Synthesis and characterization of tungsten carbide and application to electrocatalytic hydrogen evolution
Can Liua,
Dali Zhou*a,
Jiabei Zhoub,
Zhen Xiea and
Yi Xiac
aDepartment of Materials Science and Engineering, Sichuan University, Chengdu 610064, China. E-mail: zdl@scu.edu.cn
bDepartment of Chemical Engineering, Sichuan University, Chengdu 610064, China
cResearch Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming 650093, China
First published on 8th August 2016
Abstract
Tungsten carbide was synthesized and further hydrogen treated to remove excess carbon. The obtained WC with different carbon contents was investigated by XRD, TG, SEM, EDS, BET and Raman. This work evaluated the effect of excess carbon on the properties of WC as a support for platinum. The hydrogen evolution reaction (HER) activity was used as a metric to test the effect of excess carbon on the electrochemical activity in 0.5 M sulfuric acid using a linear sweep voltammogram (LSV). Pt deposited on WC after hydrogen treatment exhibited a better electrocatalytic performance than Pt deposited on untreated WC. The investigations show that the presence of excess carbon is harmful to HER activity.
1. Introduction
With zero carbon dioxide emission, hydrogen is considered to be an ideal fuel in the future.1 Nowadays, the Toyota Fuel Cell Vehicle is set to make its debut on the road. Hydrogen gas is fed into the fuel cell stack where it is combined with oxygen. The electricity produced by this chemical reaction is used to power the electric motor and to charge the battery. Splitting water into hydrogen by renewable energy sources has been researched as a promising way for large scale hydrogen production. The catalysts used in the electrocatalytic systems for H2 generation are usually comprised of noble metals such as Pt due to their low overpotential and fast kinetics for driving the hydrogen evolution reaction (HER).2 However, the high cost and scarce reserve of Pt-group metals represent barriers to practical applications.3 Therefore, it is imperative to develop low-cost and highly efficient materials to replace the noble metals or reduce their usage.Since tungsten carbide (WC) was discovered to behave similar catalytic properties to those of platinum group metals for certain reactions by Levy and Boudart,4 it has been considered as an attractive non-noble metal catalyst for PEM fuel cell applications. Although the performance of WC is inferior in direct comparison to platinum, WC has received considerable attention because of the low price and insensitivity to catalyst poisons such as H2S and CO.5,6 However, tungsten carbide alone is not the suitable catalyst for HER due to its high overpotential, which consequently leads to the low electrocatalytic activity.7 In recent years, most researches prefer to study WC as an electrocatalyst substrate combined with platinum nanoparticles because it is generally believed to have a synergistic effect which leads to a higher intrinsic activity of Pt/WC electrocatalysts.8–11
Recently researchers have made an effort to prepare WC and evaluate the activity and stability when use WC as support compared to carbon. For instance, Liu et al.12 reported the as-synthesized tungsten carbide exhibited excellent performances for the oxygen reduction reaction. Their results showed the presence of amorphous carbon was beneficial for improving the conductivity and dispersibility of tungsten carbide catalyst. On the contrary, Hara et al.13 reported that the catalyst showed poor catalytic activity for hydrogen oxidation reaction due to the surface carbon. Elezović et al.14 prepared a tungsten support with a high specific surface area of 177 m2 g−1 containing WC islands on W particles and demonstrated the synthesized Pt/WC catalyst exhibited a remarkable improvement in catalytic activity towards oxygen reduction in comparison with Pt/Vulcan XC-72 carbon. In the work performed by Moon et al.,15 as compared to Pt/C, a more negative peak potential and a higher stability for methanol electrooxidation were shown by the Pt/WC. Later, Hassan and the co-workers16 reported the synthesized WC/C showed somewhat more stable against oxidation than Vulcan XC-72 carbon, which evidenced by potential cycling experiments.
It is worthwhile to mention that most of the preparation conditions on tungsten carbide result in samples with excess carbon, especially on the surface. Although carbon is a common electrochemical catalyst support, previous literature17 pointed out that the oxidation of the carbon support to produce CO2 can occur in an electrochemical environment, resulting in the separation of Pt particles from the carbon support and loss of performance. On the other hand, WC is stable at anode potentials below 0.6 V and the presence of Pt on WC can inhibit the oxidation of WC.18–20 Moreover, theoretical calculations of the Pt/WC surface demonstrated that the surface density states of Pt is modified by the direct interaction between Pt and WC,21 therefore the existence of carbon on the surface of WC may prevent such direct interaction.
In the present work, we developed a facile approach for the synthesis of tungsten carbide and tried to remove the excess carbon existed in the prepared product. In addition, the effect of excess carbon on the WC electrocatalytic activity was investigated by comparing the HER activity of untreated WC with that of WC after hydrogen treatment.
2. Experimental
Synthesis of tungsten carbide
Sodium tungstate (Na2WO4·2H2O) was dissolved in distilled water and stirred until complete dissolution. The solution was added dropwise into nitric acid (HNO3) in a beaker under magnetic stirring. The mixture was filtered and washed to get the white tungstic acid. A certain amount of hydrogen peroxide (H2O2) was added to the white tungstic acid to form transparent yellow solution (hydrated tungsten peroxides). Then the stable tungsten-containing system was obtained by putting ethanol and glacial acetic acid into the above solution. Afterwards, an appropriate amount of phenolic resin ethanol solution was added into the above system. The suspension was ultrasonically blended for 1 h and then water bath for a period of time. The precursor powder which was obtained after aging and drying was carbonized at 1000 °C for 1 h in Ar and H2 mixed atmosphere. The heating rate was controlled at 5 °C min−1. Finally, the tungsten carbide was synthesized. For the sake of removing amorphous carbon, the synthesized tungsten carbide was heat-treated at various temperatures under pure hydrogen atmospheres and then cooled down to room temperature in argon protection. Here, the temperature of hydrogen treatment was varied to 600 °C, 700 °C, 800 °C and 900 °C (the final products were assigned as WC600, WC700, WC800 and WC900, respectively).Preparation of electrocatalysts
Pt supported on WC was prepared through a microwave-assisted ethylene glycol (EG) process.22,23 The synthesized WC as the supporting material was mixed with EG in an ultrasonic bath, to which an appropriate amount of H2PtCl6·6H2O dissolved in EG was added dropwise. After the pH value of the mixture was adjusted to 10 by the addition of 0.5 mol L−1 NaOH/EG solution, the mixture was magnetic stirred for 1 h. Afterwards, the slurry was placed into a domestic microwave oven (700 W, 2.45 GHz) for 1 min. Thereafter, 1.0 mol L−1 HCl solution was employed to accelerate the deposition of Pt onto the WC support. At last, the product was filtered, washed and then dried in vacuum at 80 °C.Sample characterizations
Powder X-ray diffraction (XRD) measurements of the materials were carried out by using a Bruker DX-1000 Discovery instrument (Cu Kα, λ = 1.5418 Å). The patterns were recorded in the scan range of 2θ = 20–80° with a step size of 0.025° and a scan step of 15 s. Thermal analysis was carried out by using a simultaneous thermal analyzer (TG-DSC, STA 449C, Netzsch, Germany) in the temperature range of 40 °C to 900 °C in air at a heating rate of 10 °C min−1. Scanning electron microscopy (SEM, Hitachi S-4800) was employed to study the morphology of the synthesized materials. Raman measurement was performed on a Raman spectrometer (Renishaw Corp., UK) with a laser wavelength of 532 nm. Specific surface areas were measured by Brunauer–Emmett–Teller (BET) nitrogen adsorption–desorption on a TristarII 3020 (Micromeritics, USA) gas adsorption analyzer.Electrochemical measurements
For linear sweep voltammetry measurements, 5 mg of catalysts and 20 μl of 5 wt% Nafion solution were added to 0.50 ml of ethanol and 0.50 ml of H2O, and ultrasonic dispersed for 30 min to form a homogeneous ink. Then, 10 μl of the catalyst ink (containing 50 μg catalysts) was loaded onto a glassy carbon electrode of 5 mm in diameter to achieve a loading density of 0.25 mg cm−2. Electrochemical measurements were carried out using a conventional three-electrode cell containing 0.5 M H2SO4 aqueous solution. A saturated calomel electrode (SCE) was used as the reference electrode and was calibrated with respect to a reversible hydrogen electrode (RHE) (RHE = SCE* + 0.241 V + 0.0591 × pH). A glassy carbon and Pt wire were used as the working and counter electrode, respectively.3. Results and discussion
The XRD patterns of the synthesized WC are shown in Fig. 1A. Three major intensive diffraction peaks are shown with 2θ values of 31.57°, 35.72° and 48.40°, which well correspond to (001), (100) and (101) facets of WC (JCPDS 65-4539), respectively. There are no impurity peaks corresponding not only to tungsten trioxide or metallic tungsten, but also to W2C. No diffraction peaks of carbon can be observed, indicating that the carbon is amorphous state. In order to remove the excess carbon, treatment of WC sample with flowing hydrogen at an elevated temperature is the most commonly used procedure. Unfortunately, rather high temperatures which are adopted can also lead to the reduction of WC into metallic W. Therefore, it is necessary to know precisely under which conditions excess carbon can be removed without formation of metallic tungsten. In this study, we treated the prepared WC with flowing hydrogen at different temperatures. The WC600 and WC700 showed the same XRD pattern as untreated WC without the impurity phases. While further raised the temperature up to 800 °C and 900 °C, metallic tungsten could be detected by XRD. As shown in Fig. 1B, the diffraction peaks at 40.41°, 58.35° and 73.32° can be assigned to the facets of W (110), (200) and (211) respectively (JCPDS 01-1204), which means the carbidic carbon was removed at this temperature. However, WC exists as the main phase of this sample. Fig. 1C shows the XRD patterns of WC sample after hydrogen treatment at 900 °C. The obvious peaks related to W indicate that WC was almost completely decarburized into metallic tungsten at 900 °C. | ||
| Fig. 1 XRD patters of tungsten carbide: (A) untreated WC; (B) WC800; (C) WC900: (◆) for WC peaks, (●) for W peaks. | ||
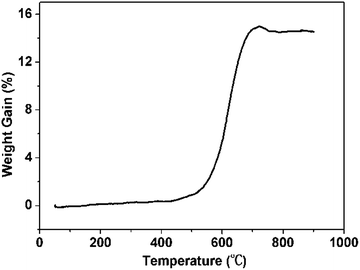
Fig. 2 shows the TGA results of the WC after hydrogen treatment at 700 °C measured under air atmosphere. We could find that the weight of the product has not changed significantly below 500 °C. In this stage, WC is very stable. As the temperature rises, the TG curve shows a significant increase, indicating that the WC sample reacted with oxygen to produce tungsten oxide. This increase reaches its maximum at around 700 °C, and then starts to decrease, which is attributed to gasification of excess carbon. Finally, the weight remains stable, which means the carbon is completely converted into in carbon dioxide and tungsten oxide exists as the final residue. As a result, the weight percentage of tungsten carbide in the product is about 97 wt% based on the TG analysis.
The SEM micrograph (Fig. 3a) of the untreated WC sample shows the presence of areas with crystalline WC and areas with amorphous carbon. It is evident that some particles are surrounded by a basically continuous phase. The particles should be the synthesized WC, and the basically continuous phase should be the polymeric carbon layers, which is derived from pyrolysis of phenolic resin. For the WC600 sample and WC700 sample, only little amorphous carbon can be seen. The WC particles exhibit slight agglomeration due to the high temperature at which carbide synthesis is performed. Table 1 shows the EDS data of WC, WC600 and WC700. The carbon content of all the samples is higher than the theoretical atomic ratio (C/W = 1), suggesting the inevitable existence of excess carbon in the final product. However, a comparison with the carbon content clearly shows that a large amount of excess carbon has been removed, indicating that the hydrogen treatment is a useful strategy for removing the excess carbon.
| The product | C atomic% | W atomic% | C/W atomic ratio |
|---|---|---|---|
| WC | 79.17 | 20.83 | 3.80 |
| WC600 | 70.79 | 29.21 | 2.42 |
| WC700 | 55.31 | 44.69 | 1.24 |
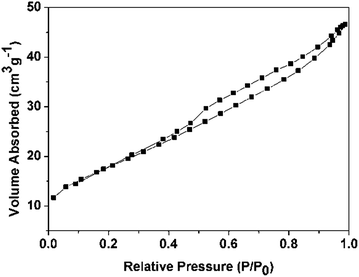
The N2 adsorption/desorption isothermal curves of untreated WC are shown in Fig. 4. The sample exhibited the representative type IV isotherms with distinct hysteresis loop which indicates the mesoporous structure of the prepared WC. The specific surface area of untreated WC is 67.587 m2 g−1. The surface area of untreated WC was measured and the obtained data was compared with those of WC after hydrogen treatment at different temperatures. As shown in Fig. 5, the surface area of WC shows a tendency to increase with raising the temperature up to 700 °C, suggesting the surface polymeric carbon has a negative effect on the specific surface area of WC. When the temperature of hydrogen treatment increases to 700 °C, the specific surface area of the WC reaches 95.286 m2 g−1. However, with further increase in temperature, the surface area starts to decrease due to destruction of WC and formation of metallic W. The larger surface area of WC is more beneficial for loading large amount of Pt nanoparticles, so it is reasonable to believe that the WC, after hydrogen treatment at 700 °C, is much more suitable for the use as a catalyst support.
Fig. 6 compares the Raman spectra of WC before and after hydrogen treatment. As shown in the graph, the peak located at 1335 cm−1 (D band) is associated with the A1g mode of diamond-like carbon with a sp3 configuration while 1582 cm−1 (G band) can be corresponded to the E2g vibration of graphitic carbon with a sp2 electronic configuration.24 The ratios of the G band to D band integrated intensity (IG/ID) are used to judge the degree of graphitization. The higher degree of graphitization helps to improve electron donor capacity of the support and consequently improve its catalytic activity. The IG/ID values for untreated WC, WC600 and WC700 are 0.86, 0.98 and 1.21, respectively, which means WC700 can serve as a kind of suitable catalyst support. The results show that the samples with the lower carbon content gave a higher degree of graphitization, which is consistent with the phenomenon observed by Lu et al.25 In this work, there are two possible reasons to explain the phenomenon, one is that the degree of graphitization increases with the treatment temperature arising, the other is WC has somewhat catalytic effect on graphitization so that the sample with a higher WC content shows a higher degree of graphitization.
The HER activities of various catalysts were determined by conducting linear sweep voltammograms (LSV) in 0.5 M H2SO4 at room temperature with a scanning rate of 5 mV s−1. Pt on untreated WC exhibited lower current density and more negative onset potential than the others, suggesting that the excess carbon have a detrimental effect on HER activity. These differences are most likely due to the increased degree of graphitization of the hydrogen-treated WC. Additionally, the increased BET surface area of the hydrogen-treated WC may lead to a higher current density than that of untreated WC. Another available way to evaluate the kinetic performance and intrinsic activity of catalysts is the comparison of Tafel slope by plotting potential versus log|j| (current density in logarithm). Tafel slope of 51 mV dec−1 for Pt on untreated WC suggests that it is inferior to both the Pt/WC600 (46 mV dec−1) and the Pt/WC700 (35 mV dec−1), as shown in Fig. 7b. Previous studies revealed that tungsten carbide promoted the catalytic activity through synergistic effects with Pt in fuel cells.26 Based on these findings, it seems that WC is not only used as a catalyst support, but also acting as a co-catalyst. Once the surface of WC was covered with excess polymeric carbon, WC could not associate with Pt at the active site, which might explain the lower HER activity of Pt on untreated WC. Since it was recognized that to achieve a high performance for the fuel cell catalyst, the catalyst support should have excellent electronic conductivity and high surface area, another possible explanation for the higher activity of Pt on WC after hydrogen treatment is that hydrogen-treated WC involved a higher degree of graphitization and a larger surface area.
 | ||
| Fig. 7 Linear sweep voltammograms (a) and Tafel plots (b) for Pt/WC, Pt/WC600 and Pt/WC700 in 0.5 M H2SO4 at the scanning rate of 5 mV s−1. | ||
Besides the catalytic activity, the durability of catalysts also has a great influence on the HER catalytic performance during the practical operation. Fig. 8 shows the polarization curve of the Pt/WC700 electrode at the 3000th potential sweep is similar to that in the first sweep and only a slight decay at high current region can be observed, thus demonstrating that the catalyst shows a good durability in an acidic environment. The promising stability of the catalyst can be attributed to neither the oxidation of Pt nor WC is likely at the negative operating potentials in HER applications.
4. Conclusions
In this work, we prepared nano-structured WC and successfully removed the excess carbon through hydrogen treatment. It was found that the carbon content decreased with the increase in temperature from 600 °C to 700 °C. However, with further increase in temperature, the undesirable metallic tungsten appeared, due to the removal of carbidic carbon. WC after hydrogen treatment showed a higher degree of graphitization and a larger surface area than untreated WC, which means the removal of excess carbon is beneficial for improving the conductivity and dispersibility of tungsten carbide support. Pt deposited on WC after hydrogen treatment had a higher HER activity than Pt on untreated WC, indicating that the amorphous carbon has a detrimental effect on HER activity.References
- S. S. Penner, Energy, 2006, 31, 33–43 CrossRef CAS.
- W. F. Chen, C. H. Wang, K. Sasaki, N. Marinkovic, W. Xu, J. T. Muckerman, Y. Zhu and R. R. Adzica, Energy Environ. Sci., 2013, 6, 943–951 CAS.
- P. Xiao, X. M. Ge, H. B. Wang, Z. L. Liu, A. Fisher and X. Wang, Adv. Funct. Mater., 2015, 25, 1520–1526 CrossRef CAS.
- R. B. Levy and M. Boudart, Science, 1973, 181, 547–549 CAS.
- V. S. Palanker and R. Gajyev, Electrochim. Acta, 1977, 22, 133–136 CrossRef CAS.
- X. Z. Zhou and Y. J. Qiu, Int. J. Hydrogen Energy, 2011, 36, 7398–7404 CrossRef CAS.
- M. Wu and P. K. Shen, J. Power Sources, 2007, 166, 310–316 CrossRef CAS.
- A. R. Ko, J. Y. Kim, J. K. Oh, H. S. Kim, Y. W. Lee, S. B. Han and K. W. Park, Phys. Chem. Chem. Phys., 2010, 12, 15181–15183 RSC.
- W. M. Zhu, A. Ignaszak, C. J. Song, R. Baker, R. Hui, J. J. Zhang, F. H. Nan, G. Botton, S. Y. Ye and S. Campbell, Electrochim. Acta, 2012, 61, 198–206 CrossRef CAS.
- Z. X. Yan, F. Li, J. M. Xie and X. L. Miu, RSC Adv., 2015, 5, 6790–6796 RSC.
- C. Y. He, J. Z. Tao, Y. B. Ke and Y. F. Qiu, RSC Adv., 2015, 5, 66695–66703 RSC.
- Z. W. Liu, P. Li, F. Q. Zhai, Q. Wan, A. A. Volinsky and X. H. Qu, RSC Adv., 2015, 5, 70743–70748 RSC.
- Y. Hara, N. Minami and H. Itagaki, Appl. Catal., A, 2007, 323, 86–93 CrossRef CAS.
- N. R. Elezović, B. M. Babić, L. Gajić-Krstajić, P. Ercius, V. R. Radmilović, N. V. Krstajić and L. M. Vračar, Electrochim. Acta, 2012, 69, 239–246 CrossRef.
- J. S. Moon, Y. W. Lee, S. B. Han and K. W. Park, Int. J. Hydrogen Energy, 2014, 39, 7798–7804 CrossRef CAS.
- A. Hassan, V. A. Paganin and E. A. Ticianelli, Appl. Catal., B, 2015, 165, 611–619 CrossRef CAS.
- Y. J. Wang, D. P. Wilkinson and J. J. Zhang, Chem. Rev., 2011, 111, 7625–7651 CrossRef CAS PubMed.
- M. Zellner and J. G. Chen, Catal. Today, 2005, 99, 299–307 CrossRef CAS.
- E. C. Weigert, D. V. Esposito and J. G. Chen, J. Power Sources, 2009, 193, 501–506 CrossRef CAS.
- K. Lee, A. Ishihara, S. Mitsushima, N. Kamiya and K. Ota, Electrochim. Acta, 2004, 49, 3479–3485 CrossRef CAS.
- D. V. Esposito, S. T. Hunt, A. L. Stottlemyer, K. D. Dobson, B. E. McCandless, R. W. Birkmire and J. G. Chen, Angew. Chem., Int. Ed., 2010, 49, 9859–9862 CrossRef CAS PubMed.
- S. Komarneni, D. S. Li, B. Newalkar, H. Katsuki and A. S. Bhalla, Langmuir, 2002, 18, 5959–5962 CrossRef CAS.
- S. Song, J. Liu, J. Shi, H. Liu, V. Maragou, Y. Wang and P. Tsiakaras, Appl. Catal., B, 2011, 103, 287–293 CrossRef CAS.
- T. Belin and F. Epron, Mater. Sci. Eng., B, 2005, 119, 105–118 CrossRef.
- J. L. Lu, Z. H. Li, S. P. Jiang, P. K. Shen and L. Li, J. Power Sources, 2012, 202, 56–62 CrossRef CAS.
- N. Liu, K. Kourtakis, J. C. Figueroa and J. G. Chen, J. Catal., 2003, 215, 254–263 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |