Zn-catalyzed hydrohydrazination of propargylamides with BocNHNH2: a novel entry into the 1,2,4-triazine core†
Alexey Lukina,
Tatiana Vedekhinaa,
Dmitry Tovpekoa,
Nikolay Zhuriloa and
Mikhail Krasavin*b
aLomonosov Institute of Fine Chemical Tehnologies, Moscow Technological University, 86 Vernadskogo Prospekt, Moscow, 117571 Russian Federation
bSaint Petersburg State University, Saint Petersburg, 199034 Russian Federation. E-mail: m.krasavin@spbu.ru; Fax: +7 812 428 6939; Tel: +7 931 3617872
First published on 6th June 2016
Abstract
Hydrohydrazination of a variety of propargylamides with BocNHNH2 under Zn(OTf)2 catalysis, unexpectedly, gave dihydro-1,2,4-triazines with a loss of the protecting group. The initial products can be efficiently aromatized in situ with K3[Fe(CN)6]. This provides a new entry into the medicinally important 1,2,4-triazine core.
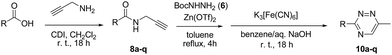
Recently, the Beller group reported a facile Zn-catalyzed hydroamination1 of propargylamides 1 with primary amines 2 leading to high yields of di- and tri-substituted imidazoles 3.2 Earlier, a similar reaction with phenylhydrazines 4 (hydrohydrazination3) had been shown to deliver 3-amidoindoles 5 via a domino alkyne-amination-Fischer-indole sequence of events.4 In our recent program aimed to identify new heterocyclic scaffolds for medicinal applications (particularly, as anti-infectives5) we sought to investigate if a similar hydrohydrazination of 1 with BocNHNH2 (6), which is not capable of entering a Fischer-type reaction, would deliver N-(Boc)amino imidazoles 7 which would be valuable building blocks for library synthesis of potential antibacterials (Fig. 1).6 However, the reaction took an unexpected (and hitherto unprecedented) course, of which we report herein.
 | ||
| Fig. 1 Examples of Zn-catalyzed hydroamination (a) and hydrohydrazination (b) of propargylamides from the Beller group and the variant involving BocNHNH2 (c) investigated in this work. | ||
A trial reaction of propargyl benzamide 8a and BocNHNH2 under Zn(OTf)2 catalysis (25 mol%) demonstrated that the principal product of it was 2,5-dihydro-1,2,4-triazine 9a. While it was isolated by chromatography in modest yield and characterized (see ESI†), its apparent instability (particularly to air oxidation) prompted us to seek an appropriate oxidant to aromatize 9a and thus obtain stable 1,2,4-triazine 10a (the instability of 9a was seen as common to partially aromatized heterocycles; at the same time many 2,5-dihydro-1,2,4-triazines are commercially available7). Among the oxidants screened, K3[Fe(CN)6]8 gave the best isolated yield of 10a (Table 1) and was, therefore, selected for further assessment of the scope of the newly identified method to prepare 1,2,4-triazines.
| Oxidant | Solvent (temperature) | Time (h) | Isolated yield (%) |
|---|---|---|---|
| O2 | Toluene (110 °C) | 8 h | 11 |
| MnO2 | CH2Cl2 (r. t.) | 12 h | 32 |
| DDQ | CH2Cl2 (r. t.) | 12 h | 42 |
| K3[Fe(CN)6] | Benzene/aq. NaOH | 12 h | 77 |
| KMnO4/SiO2 | Acetonitrile (r. t.) | 0.5 h | 60 |
| 10% Pd/C | Benzene (reflux) | 4 h | 0 |
In order to minimize the material losses potentially associated with the chemical instability of 2,5-dihydro-1,2,4-triazines, we tested the possibility of performing the oxidation in situ. This was successfully achieved by adding an alkaline aqueous solution of K3[Fe(CN)6] directly into the reaction mixture containing 9a. After stirring the biphasic reaction mixture overnight, an excellent yield of 80% was obtained, which presented a significant improvement of the total yield of 28.5% obtained with interim isolation of 9a (Scheme 1).
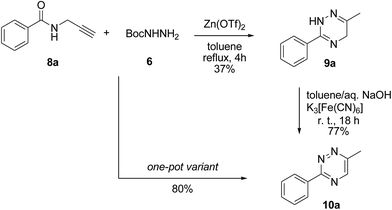 | ||
| Scheme 1 Synthesis of model 1,2,4-triazine 10a with and without the interim isolation of 2,5-dihydro-1,2,4-triazine 9a. | ||
It should be noted that besides the screening of oxidants, several reaction parameters were also altered for the synthesis of 9a (prior to its oxidation to 10a) in an attempt to see if there will be a further improvement of the overall yield. We tried to lower the temperature in the first step from reflux to 80 °C, which led to incomplete conversions even after 48 h. Solvent screening either at reflux temperatures or at 110 °C (benzene, acetonitrile, CH2Cl2, THF, DMF, methanol, xylenes) confirmed toluene was an optimal solvent for the synthesis. Interestingly, screening of other Lewis acids at 25 mol% (Cu(OTf)2, LiOTf, Sc(OTf)3, Gd(OTf)3, Yb(OTf)3, ZnCl2) provided no conversion of 8a into 9a whatsoever under the same conditions as Zn(OTf)2 was effective, thereby demonstrating the latter was a fortunate initial finding indeed.2,4 Likewise, a control reaction performed in the absence of any catalyst demonstrated no conversion of 8a.
Mechanistically, the conversion of 8a into 9a (as well as of the other propargylamides into respective 2,5-dihydro-1,2,4-triazines, vide infra) was likely to proceed via the initial hydrohydrazination of propargyl portion of 8a. Further role of Zn(OTf)2 (which turned out to be somewhat unique among other Lewis acids) could simply be in facilitating the removal of the Boc protecting group in 11. The resulting hydrazone (12) is likely to undergo cyclodehydration and deliver observed product 9a (Scheme 2). The importance of the Boc group is further substantiated by the fact that benzoic and acetic acid hydrazides did not furnish 2,5-dihydro-1,2,4-triazine products under the same conditions.
If intermediate 12 could indeed be involved in the formation of 9a, conversion of 8a into 9a should also be possible with unprotected hydrazine. We tested this idea using hydrazine hydrate as well as anhydrous hydrazine (obtained by distillation of the latter). While hydrazine hydrate delivered no product (most likely, due to catalyst hydration and de-activation), anhydrous hydrazine, to our delight, gave compound 9a. After in situ oxidation, compound 10a was isolated in 56% yield (Scheme 3). While this clearly argues for the above mechanistic vision, the yield obtained with Boc-protected hydrazine (6) was superior to that obtained using anhydrous hydrazine.
The established protocol of one-pot hydrohydrazination–cyclodehydration–oxidation leading to 1,2,4-triazines was extended to a range of propargylamines (8b–q) prepared by CDI-promoted amidation of the respective carboxylic acids (Scheme 4). This reliably yielded respective 1,2,4-triazines 10b–q in moderate to good yields (Table 2, see ESI† for characterization data). Despite the moderate yields observed in some cases, the method disclosed herein represents a practically simple and attractive, complimentary alternative to the existing methods.9 Many of the compounds obtained in this work can be considered as suitable tools for fragment-based drug discovery (especially so, considering the presence of the methyl substituent as producing a suitable signal NMR signal to observe in ligand-based fragment screens).10 The medicinal importance of the 1,2,4-triazines related to 10a–q in such areas as anti-inflammatory,11 metabolic12 and neurological13 disease (mGluR5 antagonists) has been established. Additionally, 1,2,4-triazines found utility as synthons for cycloaddition reactions14 and ligands for fluorescent15 and luminescent16 transition metal complexes.
| Compound | Structure | Isolated yield (%) |
|---|---|---|
| a Compound 10a is not included in the table as its synthesis has been described in preceding paragraphs related to the method development.b Conditions: Zn(OTf)2 (0.25 mol%), toluene, reflux, 4 h; then – aqueous K3[Fe(CN)6]/NaOH, 12 h. | ||
| 10b |  |
33 |
| 10c |  |
37 |
| 10d |  |
42 |
| 10e |  |
58 |
| 10f |  |
23 |
| 10g |  |
61 |
| 10h |  |
61 |
| 10i |  |
47 |
| 10j | 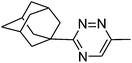 |
62 |
| 10k |  |
32 |
| 10l |  |
35 |
| 10m |  |
37 |
| 10n |  |
42 |
| 10o |  |
29 |
| 10p |  |
42 |
| 10q |  |
42 |
In summary, we have identified a practically simple, one-pot method to prepare 3,6-disubstituted 1,2,4-triazines via a Zn-catalyzed hydrohydrazination–cyclodehydration–oxidation sequence involving propargylamides and BocNHNH2, in moderate to good yields. Studies involving other N-nucleophiles in lieu of BocNHNH2 are currently underway in our laboratories and will be reported on in due course.
Acknowledgements
This research was supported by the Russian Scientific Fund (project grant 14-50-00069). We are grateful to the Center for Chemical Analysis and Materials Research of Saint-Petersburg State University for providing high-resolution mass-spectrometry data.Notes and references
- L. M. Pardo, I. Tellitu and E. Dominguez, Recent Res. Dev. Synth. Org. Chem., 2012, 12, 9–31 CAS.
- Selected reports on alkyne hydrohydrazination: (a) K. Alex, A. Tillack, N. Schwarz and M. Beller, Angew. Chem., Int. Ed. Engl., 2008, 47, 2304–2307 CrossRef CAS PubMed; (b) N. Schwarz, K. Alex, I. A. Sayyed, V. Khedkar, A. Tillack and M. Beller, Synlett, 2007, 1091–1095 CAS; (c) J. L. Peltier, R. Jazzar, M. Melami and G. Bertrand, Chem. Commun., 2016, 52, 2733–2735 RSC; (d) N. T. Patil and A. Konala, Eur. J. Org. Chem., 2010, 6831–6839 CrossRef CAS.
- A. Pews-Davtyan and M. Beller, Chem. Commun., 2011, 47, 2152–2154 RSC.
- A. Pews-Davtyan, A. Tillack, A.-C. Schmöle, S. Ortinau, M. J. Frech, A. Rolfs and M. Beller, Org. Biomol. Chem., 2010, 8, 1149–1153 CAS.
- M. Krasavin, P. Mujumdar, V. Parchinsky, T. Vinogradova, O. Manicheva and M. Dogonadze, J. Enzyme Inhib. Med. Chem., 2016 DOI:10.3109/14756366.2015.1101094 , in press.
- (a) T. L. Harris, R. J. Worthington and C. Melander, Bioorg. Med. Chem. Lett., 2011, 21, 4516–4519 CrossRef CAS PubMed; (b) J. M. Ruxer, C. Lachoux, J. B. Ousset, J. L. Torregrosa and G. Mattioda, J. Heterocycl. Chem., 1994, 31, 409–417 CrossRef CAS.
- According to SciFinder® (https://www.scifinder.cas.org/) search, seventy-five 3,6-disubstituted 2,5-dihydro-1,2,4-triazines related to 9a were available at the time of submission of this article, May 16 2016.
- S. Konno, S. Ohba, M. Sagi and H. Yamanaka, Chem. Pharm. Bull., 1987, 35, 1378–1382 CrossRef CAS.
- (a) C. W. Lindsley and M. E. Layton, Sci. Synth., 2004, 17, 357–447 CAS; (b) S. Konno, M. Sagi, Y. Yuki and H. Yamanaka, Heterocycles, 1985, 23, 2807–2810 CrossRef CAS; (c) T. V. Saraswathi and V. R. Srinivasan, Tetrahedron Lett., 1971, 12, 2315–2316 CrossRef; (d) T. Phucho, A. Nongpiur, S. Tumtin, R. Nongrum, B. Myrboh and R. L. Nongkhlaw, ARKIVOC, 2008, 79–87 CAS; (e) M. Kidwai, P. Sapra, K. R. Bhushan and P. Misra, Synth. Commun., 2001, 31, 1639–1645 CrossRef CAS; (f) A. I. Matveev and I. K. Moiseev, Khim. Geterocycl. Soed., 1989, 275–276 CAS; (g) S. Laphookhieo, S. Jones, S. A. Raw, Y. F. Sainz and R. J. K. Taylor, Tetrahedron Lett., 2006, 47, 3865–3870 CrossRef CAS; (h) N. T. Coogan, M. A. Chimes, J. Raftery, P. Mocilac and M. A. Denecke, J. Org. Chem., 2015, 80, 8684–8693 CrossRef CAS PubMed; (i) D. S. Kopchuk, N. V. Chepchugov, G. A. Kim, G. V. Zyryanov, I. S. Kovalev, V. L. Rusinov and O. N. Chupakhin, Tetrahedron Lett., 2016, 57, 296–299 CrossRef CAS.
- (a) D. A. Erlansson, Top. Curr. Chem., 2012, 317, 1–32 CrossRef; (b) B. J. Davis and D. A. Erlansson, Bioorg. Med. Chem. Lett., 2013, 23, 2844–2852 CrossRef CAS PubMed.
- (a) G. B. Bennett, R. B. Mason, L. J. Alden and J. B. Roach, J. Med. Chem., 1978, 21, 623–628 CrossRef CAS PubMed; (b) M. O'Rourke, S. A. Lang and E. Cohen, J. Med. Chem., 1977, 20, 723–726 CrossRef.
- F. I. Carroll, S. V. Kotturi, H. A. Navarro, S. W. Mascarella, B. P. Gilmour, F. L. Smith, B. H. Gabra and W. L. Dewey, J. Med. Chem., 2007, 50, 3388–3391 CrossRef CAS PubMed.
- G. Adam, E. Goetschi, J. Wichmann and T. J. Woltering, PCT Int. Appl. WO 2003066623A1, 2003 transChem. Abstr., 2003, 139, 180090 Search PubMed.
- (a) M. Ernd, M. Heuschmann and H. Zipse, Helv. Chim. Acta, 2005, 88, 1491–1518 CrossRef CAS; (b) N. Katagiri, H. Watanabe and C. Kaneko, Chem. Pharm. Bull., 1988, 36, 3354–3372 CrossRef CAS; (c) E. C. Taylor and L. G. French, J. Org. Chem., 1989, 54, 1245–1249 CrossRef CAS; (d) I. Akritopoulou-Zanze, Y. Wang, H. Zhao and S. W. Djuric, Tetrahedron Lett., 2009, 50, 5773–5776 CrossRef CAS.
- (a) D. N. Kozhevnikov, O. V. Shabunina, D. S. Kopchuk, P. A. Slepukhin and V. N. Kozhevnikov, Tetrahedron Lett., 2006, 47, 7025–7029 CrossRef CAS; (b) V. N. Kozhevnikov, O. V. Shabunina, D. S. Kopchuk, M. M. Ustinova, B. Koenig and D. N. Kozhevnikov, Tetrahedron, 2008, 64, 8963–8973 CrossRef CAS.
- (a) A. M. Prokhorov, V. N. Kozhevnikov, D. S. Kopchuk, H. Bernard, N. Le Bris, R. Tripier, H. Handel, B. Koenig and D. N. Kozhevnikov, Tetrahedron, 2011, 67, 597–607 CrossRef CAS; (b) D. N. Kozhevnikov, V. N. Kozhevnikov, M. M. Ustinova, A. Santoro, D. W. Bruce, B. Koenig, R. Czerwieniec, T. Fischer, M. Zabel and H. Yersin, Inorg. Chem., 2009, 48, 4179–4189 CrossRef CAS PubMed; (c) D. W. Bruce, V. N. Kozhevnikov and A. Santoro, PCT Int. Appl. WO 2010112799A1, 2010 transChem. Abstr., 2010, 153, 505977 Search PubMed; (d) A. Santoro, A. C. Whitwood, J. A. G. Williams, V. N. Kozhevnikov and D. W. Bruce, Chem. Mater., 2009, 21, 3871–3882 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12664b |
| This journal is © The Royal Society of Chemistry 2016 |