Recent advances in non-enzymatic electrochemical glucose sensors based on non-precious transition metal materials: opportunities and challenges
Xiangheng Niu
*ab,
Xin Li
b,
Jianming Pan
*b,
Yanfang He
b,
Fengxian Qiu
*b and
Yongsheng Yan
a
aInstitute of Green Chemistry and Chemical Technology, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: niuxiangheng@126.com
bSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: pjm@ujs.edu.cn; fxqiu@ujs.edu.cn
First published on 2nd August 2016
Abstract
With the booming requirements for diabetes management, food quality control, and bioprocess inspection, monitoring of glucose in various matrices has drawn unprecedented interest of both academic and industrial researchers recently. As a relatively new class of glucose sensors, enzyme-free detection of the target is capable of providing several fascinating characters such as ultra-high sensitivity, excellent stability, and simple fabrication. Considering the rapid expansion of the glucose determination field without using any biological enzymes, here we focus our attention on updating the latest advances in non-enzymatic electrochemical glucose sensors based on non-noble transition metal materials achieved in the past few years. In this minireview, both the superiorities and the intrinsic drawbacks of detecting glucose by employing non-precious materials including Ni, Cu, Co, Mn, and Fe are intensively highlighted, followed by a systematic discussion on the important progress harvested for enzymeless glucose sensing. Finally, the potential opportunities of non-noble transition metal materials in fabricating high-performance enzyme-free glucose sensors are given, and the current challenges for their practical applications are also summarized.
Introduction
There is no doubt that the detection of glucose has always been one of the research hotspots in analytical science. This lasting prosperity of glucose sensing is mainly driven by the huge demand for blood sugar management. According to the latest report from the World Health Organization (WHO),1 the present number of diabetics has a fourfold increase compared to that in 1980, reaching 422 million in 2014, and approximately 10% of adults (≥18 years old) in China are diabetes patients. Moreover, the increasing rate seems to have been accelerated recently because of harmful life styles and dietary habits. For these diabetics, frequent inspection of the glucose level in blood is of great importance to avoid fatal emergencies of hyperglycemia and hypoglycemia. With this enormous requirement, it is estimated that analytical devices for blood glucose detection have dominated about 85% of the whole biosensor industry.2 In addition to the above demand, other requirements including food quality control and bioprocess monitoring also promote the fast development of glucose sensors. In the former, the content of glucose in food needs to be strictly limited for specific consumers like diabetes patients, and in the latter it is necessary to control the glucose concentration in biological processes like alcoholic fermentation. For these applications, tremendous attention and interest from both academic and industrial fields have been focused on the exploration of glucose analytical devices with desired performance.For glucose sensing, several detection models have been established in the past few decades, including acoustic,3 magnetic,4 thermal,5 optical,6 and electrical7 transducers. Among these methods, electrochemical determination of the target via constructing various (bio)sensors has attracted special attention of scientists due to its favorable performance, facile fabrication, and miniaturized equipment. As categorized by the sensing element used to identify the analyte, electrochemical glucose (bio)sensors can be roughly classified into two types, i.e., enzyme-based biosensors and non-enzymatic sensors. Table 1 compares the characteristics of different electrochemical glucose (bio)sensors. Enzyme-based biosensors are based on the specific biocatalysis of enzymes (glucose oxidase, glucose dehydrogenase, etc.) toward the zymolyte. Since the first enzyme electrode was introduced by Clark and Lyons in 1962,8 three generations of enzymatic glucose biosensors have been expanded. Thanks to the continuous advances in the design of electron mediators and the immobilization of enzymes, enzyme-based biosensors are able to offer enough sensitivity and selectivity for detecting glucose in blood matrix. This kind of biosensors have been successfully commercialized for wide applications in clinical diagnosis and personal health management since the 1980s. However, the employment of biological enzymes brings some negative effects as well as positive roles. It has been widely recognized that the most serious drawback of enzyme-based biosensors is their poor stability. Due to the nature of biological enzymes, they are much sensitive to external conditions including temperature, pH, and chemical environment,9 thus leading to their insufficient long-term stability. As well, the instability of enzyme activity results in the difficulty in storing test strips.
| Category | Sensing element | Typical example | Involved reaction | Merit | Drawback |
|---|---|---|---|---|---|
| a GOD – glucose oxidase; GODox – the oxidation state of GOD; GODred – the reduction state of GOD; Mox – the oxidation state of electron mediator used; Mred – the reduction state of electron mediator used. | |||||
| Enzyme-based biosensors | Biological enzymes | GOD | Solution: (1) GODox + glucose → GODred + glucolactone. (2) GODred + O2 → GODox + H2O2. Electrode: H2O2 → O2 + 2H+ + 2e− | ♦ Excellent selectivity due to the specific biocatalysis | ✧ Much sensitive to external conditions, e.g., temperature |
| Solution: (1) GODox + glucose → GODred + glucolactone. (2) GODred + Mox→ GODox + Mred. Electrode: Mred → Mox + ne− | ♦ Sufficient sensitivity for blood glucose sensing | ✧ Long-term stability is still insufficient in some cases | |||
| Solution: GODox + glucose → GODred + glucolactone. Electrode: GODred → GODox + ne | ✧ Harsh storage conditions | ||||
| Non-enzymatic sensors | Noble metal catalysts | Pt | 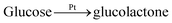 |
♦ Suitable for detecting glucose in blood directly | ✧ High cost |
| ♦ Relatively stable | ✧ Unsatisfied sensitivity | ||||
| ✧ Prone to poisoning | |||||
| ✧ Poor selectivity | |||||
| Non-precious transition metal materials | Ni | Ni(III) + glucose → Ni(II) + glucolactone | ♦ Ultra-high sensitivity | ✧ Only active in alkaline media, not suitable for detecting blood samples directly | |
| ♦ Stable under detection conditions | ✧ Poor selectivity | ||||
| ♦ Low cost | |||||
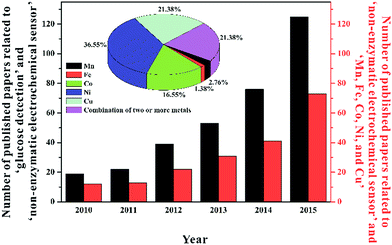
Considering the intrinsic flaws of insufficient stability and fair repeatability for enzyme-based biosensors, one moves interest to develop new electrochemical glucose sensors without using any bio-enzyme, namely non-enzymatic sensors. Different from enzyme-based biosensors, enzyme-free sensors are based on the direct electrocatalysis of electrode materials toward the analyte. With superiorities of good sensitivity, favorable stability, and facile fabrication, extensive efforts have been made to explore high-performance non-enzymatic sensors for the monitoring of glucose in the past few years, as confirmed by the increasing number of relevant papers shown in Fig. 1. Currently, there are mainly two kinds of materials widely utilized to assemble enzymeless glucose sensors. One is noble metal catalysts like Pt, which is suitable for detecting the target in blood samples directly. On Pt surface, glucose is able to be finally oxidized to glucolactone via losing two electrons.10 Although several advanced strategies have been introduced to improve the detection properties of Pt-based glucose sensors,11 the following challenges still hinder their applications in practical blood analysis:12 on the one hand, the activity of Pt-based catalysts is seriously poisoned by adsorbed Cl− and intermediates that can rapidly cover the electroactive surface; on the other hand, due to the electrocatalytic activity of Pt toward lots of small molecules, some endogenous and exogenous species including ascorbic acid (AA), dopamine (DA), uric acid (UA), and acetamidophenol (AP) can also be oxidized in the potential scope of glucose oxidation, thus interfering the selective determination of the target. Besides, the overall kinetics of glucose electro-oxidation is still too sluggish to provide remarkable amperometric responses, especially for the monitoring of glucose in non-blood physiological fluids such as tears, saliva, and sweat.13–15 In addition, the high cost and scarce resource of noble metals restrict their large-scale applications. The other is based on the electrocatalysis of non-precious transition metal materials like Ni toward glucose oxidation, where the polyvalence of these metals plays an important role in capturing electrons from the analyte and then transferring to current collectors. Because these non-noble transition metal materials can catalyze glucose in alkaline media with favorable reaction kinetics, ultra-high sensitivity (up to mA mM−1 cm−2) is usually harvested, very suitable for the low-level glucose detection in some cases. Moreover, these electrode materials are much cheap and accessible compared to noble metals. However, it has to be stated that this kind of non-enzymatic sensors only work in alkaline solutions, and can not be used for the direct detection of blood samples (blood samples should be diluted with alkaline electrolytes before measurement). Furthermore, other interfering species may produce false signals for the selective monitoring of the target. Although these, non-precious transition metal materials used for enzyme-free glucose sensing have still drawn increasing attention and interest of researchers recently, as demonstrated by Fig. 1.
Herein, we focus our attention on reviewing the latest advances in the fabrication of high-performance enzyme-free sensors for the electrochemical analysis of glucose. The reasons of doing this work are as follows: (1) although a few of comprehensive reviews have summarized the detection mechanisms of non-enzymatic glucose sensors,16 their merits over enzyme-based counterparts,10 advanced strategies for improving the analytical properties of Pt-based sensors,11 and applications of various nanostructured materials in fabricating enzyme-free sensing platforms,17,18 little specific attention has been paid to detailedly clarify the state-of-the-art research activities on non-precious transition metal-based glucose sensors; (2) it is obvious that non-enzymatic glucose sensors are currently experiencing a rapid prosperity with academic reports updating every day, and the rapid expansion of both theories and technologies requires a timely update of the research status. As a matter of fact, in the past three years, many significant advances in the field of glucose sensors based on non-noble transition metals and compounds have been obtained. In term of detection performance, ultrahigh sensitivity up to ∼100 mA mM−1 cm−2 and ultralow detection limit (LOD) down to several nM have been harvested by engineering an ideal sensing interface.19 For applications, one gradually turns attention and interest from blood sugar analysis to food, biomedical, bioengineering, and environmental applications, because some cases of the latter can be directly probed by these non-enzymatic glucose sensors, not like the case of blood glucose sensing in which samples must be treated with alkaline solutions before measurement. With respect to the fabrication of sensing interfaces, some new preparation methods, e.g., 3D printing,20 and interesting materials, e.g., metal–organic framework (MOF),21 have been explored in recent years. As well, understandings about this research field have also been advanced; (3) we believe that an illustration of the status will play some roles in directing the future development of this field. With these purposes, we are trying to exhibit a critical review of the advances in non-enzymatic glucose sensors based on non-noble transition metal materials (Mn, Fe, Co, Ni, Cu, etc.) achieved in the past three years, and intensive efforts are made to discuss the harvested achievements currently and the strategies utilized to improve the intrinsic shortfalls of this kind of enzyme-free glucose sensors. Also, a personal prospective of opportunities and challenges of these sensors in practical applications is finally described.
Non-enzymatic electrochemical glucose sensors based on non-noble transition metal materials
Non-precious transition metals and their oxides, hydroxides, sulfides, phosphides, and complexes have been widely utilized to fabricate efficient enzyme-free glucose sensors. Lying in the third line of the periodic table of elements, these metals are relatively inexpensive and can provide rapid and sensitive responses toward glucose molecules. Up date, V,22 Mn,23–25 Fe,26,27 Co,28–31 Ni,32–36 Cu,37–40 Zn,41,42 and so forth have been reported for the enzymeless detection of glucose. Here we will intensively discuss the latest developments of non-enzymatic electrochemical glucose sensors based on Mn, Fe, Co, Ni, and Cu. According to the percentage order of these metal materials, as depicted in the inset of Fig. 1, the following sections will introduce their advances in non-enzymatic glucose electrochemical sensors sequentially.Ni and its compounds
Ni and its compounds seem to be the most popular candidate used for the fabrication of non-enzymatic electrochemical glucose sensors due to their impressed catalytic activity resulting from the redox couple of Ni3+/Ni2+ in alkaline media. Making use of Ni as an electrocatalyst for glucose sensing is dependent on reforming its surface to form Ni(OH)2, thus yielding a surface rich in Ni2+ that is capable of undergoing the Ni3+/Ni2+ redox shuttle at a given potential.16 The +3 state in NiOOH induces the oxidation of glucose molecules to gluconolactone and in turn gets reduced to the +2 state in Ni(OH)2.However, the most serious shortcoming for oxides and hydroxides of Ni is their poor electrical conductivity. Therefore, substrates with promising electron transfer ability need to be developed to support these active materials. In the past three years, various carbon-based materials including CNTs,43–45 graphene,46,47 and other carbon substances48–54 and new substrates55–58 have been reported as supports for the loading of Ni-based catalysts. For example, Zhan et al. prepared a free-standing 3D graphene foam for the loading of Ni(OH)2 nanosheets for glucose sensing.59 It was believed that the 3D architecture of a graphene network without defects could effectively overcome the strong π–π interaction and intersheet contact resistance between graphene sheets and exhibit higher conductivity and larger specific surface area compared to chemically reduced graphene oxide sheets. In addition, the highly conductive 3D graphene foam synthesized on the Ni foam skeleton could also effectively increase the loading amount of catalysts, thus leading to an enhancement in electrocatalytic efficiency. Therefore, it was demonstrated that these outstanding properties of 3D graphene foam offered excellent performance for glucose biosensing, with a high sensitivity of ∼2.65 mA mM−1 cm−2 and a LOD of as low as 0.34 μM. El-Safty's group reported the first application of carbon dots as conductive electron transport channels for the electrochemical sensing of glucose.52 The authors modified nitrogen-doped carbon dot spheres onto the surface of the Ni foam electrode containing a 3D nanoarchitecture of dendritic NiO. These carbon dot spheres acted as electron transport channels between the active centers and the electrode–electrolyte interface. It was found that the addition of this promoter (carbon dot spheres) could enhance the catalytic activity toward glucose electro-oxidation by creating abundant surface defects. In addition, the coating of carbon dot spheres on the Ni foam surface effectively protected it from morphology change.
Another trend of enhancing the analytical properties of non-enzymatic glucose sensors is to explore novel nanoscale structures of Ni-based materials. Flower-like,60 hedgehog-like,61 needle-like,62 and spike-like63 Ni-based compounds have been reported for the enzyme-free detection of glucose in alkaline conditions. These structures, on the one hand, can offer large active surface and abundant sites for the electrocatalytic oxidation of glucose, as well as porous structures,64 and on the other hand, some morphologies may provide exciting catalytic properties because of exposed lattice planes. The work from Sharifi and co-workers has verified that the catalytic behavior of Ni-based nanomaterials is highly dependent on their shape.65 They obtained two NiO materials with different morphologies by electrodeposition in the presence of DNA modified on glassy carbon electrode (GCE) or not. On the bare GCE, spherical NiO nanoparticles with an average diameter of 600 nm were synthesized, while triangular nanoparticles with an average height of 50 nm and in-plane width of 500 nm uniformly distributed on the DNA-modified GCE surface were observed. Electrochemical measurements demonstrated that the electron transfer property and catalytic activity of the two materials were various.
Although advanced Ni-based nanostructures and Ni/C hybrids have been widely used for the enzyme-free glucose sensing. An apparent pitfall of these fabricated sensors is the weak mechanical stability and durability of performance, because they easily suffer from the possible agglomeration, deformation, and collapse of Ni-based nanostructures for an extended period of time under applied potential.66,67 In order to address this problem, we recently introduced a synthesized 3D porous Ni foam for glucose detection.68 The porous Ni structures were prepared via a hydrogen evolution assisted electrodeposition strategy, as depicted in Fig. 2(A). When a very large current density (up to A cm−2) was set to the electrodeposition system, much negative potential was correspondingly generated at the working electrode. In this case, both the Ni2+ precursor and H+ in electrolyte were reduced simultaneously. As bubbles from hydrogen evolution were generated, grew, coalesced, and finally left off the electrode surface dynamically, a dynamic template was formed, and the electrochemical reduction of the precursor took its way between these gas bubbles, finally resulting in the porous Ni architectures. The synthesized porous structures provided a large electrochemical surface area (much larger than commercial Ni foam), not only fasting the transport of electrolytes through the electrode/electrolyte interface due to shortened diffusion length and beneficial diffusion regime, but also allowing them to contact with more active surface through abundant pores and channels. More importantly, the 3D self-supported networks with interlaced frameworks were mechanically stable. After 100 reduplicative measurements of glucose in alkaline solutions, the self-supported Ni electrode still remained the 3D porous architecture, as demonstrated by Fig. 2(B), with no remarkable deformation and collapse of the structure under the high anodic potential (0.5 V vs. Ag/AgCl).
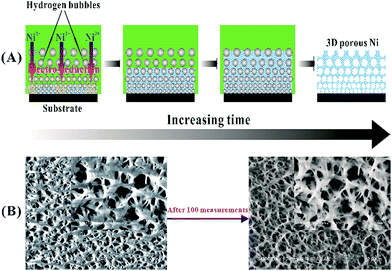 | ||
| Fig. 2 (A) Illustrates the formation process of 3D porous Ni nanostructures synthesized by the hydrogen evolution assisted electrodeposition, and (B) presents SEM images of the fabricated porous Ni material before and after 100 repetitive measurements. Adapted with permission from ref. 68. Copyright @ 2013 American Chemical Society. | ||
As can be seen from the above samples, most enzyme-free glucose sensors are based on the amperometric detection of the analyte by using efficient catalysts. In fact, other electrochemical techniques, like electrochemical impedance spectroscopy (EIS), can also be utilized as a highly sensitive tool for glucose sensing. One advantage of EIS over other direct-current techniques is that this steady-state technique is capable of probing relaxation phenomena over a wide frequency range, thus making high precision measurements since the response may be indefinitely steady and can therefore be averaged over a long period of time. Very recently, Rinaldi and Carballo fabricated an impedimetric non-enzymatic glucose sensor based on Ni(OH)2 thin film modified on Au electrode.69 They recorded the Nyquist diagrams of the fabricated sensor in 0.1 M KOH containing glucose in the concentration range of 0–1.32 mM at a potential of 0.5 V vs. Ag/AgCl. As shown in Fig. 3, the Nyquist plots consist of two slightly depressed capacitive semicircles in the high and low frequencies. The depressed semicircle in high frequency region can be related to the combination of charge transfer resistance and double layer capacitance. The low-frequency semicircle is related to the adsorption of reaction intermediates on the electrode surface. It is obvious that the resistance value decreased with the increasing content of glucose in this concentration range. Therefore, the target sensing can be finished based on the evaluation of the change of resistance as a function of glucose concentration.
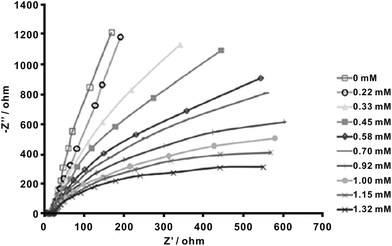 | ||
| Fig. 3 Nyquist plots of Ni(OH)2 thin film on Au electrode in 0.1 M KOH solution for glucose in the 0–1.32 mM concentration scope. Adapted with permission from ref. 69. Copyright @ 2016 Elsevier B.V. | ||
Cu and its compounds
Although the electro-oxidation of glucose on Cu-based materials in alkaline media is still under debate,70 it is still considered that Cu-based electrodes work in a similar way as Ni-based electrodes toward the electrochemical oxidation of glucose. As widely recognized, the catalytic reaction relies on the redox couple of Cu(III)/Cu(II), but the electron transfer process between Cu(III)/Cu(II) is not as evident as for Ni-based electrodes.16Similarly, many Cu-based materials with different oxidation states,71–75 shapes,72,76–81 and supports82–87 to improve the performance of non-enzymatic glucose sensors have been reported. The utilization of these strategies is to enlarge the active surface exposed, or/and improve the electronic conductivity of oxides. One has noted that the intimate interface contact between the highly electrocatalytic material and the current collector is of much significance for enhancing the electronic conductivity of electrodes and taking full advantage of electroactive substances. With this consideration, Zhao and co-workers synthesized hyper-branched Cu@Cu2O coaxial nanowires for the sensitive detection of glucose in alkaline media.71 The hierarchical architecture of branched coaxial nanowires, on the one hand, provided a large specific surface area for more active catalytic sites and easy accessibility for the target molecules, and on the other hand, was able to promote the electron transport because of the high-quality heterojunction with minimal lattice mismatch between the built-in current collector (Cu core) and the active medium (Cu2O shell). Furthermore, the adequate free space between branches and anisotropic nanowires could accommodate a large volume change to avoid collapse or distortion during the reduplicative operation processes. With these structural properties, very low LOD (40 nM) and ultra-fast response (within 0.1 s) were obtained on the fabricated sensor.71 Zhang's group also used metallic Cu to prepare nanosized Cu-based structures for efficient non-enzymatic glucose sensing.19 In their work, single crystalline mesoporous Cu2O nanothorn arrays were formed on commercial Cu foam via simple anodization in H2C2O4 and electrochemical oxidation in KOH, as shown in Fig. 4. Surprisingly, the assembled electrode provided an ultra-high sensitivity (up to 97.9 mA mM−1 cm−2) and an ultra-low LOD (down to 5 nM) for the enzyme-free electrochemical detection of glucose. These excellent analytical properties were ascribed to the synergistic action of superior electrochemical catalytic activity of nanothorn arrays with dramatically enhanced surface area and intimate contact between the active material (Cu2O) and the current collector (Cu foam), concurrently supplying good conductivity for electron/ion transport during glucose biosensing.19 We previously reported the synthesis of a porous Cu foam via hydrogen evolution assisted electrodeposition for the electrocatalysis and analysis of glucose in base solutions.88 By controlling the electrodeposition conditions, Cu foam with various porous properties could be facilely harvested. With large active surface exposed, the prepared Cu foam also exhibited fascinating sensitivities (2.57 and 1.81 mA mM−1 cm−2 in the linear concentration ranges of 2–80 μM and 0.1–5 mM, respectively) for glucose sensing.
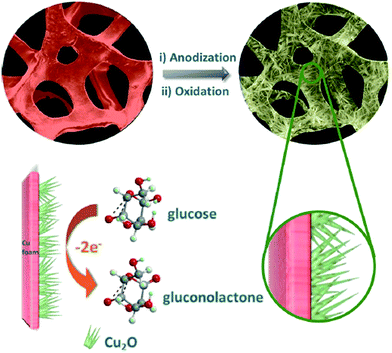 | ||
| Fig. 4 Schematic illustration of the fabrication of the 3D Cu foam-supported mesoporous Cu2O nanothorn array electrode and its function in the electrocatalytic oxidation of glucose in alkaline media. Adapted with permission from ref. 19. Copyright @ 2015 American Chemical Society. | ||
Beyond these, some emerging materials (Cu MOF etc.) and technologies (inkjet printing et al.) have also been utilized to fabricate non-enzymatic electrochemical glucose sensors. Liu and co-workers employed nanostructured [Cu3(btc)2], a Cu MOF crystal, to study its electrocatalytic behavior toward glucose in 0.1 M NaOH.21 Four Cu MOF nanocrystals with different morphologies (nanocubes, truncated cubes, cuboctahedrons, and octahedrons) were synthesized and investigated. It was interestingly found that the electrocatalytic activity of these crystals toward glucose oxidation depended on the crystal plane. By decreasing the planes from cubes to octahedra, the non-enzymatic electrocatalytic activity changed from highly sensitive to general. Considering the convenience of inkjet-printing in immobilizing nanomaterials onto a certain solid support, Ahmad et al. reported the first non-enzymatic glucose sensor based on CuO NPs that were inkjet-printed on a Si/Ag electrode.20 In comparison with the commonly used drop-casting method, the utilization of the emerging technique could produce modified electrodes with higher precision and on large scale.
Co and its compounds
In the pursuit of more efficient and economical materials for enzymeless glucose sensors, considerable interest has been focused on Co-based catalysts. Throughout the advances achieved in the past three years, it can be concluded that researchers have made many efforts to (1) synthesize different Co-based compounds for glucose sensing, (2) explore the effect of structures on the analytical properties, and (3) seek ideal supports for active catalyst loading.At present, various Co-based oxides (Co3O4,89–92 CoO,93 CoOx,94,95 etc.), phosphides,96 sulfides,97 and complexes98 have been explored for the enzyme-free electrochemical monitoring of glucose in alkaline media. For example, Li et al. reported the synthesis of CoOx NPs on reduced graphene oxide (rGO) for glucose analysis.94 Similar to that on Co3O4, several peaks corresponding to the conversion between different oxidation phases of Co(OH)2, Co3O4, CoOOH, and CoO2 were observed on the prepared CoOx. Wang's group designed and synthesized CoP nanorods for non-enzymatic glucose detection.96 The valence of Co was changeable, and Co was able to be easily exposed in the orthorhombic crystal of CoP. Therefore, high activity of the synthesized CoP toward glucose under alkaline conditions was obtained. Specially, the authors clarified the underlying electrocatalytic processes of CoP toward glucose by density-functional theory (DFT) calculations. In NaOH solution, glucose molecules, H2O, and OH− were considered as the three main reactive species in the electrocatalytic reaction system. It was calculated that the reaction of glucose with OH− was far easier than that of glucose with H2O. This result was also in good agreement with the experimental phenomenon that non-noble transition metal materials exhibited highly active electrocatalysis toward glucose with high pH values while negligible activity in neutral solutions. The authors further proposed that the main electrocatalytic reaction was controlled by the adsorbed structure of glucose molecules onto the OH−-covered CoP surface.
For high-performance glucose analysis, Qu and co-workers prepared nanoflower-like CoS decorated with porous carbon.97 In comparison with oxides, the shape of transition metal sulfides could be controlled more easily by adjusting the molar ratio of reactants. In addition, CoS compounds, as their chemical bonds Co–S had several strengths and binding mechanisms, were especially significant to form ionic bonds that were easily bound in small molecules.97 Chen's group fabricated a sensitive and selective enzyme-free amperometric glucose sensor based on cobalt phthalocyanine and CNTs.98 As reported previously, cobalt phthalocyanine could be used as electron mediators for enzyme-based glucose biosensors.99 With a reversible or quasi-reversible redox couple of Co(IV)/Co(III), the proposed Co-based complex exhibited satisfied properties for glucose electrocatalysis and electroanalysis as expected.
It is widely recognized that nanotechnology has endowed old materials with new prospects. Compared to bulk electrode, nanostructured materials not only offer large surface area, but also exhibit some unique properties for electrochemical applications. Similar to other nanomaterials, Co3O4 with different morphologies (nanocubes,91 microspheres,100,101 nanoflakes,102 nanowires,103,104 and other shapes105,106) and crystals107 have been intensively synthesized for the fabrication of non-enzymatic glucose sensors. Table 2 compares the analytical performance of various Co3O4 nanostructures toward glucose in alkaline solutions. A common purpose of these efforts is to provide larger active surface and more sites for the electrocatalysis of glucose oxidation. Of course, this purpose can be also realized by introducing abundant pores to these catalysts.90
| Shape | Linear range (μM) | Sensitivity (mA mM cm−2) | LOD (μM) | Ref. |
|---|---|---|---|---|
| Chrysalidocarpus lutescens-like | 0.2–14 | — | 0.06 | 105 |
| 15–225 | ||||
| Nanocubes | 500–3000 | — | 0.77 | 91 |
| Nanodisc-like | 500–5000 | 0.027 | 0.8 | 106 |
| Nanoflakes | 100–5000 | 1.18 | 0.7 | 102 |
| Porous nanowires | 5–570 | 0.3 | 5 | 103 |
| Hollow microspheres | 2–1480 | 0.069 | — | 101 |
| Nanowires | 0.1–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.046 | 0.0265 | 104 |
Like other metal oxides, Co-based nanostructures have two major weaknesses that impede their widespread applications. One is the poor electronic conductivity, and the other is the structure collapse. To alleviate the two problems, loading these nanostructures with conductive supports is proved to be an effective solution. With this consideration, CNTs,108,109 graphene,92–94,100 carbon matrices,110,111 conducting polymers,95 and even high-conductivity metals112 have been utilized to enhance the analytical properties of Co oxides. For instance, Bapu's group assembled a Co-MWCNT nanocomposite electrode for the enzymeless electrochemical determination of glucose.108 As a matter of fact, CNTs have been extensively used in fabricating electrochemical sensors because of their large surface-to-volume ratio, excellent electrical conductivity, and good stability. Moreover, CNTs in nanocomposites are able to promote electron transport along with electroactive sites for spatial diffusion.113 Therefore, composite materials synthesized by adding highly conductive CNTs to metal oxides can expect an performance enhancement owing to the synergistic effect between the two components rather than just one. As expected, higher sensitivity and lower onset potential for glucose detection were observed on the Co-MWCNT modified electrode compared to the bare Co modified one.108
Graphene is a 2D monolayer of sp2-hybridized carbon atoms, and has drawn enthusiastic attention and interest since its first isolation and characterization by Novoselov and Geim in 2004.114 Due to its excellent conductivity and electrocatalytic activity, graphene has been widely used in fabricating high-performance electrochemical sensors and biosensors.115 For instance, Ci and co-workers reported the enzymeless glucose detection on Co3O4/graphene microsphere hybrids.93 In their work, 3D crumpled graphene was used as a substrate for the loading of Co3O4 nanocrystals, which, on the one hand, enabled the full utilization of nanocrystals on both sides of the graphene sheet and prevented the loss of the catalyst surface area due to the natural restacking of graphene sheets, on the other hand, promoted the electron transfer between the Co3O4 particles and the glassy carbon electrode collector. As measured, the catalytic rate constant obtained on the Co3O4/crumpled graphene modified electrode was much larger than that on the bare Co3O4 modified one, confirming the enhancement role of graphene as an ideal substrate for catalyst loading.
Madhu et al. introduced honeycomb-like porous carbon for the support of Co3O4 particles.110 In comparison with the complicated synthesis of CNTs and graphene, the preparation of activated carbon seems to be much simple, eco-friendly, and cost-effective. In term of analytical properties, it was demonstrated that the loading of Co3O4 onto the activated carbon surface could improve the current response from glucose electro-oxidation.110
Beyond carbon materials, Yu and co-workers prepared a CoOx/over-oxidized polypyrrole film for the enzyme-free electrochemical determination of glucose in alkaline conditions.95 Polypyrrole, a common polyelectrolyte that transmits charge via delocalized π electrons, could bridge the electron transfer channel between the active catalyst and the collector. More importantly, over-oxidized polypyrrole was supposed to be capable of avoiding interferences caused by anions. During the over-oxidation process, oxygen-containing groups including carbonyl and carboxyl were introduced into the pyrrole unit, resulting in a cation-exchange and molecular sieve property, which would have superior anti-interference ability against negatively charged species in alkaline media.116,117 As a result, it was observed that addition of AA, DA, UA, maltose, and fructose exhibited negligible interference for the selective analysis of glucose.95
Very recently, Su and co-workers reported the used of noble metals, Au, to enhance the performance of Co3O4 for glucose detection.112 They combined electrodeposition with galvanic replacement to synthesize a controllable Co3O4/Au hierarchically nanostructure, as shown in Fig. 5(A). Loading a very low amount of Au with good electron conductivity on the surface of Co3O4 with large active surface area not only cut the cost in the case of expensive and rare Au but also improved the transportation of electrons between glucose and Co3O4 to greatly improve the sensitivity of the fabricated sensor, as demonstrated by Fig. 5(B). Additionally, the authors found that more negative onset potential was needed for glucose electro-oxidation due to the synergistic electrocatalytic activity of Au and Co3O4, thus effectively avoiding the interferences of existing species such as UA, DA, and AA.
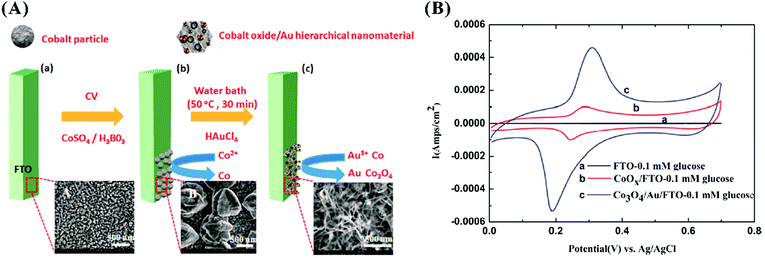 | ||
| Fig. 5 (A) Shows the fabrication of Co3O4/Au hierarchically nanostructured electrode, and (B) presents cyclic voltammograms of the bare FTO, CoOx/FTO, and Au/Co3O4/FTO in 0.1 M NaOH solution with the presence of 0.1 mM glucose. Adapted with permission from ref. 112. Copyright @ 2016 American Chemical Society. | ||
Mn and its compounds
As an important oxide of Mn, MnO2 is widely studied in the energy storage field, including supercapacitors118 and Li-ion batteries,119 due to its large specific capacitance and low cost. Usually, MnO2 can exhibit various structural forms (e.g., α-, β-, γ-, ε-, and λ-MnO2). Among these crystals, β-MnO2 is the most commonly used material in electrochemistry. β-MnO2 shows excellent chemical stability, with negligible dissolution even in strong acid. With a redox pair of Mn(IV)/Mn(III), MnO2 is able to capture electrons from glucose molecules and catalyze the electro-oxidation of the target. However, an apparent shortcoming of MnO2 is its low electronic conductivity (∼10−6 S cm−1), which significantly hinders its practical applications in enzyme-free glucose sensing.In order to enhance the electron transfer performance of MnO2, materials with outstanding electro-conductivity are supposed to be beneficial supports to load the active catalyst. In this term, Guo and co-workers studied the behavior of a 3D porous carbon nanotube (CNT)/MnO2 composite electrode for enzymeless glucose detection.25 They first modified CNTs onto a commercial Ni foam, and then electrodeposited MnO2 nanoflakes on the composite surface. As they stated, the prepared CNT/MnO2 electrode presented the following features: on the one hand, the utilization of CNTs allowed for the fast electron transport from the sensing interface to the Ni-based current collector; on the other hand, the porous structure of the composite electrode provided short and direct paths for mass diffusion and charge transfer with rapid reaction kinetics. As a result, the authors found that the response increasement caused by addition of 0.5 mM glucose on bare MnO2 was much lower than that on CNT/MnO2, demonstrating the positive role of CNTs in improving enzyme-free detection properties. Based on this case, other supports with excellent electron transport ability, such as polyelectrolytes and emerging graphene, are believed finding future applications to improve the performance of MnO2 for glucose sensing.
Beyond MnO2, Mn3O4 is also utilized for the enzyme-free electrochemical detection of glucose. Yang's group reported the analytical performance of Mn3O4 nanoparticles (NPs) toward glucose in alkaline media.120 Considering the aggregation phenomenon commonly occurred during the synthesis of small-sized nanostructures, which would result in the decrease of accessible active surface unfavorably, they used nitrogen-doped graphene (NG) as an effective additive to block the aggregation of NPs for retaining their large surface area. Besides, enhanced electron transfer properties of Mn3O4 NP/NG were also demonstrated by electrochemical impedance spectroscopy (EIS). In addition, Zhang et al. electrospun graphene-modified MnCo2O4 nanofibers for the enzymeless sensing of glucose.121 With a spinel structure, MnCo2O4 could exhibit synergistic enhancement in electrocatalytic activity over a simple combination of individual metal oxide.122 With the perfect incorporation of graphene with excellent conductivity and spinel-type MnCo2O4 with outstanding electrocatalytic activity, the synthesized composite provided an ultra-low limit of detection (LOD) for glucose sensing.
With the promising activity toward glucose electro-oxidation, various Mn-based materials are supposed to be prepared and utilized in future for the enzyme-free detection of the analyte. On the one hand, conductive materials beyond CNTs and graphene will be explored to further enhance the electron transfer ability of Mn-based catalysts. On the other hand, much attention may be paid on comparing the analytical performance of oxides (e.g., MnO2) with different crystals and shapes and further discussing the underlying mechanisms.
Fe and its compounds
As the most abundant transition metal on earth, Fe is considered as a distinguished electron mediator due to its reversible pair of Fe(III)/Fe(II). Because metallic Fe is unstable in air, various oxides of Fe (FeO, Fe2O3, Fe3O4, etc.) are usually prepared and utilized in analytical science. Among these, Fe3O4 NPs are the most commonly used magnetic materials for separation, drug delivery, and resonance imaging, thanks to their strong super-paramagnetic property, good biocompatibility, low toxicity, and easy preparation.123 Fe3O4 NPs are also found capable of catalyzing hydrogen peroxide as a mimetic peroxidase.124 In addition to these, Fe3O4 nanostructures with the redox of Fe(III)/Fe(II) can directly electro-catalyze the oxidation of glucose.Massomi-Godarzi and co-workers reported the utilization of Fe3O4 NPs supported on CNTs for the non-enzymatic electrochemical detection of glucose.26 Specifically, the authors carried out the measurements in phosphate buffer solution (PBS) at pH 7.0. In the absence of the analyte, a pair of redox peaks in the potential scope of −0.5 to −0.2 V vs. Ag/AgCl were observed, attributing to the Fe(III)/Fe(II) couple. When glucose was added into the electrolyte, both the anodic and cathodic responses increased dramatically, demonstrating the strong catalytic effect of Fe3O4/CNTs on the direct oxidation of glucose. In neutral media, the fabricated sensor was highly stable and selective, with 90% of activity retained after 40 days and no remarkable interference from AA and UA.
Beyond nanoparticles, other Fe3O4 structures were also prepared for enzyme-free glucose sensing. Zhang et al. combined the anodization of Fe foil with successive anneals in different atmospheres and synthesized two kinds of 1D structures.27 After anodization and anneal in air, α-Fe2O3 nanotube arrays (NTAs) grown on the foil were achieved, as shown in Fig. 6. With further anneal in H2 atmosphere, these α-Fe2O3 NTAs would transform to Fe3O4 nanorod arrays (NRAs). These 1D nanostructures of Fe3O4 not only provided direct channels for electron transport, but also survived from the irreversible aggregation usually occurring in nanoparticles. Interestingly, it was found that the 1D Fe3O4 NRAs were much more sensitive toward glucose than the α-Fe2O3 NTAs. The authors ascribed the underlying reason to the hopping between Fe3+ and Fe2+ in Fe3O4.
 | ||
| Fig. 6 Illustration of the preparation of the 1D Fe3O4 NRAs for non-enzymatic glucose detection. Adapted with permission from ref. 27. Copyright @ 2015 Springer-Verlag Wien. | ||
Potential opportunities and current challenges
Considering the insufficient stability of enzyme-based glucose biosensors, much attention is now focused on the fabrication of non-enzymatic electrochemical glucose sensors. In this context, non-noble transition metals, such as Ni, Cu, and Co, start to find promising applications in this electroanalytical field. Compared to other materials, these non-precious transition metal materials exhibit the following advantages in preparing high-performance glucose detection devices:(1) Highly catalytic activity. Both theoretical and experimental evidences have confirmed that non-precious transition metals, including Mn, Fe, Co, Ni, and Cu, can catalyze the electrochemical oxidation of glucose in high pH solutions with much fast reaction kinetics. With this, very high sensitivity ranging from hundreds of μA mM−1 cm−2 to tens of mA mM−1 cm−2 has been harvested, which is sufficient for the analysis of glucose in most samples. Moreover, the sensitivity can be further enhanced by introducing favorable supporting substrates, porous structures, and novel shapes with large active surface.
(2) Promising selectivity and stability. The difference in the reaction kinetics of common species including AA, DA, AP, and other sugars with that of the target makes it possible to detect glucose with good selectivity. In addition, these catalysts will not be poisoned by chloride ions and intermediates that usually block the active surface in the Pt-based catalyst case.
(3) The low cost and abundant resource of these materials. Different from noble metals like Pt, these transition metal materials including Mn, Fe, Co, Ni, and Cu are much inexpensive, and their resources are also much abundant on earth. This advantage will not limit the large-scale utilization of them in practical applications.
Although the above prospects, it should be stated that practical applications of this kind of glucose sensors still meet the following challenges:
(a) As demonstrated by both theoretical and experimental results, the catalysis of most non-precious transition metal materials toward glucose is highly dependent on the presence of concentrated OH− anions. In other words, these materials exhibit highly electrocatalytic activity for the oxidation of the target only in strong base conditions, and their catalytic capacity turns to be negligible in either neutral or acid solutions. Therefore, their use in glucose sensing is very limited. For instance, these fabricated sensors can not be used for the direct analysis of neutral blood samples. Instead, these samples need to be diluted into an alkaline electrolyte for further measurements. This drawback not only makes the measurement process more complicated, but also requires for sensors with higher sensitivity for the analysis of blood samples.
(b) Although some common species including AA, DA, UA, AP, and other monosaccharides have been reported providing very weak interference on the detection of glucose, the selectivity of this kind of sensors remains a huge problem. It has been found that all small organic molecules can be oxidized at the same potential, immediately after the formation of active sites. As a major oxidizable component, ethanol, is often present in many samples, the presence of even low alcohol levels will affect the glucose determination significantly.
(c) These non-precious transition metal materials have proved to be unaffected by Cl− (a common species that can significantly poison noble metals like Pt for glucose analysis), and the fouling of species on a suitable electrode substrate can also be ignorable, a promising long-term stability much better than that of enzyme-based biosensors can be achieved. Although these, the present durability is still not enough for practical applications in some cases. Moreover, the nanomaterials used for the fabrication of non-enzymatic glucose sensors easily suffer from the agglomeration, deformation, and collapse of structures in an extended period of time under high applied potential, thus resulting in the decreased durability of performance.
(d) Although many nanosized materials and structures have been reported providing excellent properties for the enzyme-free determination of glucose, the repeatable synthesis of these nanostructures on a large scale is still a challenge. Similar to other fields, many practical applications of nanostructured materials have been restricted by their reproducible preparation with low cost, and it is also so for non-enzymatic electrochemical glucose sensors.
(e) As shown in this minireview, most of the proposed sensors are first tested and studied in buffers, which simulate similar conditions as in biological matrices. The development of such sensor for clinical applications with biological matrices is far more complicated, and there is nor always a perfect match between the normal concentrations and the analytical ranges of the sensors developed. That is, the performance of sensors using buffers in simulated conditions cannot be directly transposed to clinical conditions. Once developed on the lab scale, there is room for further improvements for non-enzymatic glucose sensors at the clinical level and their application to real biological samples.
(f) By comparing the reported results of non-enzymatic glucose sensors, it is found that the analytical properties (sensitivity, etc.) of these devices have been gradually improved through various strategies. However, these results are obtained by different research groups under different conditions. It is known that the measurement conditions (applied potential, electrolyte, diffusion, etc.) will have important effect on the analytical performance. Up today, a universal performance evaluation protocol is still lack, which also hinder the development of the field.
In our opinion, many efforts in near future will be focused on the following aspects to minimize the intrinsic disadvantages:
(1) The biggest shortcoming of transition metal oxides and compounds used for electrochemical biosensing is their poor electrical conductivity. Therefore, promoting the electron transport between active catalysts and current collectors is of great importance. At present, one mainly supports these active catalysts with carbon-based materials (CNTs, graphene, etc.). Although these carbon materials exhibit good performance in enhancing the analytical properties of non-enzymatic glucose sensor. The complex preparation and comparatively high cost of them make it not perfect for large-scale applications. Developing new and effective conductive supports will still draw much interest in the near future.
(2) In order to enhance the analytical performance of a non-enzymatic glucose sensor, increasing the exposed active surface has been demonstrated an efficient routine. Therefore, various materials with beneficial porosity (macroporous, mesoporous, and even microporous) are believed finding wide applications in fabricating satisfied glucose detection devices.
(3) As demonstrated by some experimental results, the electrocatalytic activity of non-precious transition metal materials toward the oxidation of glucose molecules depends on their shapes and crystals. However, the underlying mechanisms are still not clear. Therefore, more attention need to be focused on this question to further develop high-performance glucose sensors.
(4) Though some detection protocols have been proposed for the selective sensing of the target, these non-enzymatic glucose themselves have limited selectivity. Therefore, the design and development of non-precious transition metal materials with high selectivity toward the target in complex conditions will be one of the greatest challenges to be tackled.
(5) Although nanostructures play an important role in enhancing the analytical properties of enzyme-free electrochemical glucose sensors, a pitfall of these structures, especially nanoparticles, as mentioned before, is the weak mechanical stability and durability of performance. Therefore, developing nanomaterials with highly mechanical stability to improve the detection durability needs more attention in the near future.
Conclusions
This minireview has highlighted the latest advances in the field of enzyme-free electrochemical glucose sensing achieved in the past three years. As can be seen from the rapidly growing number of publications, the research in this field is highly active. Although several advanced strategies have been introduced to successfully improve the detection properties of non-enzymatic glucose sensors, it must be recognized that the real application of these fabricated devices is still a great challenge. Therefore, co-efforts from researchers around the world are required to further minimize the drawbacks and ultimately realize the dream of applying high-performance non-enzymatic electrochemical glucose sensors to various practical analyses.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21305044, 21574091, and 21576120), the Natural Science Foundation of Jiangsu Province (No. BK20131223 and BK20150433), the National Postdoctoral Science Foundation (No. 2013M530240), the Special National Postdoctoral Science Foundation (No. 2014T70480), the Postdoctoral Science Foundation of Jiangsu Province (No. 1202002B), the Programs of Senior Talent Foundation of Jiangsu University (No. 12JDG090), and the Start-Up Research Fund of Jiangsu University (No. 15JDG143).Notes and references
- http://www.who.int/diabetes/global-report.
- S. K. Vashist, Anal. Chim. Acta, 2012, 750, 16–27 CrossRef CAS PubMed.
- J. T. Luo, P. X. Luo, M. Xie, K. Du, B. X. Zhao, F. Pan, P. Fan, F. Zeng, D. P. Zhang, Z. H. Zheng and G. X. Liang, Biosens. Bioelectron., 2013, 49, 512–518 CrossRef CAS PubMed.
- M. Gourzi, A. Rouane, R. Guelaz, M. S. Alavi, M. B. McHugh, M. Nadi and P. Roth, J. Med. Eng. Technol., 2005, 29, 22–26 CrossRef CAS PubMed.
- C. D. Malchoff, K. Shoukri, J. I. Landau and J. M. Buchert, Diabetes Care, 2002, 25, 2268–2275 CrossRef CAS PubMed.
- M. S. Steiner, A. Duerkop and O. S. Wolfbeis, Chem. Soc. Rev., 2011, 40, 4805–4839 RSC.
- J. Wang, Chem. Rev., 2008, 108, 814–825 CrossRef CAS PubMed.
- L. C. Clark and C. Lyons, Ann. N. Y. Acad. Sci., 1962, 102, 29–45 CrossRef CAS PubMed.
- R. Wilson and A. P. F. Turner, Biosens. Bioelectron., 1992, 7, 165–185 CrossRef CAS.
- S. Park, H. Boo and T. D. Chung, Anal. Chim. Acta, 2006, 556, 46–57 CrossRef CAS PubMed.
- X. H. Niu, L. B. Shi, H. L. Zhao and M. B. Lan, Anal. Methods, 2016, 8, 1755–1764 RSC.
- J. P. Wang, D. F. Thomas and A. C. Chen, Anal. Chem., 2008, 80, 997–1004 CrossRef CAS PubMed.
- A. Soni and S. K. Jha, Biosens. Bioelectron., 2015, 67, 763–768 CrossRef CAS PubMed.
- M. X. Chu, K. Miyajima, D. Takahashi, T. Arakawa, K. Sano, S. Sawada, H. Kudo, Y. Iwasaki, K. Akiyoshi, M. Mochizuki and K. Mitsubayashi, Talanta, 2011, 83, 960–965 CrossRef CAS PubMed.
- O. Olarte, J. Chilo, J. Pelegri-Sebastia, K. Barbé and W. V. Moer, 2013 35th Ann. Int. Conf. IEEE Eng. Med. Biol. Soc., 2012, pp. 1462–1465 Search PubMed.
- K. E. Toghill and R. G. Compton, Int. J. Electrochem. Sci., 2010, 5, 1246–1301 CAS.
- P. Si, Y. J. Huang, T. H. Wang and J. M. Ma, RSC Adv., 2013, 3, 3487–3502 RSC.
- G. F. Wang, X. P. He, L. L. Wang, A. X. Gu, Y. Huang, B. Fang, B. Y. Geng and X. J. Zhang, Microchim. Acta, 2013, 180, 161–186 CrossRef CAS.
- C. Q. Dong, H. Zhong, T. Y. Kou, J. Frenzel, G. Eggeler and Z. H. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 20215–20223 CAS.
- R. Ahmad, M. Vaseem, N. Tripathy and Y. B. Hahn, Anal. Chem., 2013, 85, 10448–10454 CrossRef CAS PubMed.
- Y. Y. Liu, Y. J. Zhang, J. Chen and H. Pang, Nanoscale, 2014, 6, 10989–10994 RSC.
- A. Doménech and J. Alarcón, Anal. Chem., 2007, 79, 6742–6751 CrossRef PubMed.
- J. Chen, W. D. Zhang and J. S. Ye, Electrochem. Commun., 2008, 10, 1268–1271 CrossRef CAS.
- S. L. Yang, L. X. Liu, G. F. Wang, G. Li, D. H. Deng and L. B. Qu, J. Electroanal. Chem., 2015, 755, 15–21 CrossRef CAS.
- C. Y. Guo, H. Li, X. Zhang, H. H. Huo and C. L. Xu, Sens. Actuators, B, 2015, 206, 407–414 CrossRef CAS.
- S. Massomi-Godarzi, A. A. Khodadadi, M. Vesali-Naseh and Y. Mortazzavi, J. Electrochem. Soc., 2014, 161, B19–B25 CrossRef.
- C. Zhang, H. W. Ni, R. S. Chen, W. T. Zhan, B. W. Zhang, R. Lei, T. P. Xiao and Y. X. Zha, Microchim. Acta, 2015, 182, 1811–1818 CrossRef CAS.
- C. W. Kung, C. Y. Lin, Y. H. Lai, R. Vittal and K. C. Ho, Biosens. Bioelectron., 2011, 27, 125–131 CrossRef CAS PubMed.
- K. K. Lee, P. Y. Loh, C. H. Sow and W. S. Chin, Electrochem. Commun., 2012, 20, 128–132 CrossRef CAS.
- X. W. Wang, X. C. Dong, Y. Q. Wen, C. M. Li, Q. H. Xiong and P. Chen, Chem. Commun., 2012, 48, 6490–6492 RSC.
- L. Ozcan, Y. Sahin and H. Turk, Biosens. Bioelectron., 2008, 24, 512–517 CrossRef CAS PubMed.
- A. L. Sun, J. B. Zheng and Q. L. Sheng, Electrochim. Acta, 2012, 65, 64–69 CrossRef CAS.
- N. Q. Qiao and J. B. Zheng, Microchim. Acta, 2012, 177, 103–109 CrossRef CAS.
- G. M. Wang, X. H. Lu, T. Zhai, Y. C. Ling, H. Y. Wang, Y. X. Tong and Y. Li, Nanoscale, 2012, 4, 3123–3127 RSC.
- T. Watanabe and Y. Einaga, Biosens. Bioelectron., 2009, 24, 2684–2689 CrossRef CAS PubMed.
- C. Y. Guo, Y. M. Wang, Y. Q. Zhao and C. L. Xu, Anal. Methods, 2013, 5, 1644–1647 RSC.
- Z. J. Zhuang, X. D. Su, H. Y. Yuan, Q. Sun, D. Xiao and M. M. F. Choi, Analyst, 2008, 133, 126–132 RSC.
- J. Luo, S. S. Jiang, H. Y. Zhang, J. Q. Jiang and X. Y. Liu, Anal. Chim. Acta, 2012, 709, 47–53 CrossRef CAS PubMed.
- J. Liu and D. F. Xue, J. Mater. Chem., 2011, 21, 223–228 RSC.
- X. J. Zhang, G. F. Wang, W. Zhang, N. J. Hu, H. Q. Wu and B. Fang, J. Phys. Chem. C, 2008, 112, 8856–8862 CAS.
- G. N. Dar, A. Umar, S. A. Zaidi, S. Baskoutas, S. H. Kim, M. Abaker, A. Al-Hajry and S. A. Al-Sayari, Sci. Adv. Mater., 2011, 3, 901–906 CrossRef CAS.
- T. T. Baby and S. Ramaprabhu, J. Nanosci. Nanotechnol., 2011, 11, 4684–4691 CrossRef CAS PubMed.
- T. J. Choi, S. H. Kim, C. W. Lee, H. Kim, S. K. Choi, S. H. Kim, E. Kim, J. Park and H. Kim, Biosens. Bioelectron., 2015, 63, 325–330 CrossRef CAS PubMed.
- K. Z. Qiu, X. Chen, S. Q. Ci, W. P. Li, Z. Bo, K. F. Cen and Z. H. Wen, Anal. Lett., 2016, 49, 568–578 CrossRef CAS.
- R. Prasad and B. R. Bhat, Sens. Actuators, B, 2015, 220, 81–90 CrossRef CAS.
- I. Shackery, U. Patil, M. J. Song, J. S. Sohn, S. Kulkarni, S. Some, S. C. Lee, M. S. Nam, W. Lee and S. C. Jun, Electroanalysis, 2015, 27, 2363–2370 CrossRef CAS.
- B. Zhang, Y. He, B. Q. Liu and D. P. Tang, Microchim. Acta, 2015, 182, 625–631 CrossRef CAS.
- W. Y. Jeon, Y. B. Choi and H. H. Kim, Sensors, 2015, 15, 31083–31091 CrossRef CAS PubMed.
- M. Hjiri, R. Dhahri, N. B. Mansour, L. E. Mir, M. Bonyani, A. Mirzaei, S. G. Leonardi and G. Neri, Mater. Lett., 2015, 160, 452–455 CrossRef CAS.
- R. M. A. Hameed, Biosens. Bioelectron., 2013, 47, 248–257 CrossRef PubMed.
- V. Veeramani, R. Madhu, S. M. Chen, P. Veerakumar, C. T. Hung and S. B. Liu, Sens. Actuators, B, 2015, 221, 1384–1390 CrossRef CAS.
- N. Akhtar, S. A. El-Safty, M. E. Abdelsalam and H. Kawarada, Adv. Healthcare Mater., 2015, 4, 2110–2119 CrossRef CAS PubMed.
- C. Y. Ko, J. H. Huang, S. Raina and W. P. Kang, Analyst, 2013, 138, 3201–3208 RSC.
- Y. Y. Fu, W. Su, T. Wang and J. B. Hu, RSC Adv., 2015, 5, 54777–54782 RSC.
- Z. D. Gao, J. Guo, N. K. Shrestha, R. Hahn, Y. Y. Song and P. Schmuki, Chem.–Eur. J., 2013, 19, 15530–15534 CrossRef CAS PubMed.
- H. H. Huo, Y. Q. Zhao and C. L. Xu, J. Mater. Chem. A, 2014, 2, 15111–15117 CAS.
- P. Sivasakthi, G. N. K. R. Bapu and M. Chandrasekaran, Mater. Sci. Eng., C, 2016, 58, 782–789 CrossRef CAS PubMed.
- J. Chen and J. B. Zheng, J. Electroanal. Chem., 2015, 749, 83–88 CrossRef CAS.
- B. B. Zhan, C. B. Liu, H. P. Chen, H. X. Shi, L. H. Wang, P. Chen, W. Huang and X. C. Dong, Nanoscale, 2014, 6, 7424–7429 RSC.
- A. Rengaraj, Y. Haldorai, C. H. Kwak, S. Ahn, K. J. Jeon, S. H. Park, Y. K. Han and Y. S. Huh, J. Mater. Chem. B, 2015, 3, 6301–6309 RSC.
- R. A. Soomro, Z. H. Ibupoto, M. I. Abro and M. Willander, Sens. Actuators, B, 2015, 209, 966–974 CrossRef CAS.
- T. Marimuthu, S. Mohamad and Y. Alias, Synth. Met., 2015, 207, 35–41 CrossRef CAS.
- X. Zhao, F. Muench, S. Schaefer, J. Brotz, M. Duerrschnabel, L. Molina-Luna, H. J. Kleebe, S. X. Liu, J. Tan and W. Ensinger, Electrochem. Commun., 2016, 65, 39–43 CrossRef CAS.
- B. Zhao, T. Wang, L. Jiang, K. Zhang, M. M. F. Yuen, J. B. Xu, X. Z. Fu, R. Sun and C. P. Wong, Electrochim. Acta, 2016, 192, 205–215 CrossRef CAS.
- E. Sharifi, A. Salimi, E. Shams, A. Noorbakhsh and M. K. Amini, Biosens. Bioelectron., 2014, 56, 313–319 CrossRef CAS PubMed.
- C. X. Guo and C. M. Li, Phys. Chem. Chem. Phys., 2010, 12, 12153–12159 RSC.
- J. H. Zhu, J. Jiang, J. P. Liu, R. M. Ding, Y. Y. Li, H. Ding, Y. M. Feng, G. M. Wei and X. T. Huang, RSC Adv., 2011, 1, 1020–1025 RSC.
- X. H. Niu, M. B. Lan, H. L. Zhao and C. Chen, Anal. Chem., 2013, 85, 3561–3569 CrossRef CAS PubMed.
- A. L. Rinaldi and R. Carballo, Sens. Actuators, B, 2016, 228, 43–52 CrossRef CAS.
- S. K. Meher and G. R. Rao, Nanoscale, 2013, 5, 2089–2099 RSC.
- Y. X. Zhao, L. L. Fan, Y. Zhang, H. Zhao, X. J. Li, Y. P. Li, L. Wen, Z. F. Yan and Z. Y. Huo, ACS Appl. Mater. Interfaces, 2015, 7, 16802–16812 CAS.
- S. Felix, P. Kollu, B. P. C. Raghupathy, S. K. Jeong and A. N. Grace, J. Chem. Sci., 2014, 126, 25–32 CrossRef CAS.
- B. Qi, H. J. Yang, K. Y. Zhao, M. M. Bah, X. J. Bo and L. P. Guo, J. Electroanal. Chem., 2013, 700, 24–29 CrossRef CAS.
- X. H. Gao, Y. Z. Lu, M. M. Liu, S. J. He and W. Chen, J. Mater. Chem. C, 2015, 3, 4050–4056 RSC.
- M. J. Song, S. K. Lee, J. H. Kim and D. S. Lim, J. Electrochem. Soc., 2013, 160, B43–B46 CrossRef CAS.
- S. H. Weng, Y. J. Zheng, C. F. Zhao, J. Z. Zhou, L. Q. Lin, Z. F. Zheng and X. H. Lin, Microchim. Acta, 2013, 180, 371–378 CrossRef CAS.
- L. Xu, Q. Yang, X. J. Liu, J. F. Liu and X. M. Sun, RSC Adv., 2014, 4, 1449–1455 RSC.
- P. Gao and D. W. Liu, RSC Adv., 2015, 5, 24625–24634 RSC.
- Y. C. Zhang, Y. X. Liu, L. Su, Z. H. Zhang, D. Q. Huo, C. J. Hou and Y. Lei, Sens. Actuators, B, 2014, 191, 86–93 CrossRef CAS.
- M. M. Guo, Y. Xia, W. Huang and Z. L. Li, Electrochim. Acta, 2015, 151, 340–346 CrossRef CAS.
- S. Zhao, T. T. Wang, L. L. Wang and S. K. Xu, J. Solid State Electrochem., 2015, 19, 831–839 CrossRef CAS.
- D. Jiang, Q. Liu, K. Wang, J. Qian, X. Y. Dong, Z. T. Yang, X. J. Du and B. J. Qiu, Biosens. Bioelectron., 2014, 54, 273–278 CrossRef CAS PubMed.
- C. Z. Wei, Y. Y. Liu, X. R. Li, J. H. Zhao, Z. Ren and H. Pang, ChemElectroChem, 2014, 1, 799–807 CrossRef CAS.
- Y. Li, J. J. Fu, R. S. Chen, M. Huang, B. Gao, K. F. Huo, L. Wang and P. K. Chu, Sens. Actuators, B, 2014, 192, 474–479 CrossRef CAS.
- N. Q. Dung, D. Patil, H. Jung and D. Kim, Biosens. Bioelectron., 2013, 42, 280–286 CrossRef PubMed.
- Y. C. Li, Y. M. Zhong, Y. Y. Zhang, W. Weng and S. X. Li, Sens. Actuators, B, 2015, 206, 735–743 CrossRef CAS.
- A. A. Ensafi, M. M. Abarghoui and B. Rezaei, Electrochim. Acta, 2014, 123, 219–226 CrossRef CAS.
- X. H. Niu, Y. X. Li, J. Tang, Y. L. Hu, H. L. Zhao and M. B. Lan, Biosens. Bioelectron., 2014, 51, 22–28 CrossRef CAS PubMed.
- S. Ahmed, K. James and C. P. Owen, J. Steroid Biochem., 2002, 82, 425–435 CrossRef CAS PubMed.
- L. Han, D. P. Yang and A. H. Liu, Biosens. Bioelectron., 2015, 63, 145–152 CrossRef CAS PubMed.
- F. Hong, Y. H. Ni, Y. M. Zhong and H. Wu, J. Alloys Compd., 2016, 659, 112–121 CrossRef CAS.
- Y. L. Zheng, P. Li, H. B. Li and S. H. Chen, Int. J. Electrochem. Sci., 2014, 9, 7369–7381 Search PubMed.
- S. Q. Ci, S. Mao, T. Z. Huang, Z. H. Wen, D. A. Steeber and J. H. Chen, Electroanalysis, 2014, 26, 1326–1334 CrossRef CAS.
- S. J. Li, J. M. Du, J. Chen, N. N. Mao, M. J. Zhang and H. Pang, J. Solid State Electrochem., 2014, 18, 1049–1056 CrossRef CAS.
- H. Yu, J. Jin, X. Jian, Y. Wang and G. C. Qi, Electroanalysis, 2013, 25, 1665–1674 CrossRef CAS.
- Q. Q. Sun, M. Wang, S. J. Bao, Y. C. Wang and S. Gu, Analyst, 2016, 141, 256–260 RSC.
- P. P. Qu, Z. N. Gong, H. Y. Cheng, W. Xiong, X. Wu, P. Pei, R. F. Zhao, Y. Zeng and Z. H. Zhu, RSC Adv., 2015, 5, 106661–106667 RSC.
- R. Devasenathipathy, C. Karuppiah, S. M. Chen, S. Palanisamy, B. S. Lou, M. A. Ali and F. M. A. Al-Hemaid, RSC Adv., 2015, 5, 26762–26768 RSC.
- E. Crouch, D. C. Cowell, S. Hoskins, R. W. Pittson and J. P. Hart, Anal. Biochem., 2005, 347, 17–23 CrossRef CAS PubMed.
- P. P. Liang, M. T. Sun, P. M. He, L. Y. Zhang and G. Chen, Food Chem., 2016, 190, 64–70 CrossRef CAS PubMed.
- H. Y. Yin, X. L. He, Z. Z. Cui and Q. L. Nie, Micro Nano Lett., 2016, 11, 151–155 Search PubMed.
- R. A. Soomro, Z. H. Ibupoto, S. T. H. Sherazi, M. I. Abro, M. Willander, S. A. Mahesar and N. H. Kalwar, Mater. Express, 2015, 5, 437–444 CrossRef CAS.
- L. Q. Kang, D. P. He, L. L. Bie and P. Jiang, Sens. Actuators, B, 2015, 220, 888–894 CrossRef CAS.
- K. Khun, Z. H. Ibupoto, X. Liu, V. Beni and M. Willander, Mater. Sci. Eng., B, 2015, 194, 94–100 CrossRef CAS.
- R. X. Liu and J. B. Zheng, Mater. Lett., 2014, 125, 179–182 CrossRef CAS.
- R. A. Soomro, A. Nafady, Z. H. Ibupoto, S. T. H. Sherazi, M. Willander and M. I. Abro, Mater. Sci. Semicond. Process., 2015, 34, 373–381 CrossRef CAS.
- K. H. Wu, X. Leng, I. R. Gentle and D. W. Wang, J. Mater. Sci. Technol., 2016, 32, 24–27 Search PubMed.
- S. Premlatha, P. Sivasakthi and G. N. K. R. Bapu, RSC Adv., 2015, 5, 74374–74380 RSC.
- R. Prasad and B. R. Bhat, New J. Chem., 2015, 39, 9735–9742 RSC.
- R. Madhu, V. Veeramani, S. M. Chen, A. Manikandan, A. Y. Lo and Y. L. Chueh, ACS Appl. Mater. Interfaces, 2015, 7, 15812–15820 CAS.
- M. Li, C. Han, Y. F. Zhang, X. J. Bo and L. P. Guo, Anal. Chim. Acta, 2015, 861, 25–35 CrossRef CAS PubMed.
- Y. Y. Su, B. B. Luo and J. Z. Zhang, Anal. Chem., 2016, 88, 1617–1624 CrossRef CAS PubMed.
- Y. H. Lin, F. Lu, Y. Tu and Z. F. Ren, Nano Lett., 2004, 4, 191–195 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- M. Pumera, A. Ambrosi, A. Bonanni, E. L. K. Chng and H. L. Poh, TrAC, Trends Anal. Chem., 2010, 29, 954–965 CrossRef CAS.
- J. Li and X. Q. Lin, Biosens. Bioelectron., 2007, 22, 2898–2905 CrossRef CAS PubMed.
- K. Pihel, Q. D. Walker and R. M. Wightman, Anal. Chem., 1996, 68, 2084–2089 CrossRef CAS PubMed.
- N. Yu, H. Yin, W. Zhang, Y. Liu, Z. Y. Tang and M. Q. Zhu, Adv. Energy Mater., 2016, 6, 1501458 Search PubMed.
- L. Li, A. R. O. Raji and J. M. Tour, Adv. Mater., 2013, 25, 6298–6302 CrossRef CAS PubMed.
- S. L. Yang, G. Li, G. F. Wang, J. H. Zhao, X. H. Gao and L. B. Qu, Sens. Actuators, B, 2015, 221, 172–178 CrossRef CAS.
- Y. T. Zhang, S. Liu, Y. Li, D. M. Deng, X. J. Si, Y. P. Ding, H. B. He, L. Q. Luo and Z. X. Wang, Biosens. Bioelectron., 2015, 66, 308–315 CrossRef CAS PubMed.
- T. R. Paudel, A. Zakutayev, S. Lany, M. D'Avezac and A. Zunger, Adv. Funct. Mater., 2011, 21, 4493–4501 CrossRef CAS.
- M. Mohapatra and S. Anand, Int. J. Eng. Sci. Technol., 2010, 2, 127–146 Search PubMed.
- L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang, N. Gu, T. H. Wang, J. Feng, D. L. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |