corresponding to the synthesis of AAm/AN-2. After the mixture was transferred into the cylinder of 12.2 mm in diameter and placed under nitrogen atmosphere for a few minutes, TEMED was added (500 μL). The mixture was immediately mixed vigorously and polymerization under ambient conditions was allowed to proceed for different times. After polymerization, the hydrogel was collected, purified and dried using the same procedure as described above.
2.3. Characterizations
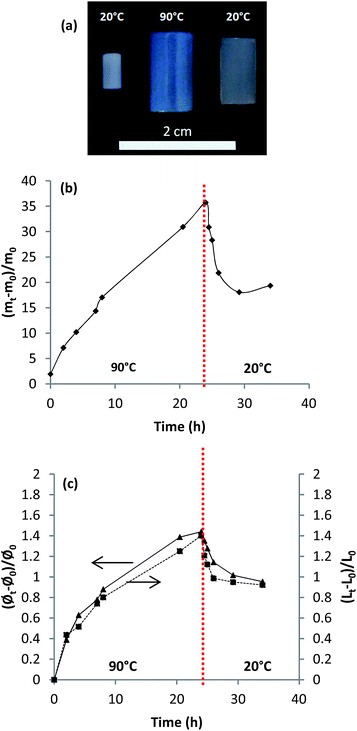
The compositions of the synthesized hydrogels were determined by infrared spectroscopy. The spectra were recorded on a FTIR (ABB Bomem, MB Series) between 800 and 4000 cm−1 at a resolution of 4 cm−1. The viscoelastic properties of the hydrogel were measured using a dynamic mechanical analyser (Perkin-Elmer DMA 8000) in shear mode. The frequency scan was conducted over the range of 1–100 Hz at a constant temperature. The cloud point of copolymers (0.25 mg mL−1 in water) synthesized without crosslinker was determined using Agilent Cary Series UV-Vis-NIR spectrophotometer. The absorbance at 700 nm was recorded as function of solution temperature between 20 and 90 °C. The absorbance was recorded every 1 °C and the heating rate was 0.25 °C min−1. The swelling ratio was determined by a gravimetric method. The weight of dry hydrogel was measured before it was immersed in large volume of thermostated MilliQ water for 48 hours. After that time, the swollen hydrogel was taken out of water and quickly dried on an absorbent paper to remove the water on the hydrogel surface. Afterwards, the swollen hydrogel was weighed. The swelling ratio, SR, was calculated from the following relation:where SR represents the swelling ratio expressed in gram of water absorbed per gram of dry hydrogel; ws is the mass of hydrogel in swollen state and wd the mass of hydrogel in dry state.
3 Results and discussion
3.1. Influence of crosslinker concentration
We first investigated the effect of crosslinking density on the volume transition temperature and swelling ratio of the UCST hydrogels. To the end, a total of five hydrogels were synthesized using a constant monomer concentration and a molar ratio of 2 between AAm and AN, while varying the concentration of the crosslinker MBA (Table 1, samples #2 and 5–8). Fig. 3 shows the plots of swelling ratio as a function of temperature for the five hydrogels. It is seen that the crosslinker concentration affects profoundly the positive thermosensitivity of the hydrogel. On one hand, with increasing the crosslinker concentration, the apparent volume transition, i.e., sharp increase in swelling ratio, takes place at higher temperature, ranging from about 40 °C to 80 °C. At the lowest MBA concentration, the volume transition temperature is close to the cloud point of poly(AAm-co-AN)-2, the control polymer without crosslinking (Table 1, sample #10), and then increases significantly with higher MBA concentrations. The volume transition temperature, defined as the temperature corresponding to the intersection of the two lines around the rise of the swelling ratio, was measured for all gels and shown in Table 1. On the other hand, the swelling ratio over the temperature range is smaller for hydrogel obtained at higher crosslinker concentration. This observation is consistent with the assumption that higher crosslinker concentration results in greater crosslinking density that reduces the amount of absorbed water in the hydrogel. | ||
| Fig. 3 Swelling ratio vs. solution temperature for hydrogels with different crosslinker concentrations (mol% of MBA indicated in the figure) at the same AAm/AN molar ratio of 2. | ||
Comparing the results obtained with the five hydrogels and the non-crosslinked random copolymer (sample #10 in Table 1), which were all prepared with the same molar ratio of 2 between AAm and AN, it is quite surprising to see how sensitive the volume transition temperature is affected by the crosslinker concentration. Indeed, the apparent transition temperature increases from 38 °C (no crosslinking) to 77 °C with only 1.5 mol% MBA. Normally, the UCST is governed by the molar ratio between AAm and AN, while infrared spectroscopic analysis shows no significant difference of the composition of all the samples. Thus the variation of transition temperature is possibly due to the nature of crosslinker. Indeed, the solubility of the crosslinker in water (20 g L−1 at 20 °C – data provided by Sigma-Aldrich) is lower than the solubility of acrylamide (2040 g L−1 at 25 °C)26 and acrylonitrile (73.5 g L−1 at 20 °C).27 The high hydrophobicity of the crosslinker may affect the volume transition temperature, since higher crosslinker concentration means more hydrophobic content in the hydrogel, which shifts the volume transition to higher temperatures. The variation of transition temperature with crosslinker concentration is not observed if the crosslinker is not significantly more hydrophobic than the monomers used to prepare the hydrogel. In that case the transition temperature is mainly governed by the molar ratio of the monomers. In other cases, the effect of crosslinker concentration is difficult to assess if the volume transition is not sufficiently sharp for comparison.28
3.2. Influence of molar ratio AAm/AN
Several hydrogels were synthesized with different compositions between AAm and AN while keeping the same crosslinker concentration. Fig. 4 shows the change in swelling ratio as function of temperature for the four hydrogels listed in Table 1 (samples # 1–4) with the AAm/AN molar ratio ranging from 1.5 to 5.0. The first surprising observation is that the apparent volume transition temperature doesn't seem to be strongly influenced by varying the ratio between AAm and AN, being around 60 °C for all samples. However, the infrared spectroscopy analysis confirmed clearly a modification of the hydrogel composition when the molar ratio between AAm and AN varied. As seen from the infrared spectra in Fig. 5, the relative content of acrylonitrile in the hydrogel increases as the used AAm/AN ratio decreases, which is indicated by the increasing absorption of the characteristic C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N band at 2244 cm−1. Despite the difference in hydrogel composition, the transition temperature does not change significantly. This is in sharp contrast which the copolymers having the same AAm/AN ratios and polymerized under the same conditions but without the crosslinker MBA (samples #9–12 in Table 1). For them, the UCST-related cloud point increases when the molar ratio between AAm and AN decreases because the copolymer composition becomes more hydrophobic, which is consistent with the report by Seuring et al.24 This is quite surprising because we expected to observe a decrease of the volume transition temperature when the percentage of acrylamide in the hydrogel increases. Again, the constant crosslinker concentration for all the hydrogels seems to be the determining parameter for the volume transition temperature.
N band at 2244 cm−1. Despite the difference in hydrogel composition, the transition temperature does not change significantly. This is in sharp contrast which the copolymers having the same AAm/AN ratios and polymerized under the same conditions but without the crosslinker MBA (samples #9–12 in Table 1). For them, the UCST-related cloud point increases when the molar ratio between AAm and AN decreases because the copolymer composition becomes more hydrophobic, which is consistent with the report by Seuring et al.24 This is quite surprising because we expected to observe a decrease of the volume transition temperature when the percentage of acrylamide in the hydrogel increases. Again, the constant crosslinker concentration for all the hydrogels seems to be the determining parameter for the volume transition temperature.
 | ||
| Fig. 4 Swelling ratio vs. solution temperature for hydrogels with different AAm/AN molar ratios (indicated in the figure) at the same crosslinker concentration (0.76 mol% of MBA). | ||
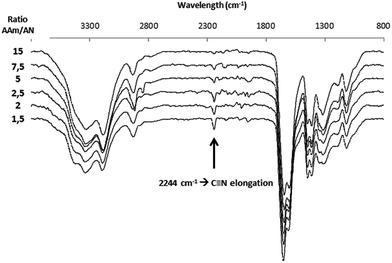 | ||
| Fig. 5 Infrared spectra for hydrogels with various AAm/AN molar ratios (indicated in the figure) at the same crosslinker concentration (0.76 mol% MBA). | ||
On the other hand, it can be noticed that changing the molar ratio between AAm and AN affects the swelling ratio of the hydrogel. Two regions in Fig. 4 can be identified. In the first region for temperatures lower than the volume transition temperature, the hydrogels are able to absorb a significant amount of water. However, at a given temperature, a higher molar ratio AAm/AN corresponds to a higher swelling ratio indicating a larger amount of water which the hydrogel can absorb. This observation may be explained by the hydrophilic nature of hydrogel based on acrylamide. Indeed, the hydrogels are composed of acrylamide monomer units in majority that are able to form hydrogen bonding with water molecules. Thus more the content of acrylamide in the hydrogel is dominant; more the hydrogel is hydrophilic in water even at low temperatures. Ozturk et al. studied the thermosensitive behaviour of hydrogels based on acrylamide and N-t-butylacrylamide (TBA).29 Their elemental analysis showed that the hydrogel contains residual water even after drying due to the hydrogen bonding formed between acrylamide and water molecule. However, the quantity of residual water decreases when the molar fraction of TBA increases. Since TBA possesses a hydrophobic character, the number of hydrogen bonds between AAm and water molecules is lower when there are more TBA units increasing the hydrophobicity of the hydrogel. Similarly, in the case of our hydrogels, more acrylamide means more hydrogen bonds with water, which accounts for a larger swelling ratio even at temperatures below the UCST volume transition.
On the other hand, the second region in Fig. 4 covers the range of temperatures above the transition temperature, where the trend is reversed. At a given temperature, the swelling ratio is higher for hydrogel with a lower molar ratio between AAm and AN. Hou et al. studied the thermosensitive behaviour of acrylamide and acrylonitrile copolymers in aqueous solution using dynamic light scattering (DLS).30 They found that when temperature increases above UCST, the hydrodynamic diameter increases between 10 and 40 nm whereas the usual diameter is smaller than 10 nm. This observation suggests that above UCST all hydrogen bonds between amide groups, which are responsible for chain collapse below UCST, are not broken by hydrogen bonding with surrounding water molecules, which results in the apparent network-like swelling above UCST. This is probably what happens in our hydrogels. When the molar fraction of acrylamide increases, the probability of having hydrogen-bonded acrylamide groups is higher, which adds up to the covalent crosslinking to increase the effective network density and, consequently, to restraint the amount of absorbable water by the hydrogel. This would explain the observed lower swelling ratio for hydrogel with a greater molar fraction of acrylamide units.
As shown by the series of photos in Fig. 6, the hydrogels of various AAm/AN molar ratios at room temperature are visually different, from opaque to translucent to completely transparent with increasing the molar fraction of acrylamide. Hou et al. reported the reactivity ratios for the acrylamide–acrylonitrile system for several experimental conditions (solvent, polymerization temperature, method of polymerization).31 The reactivity ratios for all conditions are rAN < 1 and rAAm > 1, meaning that the polymer formed at beginning of polymerization is rich in AAm and is enriched to AN over polymerization time. In the present case, these reactivity ratios cannot be applied directly, because the presence of crosslinker modifies the reactivity of each monomer with respect to other. The determination of reactivity ratios becomes more complicated when there are more than two monomers in polymerization (9 propagation reactions and 6 reactivity ratios instead of 4 and 2, respectively, for polymerization of a binary system). Nevertheless, the different reactivity ratios may help explaining the gradual change of the hydrogel appearance (Fig. 6). Indeed, if the polymer is richer in acrylamide at the beginning and richer in acrylonitrile at the end of polymerization, different domains with various compositions would be created. This should give rise to heterogeneities inside of the hydrogel, which increases scattering of light and causes the opacity of hydrogel. For example, Okay et al. studied the heterogeneities of acrylamide hydrogels synthesized with various crosslinker concentrations.32
 | ||
| Fig. 6 Photos showing the changing appearance of the hydrogel at room temperature upon increase of the AAm/AN molar ratio (indicated in the figure). | ||
It was found that beyond a certain crosslinker concentration, the hydrogel became opaque due to the formation of heterogeneities in spatial scale of submicrometer to micrometer that are responsible for strong light scattering leading to opacity. Thus, the changing appearance of our hydrogels may be caused by different degrees of heterogeneity at different AAm/AN molar ratios.
The most significant result from Fig. 4 is that the apparent UCST volume transition temperature of covalently crosslinked AAm/AN hydrogels is not sensitive to the AAm/AN molar ratio and appears around 60 °C, but the magnitude of the volume transition through UCST, i.e., the difference between the swelling ratios below and above the phase transition, is very much dependent upon the hydrogel composition. In order to have a large volume transition between most dehydrated state below UCST and most hydrated state above UCST, it is necessary to prepared hydrogel with a small molar ratio between AAm and AN such as 1.5 or 2.
3.3. Influence of polymerization time and TEMED accelerator
Several hydrogels were also synthesized with the same AAm/AN molar ratio equal to 2 while varying the polymerization time between 10 and 60 minutes, no TEMED being used. Fig. 7 shows the swelling ratio as a function of temperature for those hydrogels. Despite some data scattering, the trend is clear. With increasing the polymerization time, the swelling ratio of the resulting hydrogel decreases and its apparent volume transition temperature increases. This observation implies that the crosslinking degree of the hydrogel, i.e., its network density, increases by using a prolonged polymerization time. Being consistent with the results in Fig. 3, an increased crosslinking degree has as effect to reduce the swelling ratio but increase the volume transition temperature. | ||
| Fig. 7 Swelling ratio vs. solution temperature for hydrogels synthesized with different polymerization times (indicated in the figure) (AAm/AN = 2 and 0.76 mol% of MBA). | ||
Faster polymerization kinetics can be realized by doing polymerization in presence of the TEMED accelerator at room temperature. Another series of hydrogels were prepared under these conditions. Fig. 8 shows their swelling ratio as a function of solution temperature. Interestingly, under fast polymerization kinetics it seems that varying the polymerization time has no effect on the volume transition temperature and the swelling ratio. Indeed the swelling ratio and the transition temperature appear essentially the same for the hydrogels obtained after 2 and 60 minutes polymerization, respectively, as well as those between in. It was shown that the polymerization initiation is five times faster in the presence of TEMED.25 When TEMED is added in solution, the concentration of free radicals is significantly higher leading to faster polymerization. The results in Fig. 8 suggest that the hydrogel could be formed quickly in 2 minutes, and that the effective crosslinking degree remained the same for longer reaction times.
 | ||
| Fig. 8 Swelling ratio vs. solution temperature for hydrogels synthesized with different polymerization times (indicated in the figure) in the presence of TEMED (AAm/AN = 2 and 0.76 mol% of MBA). | ||
3.4. Swelling reversibility
The reversibility tests were realized with hydrogels with similar compositions of AAm/AN ranging from 1.5 to 5 described in Table 1. The synthesis is the same as that described above, with only the diameter of cylinder changed to 4.50 mm. After polymerization, the hydrogels were immersed in MilliQ water thermostated to 20 °C for 24 hours. The mass m0, diameter ∅0 and length L0 were measured and these values served as reference. To observe the change in size or swelling ratio, the hydrogels were successively immersed in MilliQ water thermostated to 90 °C for 24 h and then again to 20 °C. The mass mt, diameter ∅t and length Lt were measured to several time intervals in order to monitor the swelling/deswelling kinetics at a given temperature. The relative variation of mass, diameter and length were respectively given by (mt − m0)/m0, (∅t − ∅0)/∅0, and (Lt − L0)/L0.The representative results are shown in Fig. 9 using the hydrogel AAm/AN-1.5 as example. Similar trends were observed for other hydrogels of varying compositions (AAm/AN-2.5 to AAm/AN-5, data not shown).
The photos in Fig. 9a give the visual observation of the hydrogel volume expansion at 90 °C and the subsequent partial contraction at 20 °C. Fig. 9b plots the increase in mass of the hydrogel immersed in water at 90 °C and the decrease upon removal of the hydrogel from water at 90 °C to 20 °C. Two observations can be made. First, the increase in mass (and volume) of the bulky hydrogel is slow and continuous over the 24 hours period of time. Secondly, when put at 20 °C, a relatively fast contraction took place and the hydrogel lost some 35% of its mass after 2 hours. However, after this time its mass appeared to stabilize over time, showing only a partial reversibility. As seen in Fig. 9c, the same trend was observed for the relative variations of length and diameter of the hydrogel cylinder, indicating an isotropic volume transition. At this point, the reasons for the partial reversibility are unclear, and future studies should address this issue.
3.5. Viscoelastic behaviour
The viscoelastic properties of the hydrogel AAm/AN-1.5 were measured using DMA in shear mode. In order to avoid the problem of water evaporation during the measurement, a frequency scan from 1 to 100 Hz at a chosen temperature (below 50 °C) was utilized. Fig. 10 shows the data recorded at two temperatures: 23 and 30 °C, at which the hydrogel contains >60% water. The main observations are the following. First, the storage modulus (G′) remains higher than the loss modulus (G′′) over the entire frequency range. Second, with increasing the frequency, corresponding to decreasing temperature at a constant frequency, the storage modulus increases and the loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = G′′/G′) remains relatively constant. Thirdly, the storage modulus is higher at 23 °C than at 30 °C, but the loss tangent is smaller at the lower temperature. All these results are consistent with the feature of positive thermosensitivity of the hydrogels. They are expected to be more solid-like at lower temperatures.
δ = G′′/G′) remains relatively constant. Thirdly, the storage modulus is higher at 23 °C than at 30 °C, but the loss tangent is smaller at the lower temperature. All these results are consistent with the feature of positive thermosensitivity of the hydrogels. They are expected to be more solid-like at lower temperatures.
 | ||
| Fig. 10 Storage modulus (G′), loss modulus (G′′) and loss tangent vs. frequency for the AAm/AN-1.5 hydrogel at 23 °C (a) and 30 °C (b). | ||
4 Conclusions
We have investigated the positive thermosensitivity of AAm/AN copolymer hydrogels by monitoring their volume change in aqueous solution as a function of temperature. In order to unveil and understand what determines the UCST volume phase transition, a systematic study was conducted on hydrogel samples synthesized by varying a number of parameters such as crosslinker concentration, AAm/AN molar ratio, and polymerization time as well as kinetics with or without TEMED accelerator. The results show that with increasing the solution temperature, the AAm/AN hydrogels can undergo a large volume expansion characterized by a swelling ratio of dozens or even higher. The most significant finding of this study is that the composition-tunable UCST of uncrosslinked AAm/AN copolymers cannot be translated into their covalently crosslinked hydrogels. Indeed, the apparent volume transition temperature is sensitive to the used crosslinker concentration that determines the crosslinking density, but insensitive to the copolymer composition given by the AAm/AN molar ratio, which is in sharp contrast with the uncrosslinked AAm/AN copolymer whose UCST (or solution cloud point) is governed by the relative amounts of both monomers. While the volume transition temperature doesn't vary significantly with the hydrogel composition appearing at around 60 °C, the magnitude of the volume increase going through the phase transition is greater for hydrogel with a higher AN content, as a result of a more dehydrated and hydrated state at temperatures below and above UCST respectively. This finding is important and points out the way to prepare UCST hydrogels with a thermally induced straightforward and large volume variation. In addition, it was found that the volume change of the AAm/AN hydrogels displays only a partial reversibility. To explore potential applications of the hydrogels, future studies need to understand what hampers the tunability of their volume transition temperature, how to speed up the thermally induced volume change and how to improve the volume transition reversibility.Acknowledgements
The authors acknowledge the financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC) and le Fonds de Recherche du Québec: Nature et Technologies (FRQNT). YZ is a member of the FQRNT-funded Center for Self-Assembled Chemical Structures (CSACS) and the Centre Québécois sur les Matériaux Fonctionnels (CQMF).Notes and references
- M. C. Koetting, J. T. Peters, S. D. Steichen and N. A. Peppas, Mater. Sci. Eng., R, 2015, 93, 1–49 CrossRef PubMed.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2013, 65, 10–16 CrossRef CAS PubMed.
- M. Shibayama and T. Tanaka, Adv. Polym. Sci., 1993, 109, 1–62 CrossRef CAS.
- X. Gao, C. He, C. Xiao, X. Zhuang and X. Chen, Polymer, 2013, 54, 1786–1793 CrossRef CAS.
- A. Arizaga, G. Ibarz and R. Piñol, J. Colloid Interface Sci., 2010, 348, 668–672 CrossRef CAS PubMed.
- M. P. Montero-Rama, M. Liras, O. García and I. Quijada-Garrido, Eur. Polym. J., 2015, 63, 37–44 CrossRef CAS.
- M. Xia, W. Wu, F. Liu, P. Theato and M. Zhu, Eur. Polym. J., 2015, 69, 472–482 CrossRef CAS.
- R. Muzzalupo, L. Tavano, C. O. Rossi, N. Picci and G. A. Ranieri, Colloids Surf., B, 2015, 134, 273–278 CrossRef CAS PubMed.
- C. Y. Chiang and C. C. Chu, Carbohydr. Polym., 2015, 119, 18–25 CrossRef CAS PubMed.
- S. Il Kang and Y. H. Bae, J. Controlled Release, 2003, 86, 115–121 CrossRef.
- E. N. R. Koningsveld and W. H. Stockmayer, Polymer Phase Diagrams: A Textbook, Oxford Univ. Press, 2001 Search PubMed.
- F. M. W. Vladimir Aseyev and H. Tenhu, Adv. Polym. Sci., 2011, 242, 29–89 CrossRef.
- J. Seuring and S. Agarwal, Macromol. Rapid Commun., 2012, 33, 1898–1920 CrossRef CAS PubMed.
- Y. C. Bae, S. M. Lambert, D. S. Soane and J. M. Prausnitz, Macromolecules, 1991, 24, 4403–4407 CrossRef CAS.
- B. Jin, Korea Polym. J., 1997, 5, 126–130 Search PubMed.
- R. Longenecker, T. Mu, M. Hanna, N. a D. Burke and H. D. H. Stöver, Macromolecules, 2011, 44, 8962–8971 CrossRef CAS.
- Y. Chang, W. Y. Chen, W. Yandi, Y. J. Shih, W. L. Chu, Y. L. Liu, C. W. Chu, R. C. Ruaan and A. Higuchi, Biomacromolecules, 2009, 10, 2092–2100 CrossRef CAS PubMed.
- P. Mary, D. D. Bendejacq, M. P. Labeau and P. Dupuis, J. Phys. Chem. B, 2007, 111, 7767–7777 CrossRef CAS PubMed.
- G. Bokias, G. Staikos and I. Iliopoulos, Polymer, 2000, 41, 7399–7405 CrossRef CAS.
- H. Katono, A. Maruyama, K. Sanui, N. Ogata, T. Okano and Y. Sakurai, J. Controlled Release, 1991, 16, 215–227 CrossRef CAS.
- D. Serrano-Ruiz, P. Alonso-Cristobal, M. Laurenti, B. Frick, E. López-Cabarcos and J. Rubio-Retama, Polymer, 2013, 54, 4963–4971 CrossRef CAS.
- F. Liu, J. Seuring and S. Agarwal, Macromol. Chem. Phys., 2014, 215, 1466–1472 CrossRef CAS.
- F. Liu, S. Jiang, L. Ionov and S. Agarwal, Polym. Chem., 2015, 6, 2769–2776 RSC.
- J. Seuring and S. Agarwal, Macromolecules, 2012, 45, 3910–3918 CrossRef CAS.
- X. Guo, K. Qiu and X. Feng, Chin. J. Polym. Sci., 1989, 7, 165–173 CAS.
- I. M. El-Naggar, G. M. Ibrahim, B. El-Gammal and E. a. El-Kady, Desalin. Water Treat., 2013, 3994 Search PubMed , Ahead of Print.
- S. Zhou, Z. Weng, Z. Huang and Z. Pan, 2001, 1431–1438.
- T. Caykara, S. Kiper and G. Demirel, Eur. Polym. J., 2006, 42, 348–355 CrossRef CAS.
- V. Ozturk and O. Okay, Polymer, 2002, 43, 5017–5026 CrossRef CAS.
- L. Hou and P. Wu, Soft Matter, 2015, 11, 7059–7065 RSC.
- C. Hou, L. Ying and C. Wang, J. Mater. Sci., 2005, 40, 609–612 CrossRef CAS.
- N. Orakdogen and O. Okay, Polym. Bull., 2006, 57, 631–641 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |