PtCu bimetallic alloy nanotubes with porous surface for oxygen reduction reaction†
Shaofang Fu,
Chengzhou Zhu*,
Qiurong Shi,
Dan Du and
Yuehe Lin*
The School of Mechanical and Materials Engineering, Washington State University, Pullman, WA 99164, USA. E-mail: chengzhou.zhu@wsu.edu; yuehe.lin@wsu.edu
First published on 12th July 2016
Abstract
A facile wet chemistry method was developed for the synthesis of PtCu bimetallic alloy nanotubes with controllable composition and porous surfaces by employing Te nanowire as a hard template. Due to the synergetic effect among the components as well as their one-dimensional porous nanostructure, the PtCu nanotubes present enhanced electrocatalytic activity and durability for oxygen reduction reaction in acid solution. The mass activity and specific activity of Pt76Cu24 reach 0.41 A mgPt−1 and 0.83 mA cm−2, and are about 3 and 5 times higher than that of Pt/C catalysts. The bimetallic alloys could be considered as an excellent candidate for electrocatalysts and other potential applications.
Introduction
Proton exchange membrane fuel cells (PEMFCs), as one of the most promising energy resources, have been applied in automotive vehicles and portable devices. Pt-based materials are still the commonly used catalysts on PEMFCs due to their high catalytic activity. However, the commercialization of PEMFCs is still hindered by some critical issues, including high cost and instability of Pt-based catalysts, as well as low oxygen reduction reaction (ORR) activity.1–5 Therefore, it is the highest priority to investigate novel catalysts with high activity, stability as well as Pt utilization. Previous investigations have demonstrated that alloying Pt with other transition metals, such as Fe, Co, and Ni, is one effective strategy to improve the activity.4 The enhanced activity could be attributed to some synergetic effects including geometric and electronic effects, which are associated with the downshift of the d-band center of Pt as well as the lattice contraction in the alloy structure. These effects lead to a decrease of the bonding strength between Pt and the oxygenated species, which is expected to result in an increase in catalytic activity for ORR.6–8In addition, the morphology of Pt-based bimetallic/multimetallic alloys is also considered to be an effective strategy to improve the electrocatalytic performance of catalysts. Therefore, a large variety of morphologies were developed to tailor the properties of nanomaterials.9–11 Among them, one-dimensional (1D) Pt-based nanostructures have been demonstrated to be more advantageous compared to zero-dimensional (0D) nanoparticles because the elongated Pt nanostructures are less vulnerable to aggregation, migration, dissolution and Ostwald ripening under the harsh fuel cell operation condition,12–15 thus significantly minimizing the loss of electrochemical surface area (ECSA).16 For instance, Pt-based 1D nanostructures have been rationally designed in many works by using Cu or Te as hard templates.9,10,14 The as-prepared nanomaterials present remarkably high stability and good catalytic activity for ORR due to their unique structure. Among these 1D nanostructures, nanotubes (NTs) are considered to be more efficient to catalyze ORR because of their hollow morphology, which is expected to provide more surface area and active site for the reaction. Furthermore, by combining hollow feature with porous surface, the 1D nanomaterials are expected to provide special properties on electrocatalytic performances.17–19 With this interest, various porous materials with different compositions have attracted extensive attentions owing to their wide range of potential applications, including sensing, catalysis, gas storage and drug delivery.20–23 Especially, formation of well-defined Pt-based nanoporous structures has already presented superior electrocatalytic performance due to their high porosity and large surface area.24,25 Additionally, for the self-supported 1D porous NTs, the elimination of carbon support would enable a thinner electrode catalyst layer and improve mass transport and Pt utilization within the catalyst layer due to the direct contact between the catalyst layer and the gas diffusion layer.4 Therefore, the synthesis of 1D NTs with porous surface should be a new direction to fabricate superior electrocatalysts.
Herein, we proposed a facile approach to synthesize PtCu bimetallic alloy nanotubes (BANTs) with porous surface using Te nanowires (NWs) as template, where the composition of PtCu BANTs could be adjusted by simply changing the ratio between Pt and Cu precursors. The diameter of as-prepared PtCu BANTs is around 20 nm, while the length could reach to several microns. More importantly, the electrocatalytic performance towards ORR revealed that the mass activity (MA) and specific activity (SA) of PtCu BANTs with optimized composition are 0.41 A mgPt−1 and 0.83 mA cm−2, which are about 3 and 5 times higher than that of Pt/C catalyst. Moreover, the PtCu BANTs also showed a better stability compared to commercial Pt/C. The enhanced electrocatalytic performance for ORR should be attributed to the 1D unsupported feature, high specific surface area, as well as the hollow structure.
Experimental sections
Chemicals and reagents
Commercial platinum/carbon (Pt/C) 20 wt% (Pt loading: 20 wt%, Pt on carbon black), sodium tellurite (IV) (Na2TeO3, 99.5%), hydrazine monohydrate (H2NNH2·H2O, 98%) were purchased from Alfa Aesar. Copper(II) chloride (CuCl2, powder, 99%), nafion perfluorinated resin solution (5 wt% in mixture of lower aliphatic alcohols and water, contains 45% water), poly-(N-vinyl-2-pyrrolidone) (PVP, molecular weight: 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), and perchloric acid (HClO4) were obtained from Sigma-Aldrich. Chloroplatinic acid hexahydrate (H2PtCl6·6H2O, 99.9% Pt) was bought from Strem Chemicals. Acetone, ammonium hydroxide (NH4OH, 28–30%) were from JT Baker. L(+)-ascorbic acid (AA, C6H8O6) was obtained from Acros organics. All aqueous solutions were prepared with ultrapure water (>18 MΩ cm) from Barnstead Nanopure water system.
000), and perchloric acid (HClO4) were obtained from Sigma-Aldrich. Chloroplatinic acid hexahydrate (H2PtCl6·6H2O, 99.9% Pt) was bought from Strem Chemicals. Acetone, ammonium hydroxide (NH4OH, 28–30%) were from JT Baker. L(+)-ascorbic acid (AA, C6H8O6) was obtained from Acros organics. All aqueous solutions were prepared with ultrapure water (>18 MΩ cm) from Barnstead Nanopure water system.
Apparatus
FEI Sirion field emission scanning electron microscope (FESEM) was used for energy-dispersive X-ray analysis (EDX). Transmission electron microscopy (TEM) images were obtained by Philips CM200 UT (Field Emission Instruments, USA). X-ray diffraction (XRD) characterization was carried out by Rigaku Miniflex 600. The tube was operated at 40 kV accelerating voltage and 15 mA current. X-ray photoelectron spectroscopy (XPS) measurements were performed on a Kratos AXIS-165 multi-technique electron spectrometer system with a base pressure of 1 × 10−9 torr. The spectra of the surfaces were obtained with an AXIS-165 manufactured by Kratos Analytical Inc. (Spring Valley, NY, USA) using a monochromatic X-ray radiation of 1487 eV (Al Kα). The spectrometer was calibrated against both the Au 4f7/2 peak at 84.0 eV and the Ag 3d5/2 peak at 368.3 eV. Static charging when present was corrected with a neutralizer (flood gun) by placing the carbon peak (C 1s) at about 285 eV.Synthesis of Te NWs
Te NWs were synthesized according to previous reports.26 Briefly, 0.2 g PVP was dissolved in 6.563 mL of water under magnetic stirring to form homogeneous solution. After that, 445 mg Na2TeO3 was added into the PVP solution and dissolved, which was followed by the addition of hydrazine hydrate (313 μL) and NH4OH (513 μL). After thoroughly mixing, the solution was transferred into a Teflon-lined stainless steel autoclave and maintained at 180 °C for 4 h in a forced air oven (Fisher Scientific Isotemp). Finally, the product was centrifuged with acetone, washed with water, and dispersed into 3 mL water.Synthesis of PtCu BANTs and Pt NTs
1 mL Te NWs was first added into 30 mL water. In a 20 mL glass vial, 9 mL of above Te solution was added and kept in 60 °C water bath followed by the addition of 2 mL ascorbic acid AA (0.1 M). After that 300 μL H2PtCl6 (0.1 M) and 100 μL CuCl2 (0.1 M) were injected into the previous solution and stirred at this temperature for 1 hour. Furthermore, the concentration of Pt and Cu precursors was changed to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to obtain the other two PtCu BANTs with the same procedure. For comparison, Pt NTs were also synthesized without the addition of Cu precursor. The obtained products were centrifuged and dispersed into 1 M NaOH for 5 h to get rid of Te NWs. Then the products were centrifuged and washed by water for several times. The final products were re-dispersed into water for electrocatalytic experiments.
1 to obtain the other two PtCu BANTs with the same procedure. For comparison, Pt NTs were also synthesized without the addition of Cu precursor. The obtained products were centrifuged and dispersed into 1 M NaOH for 5 h to get rid of Te NWs. Then the products were centrifuged and washed by water for several times. The final products were re-dispersed into water for electrocatalytic experiments.
Electrocatalytic experiments
Glassy carbon rotating disk electrode (RDE, 5 mm in diameter) was polished and cleaned before surface coating. The PtCu BANTs and Pt NTs (10 μL, 0.5 mg mL−1 with respect to Pt) were coated on the surface of pretreated RDE surface and dried at room temperature. After that, the modified RDE was covered with a layer of nafion (5 μL, 0.05%), followed by drying at room temperature. For commercial Pt/C catalyst (2 mg mL), Pt/C powder was first dissolved into nafion solution containing nafion, 2-proponal, and water (v/v/v = 0.025/1/4). 10 μL of the obtained Pt/C catalyst was then dropped on the surface of pretreated RDE surface and dried before the electrocatalytic tests.The electrochemical measurements were carried out by an electrochemical workstation (CHI 630E) coupled with a three-electrode system. A saturated calomel electrode (SCE) filled with saturated KCl aqueous solution and Pt wire were used as reference electrode and counter electrode, respectively.
Results and discussions
Template-directed approach is considered to be a promising alternative for the synthesis of metallic alloy nanostructures because of the controllable size and shape of the template.27 Currently, the inorganic templates used to synthesize Pt-based NTs include Te, Ag, ZnO, Se NWs, etc.28–31 In this study, Te NWs were employed to construct the PtCu BANTs as a result of their good dispersion, high aspect-ratio, small diameter and convenience of synthesis.32–34 The final compositions of as-prepared PtCu BANTs were 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37, 76
37, 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, and 82
24, and 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 for the samples with Pt and Cu precursor compositions ranging from 1
18 for the samples with Pt and Cu precursor compositions ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Based on this result, the products are denoted as Pt63Cu37, Pt76Cu24, and Pt82Cu18 BANTs, respectively. It should be pointed out that the ratio between Pt and Cu in the final product is higher than that of the precursor, which was attributed to the lower standard reduction potential of Cu2+/Cu (+0.337 V vs. SHE). Therefore, Cu2+ is harder to be reduced by AA compared to PtCl62−, and thus PtCu BANTs contain more Pt content.
1. Based on this result, the products are denoted as Pt63Cu37, Pt76Cu24, and Pt82Cu18 BANTs, respectively. It should be pointed out that the ratio between Pt and Cu in the final product is higher than that of the precursor, which was attributed to the lower standard reduction potential of Cu2+/Cu (+0.337 V vs. SHE). Therefore, Cu2+ is harder to be reduced by AA compared to PtCl62−, and thus PtCu BANTs contain more Pt content.
Fig. 1 and S1† present the typical TEM images of PtCu BANTs and Pt NTs with different magnification. Low magnification TEM image (Fig. 1A) shows that Pt76Cu24 BANTs could be produced in high yield with a length of several micrometers. The high magnification TEM images (Fig. 1B) reveal the hollow structure of PtCu BANTs with porous surface, which show a tube diameter of ∼20 nm. Among them, the hollow part with diameter of ∼10 nm was observed, which was derived from the corrosion of the pristine Te nanowires. In addition to being a reducing agent, AA also plays a significant role in controlling the final branched Pt structures, which is closely associated with the fact that its byproduct, 2,3-diketo-L-gulonic acid (DGA), can serves as a shape-directing agent to direct the branched growth.35 In addition, the prepared PtCu BANTs could be well dispersed in water and ethanol, as shown in Fig. S2.† The high resolution TEM (HRTEM) image in Fig. 1C indicates that the Pt76Cu24 BANTs was faced-centered cubic (fcc) PtCu alloy with a lattice spacing of 0.22 nm.9,14
 | ||
| Fig. 1 TEM images of Pt76Cu24 BANTs with different magnification (A and B). (C) HRTEM image of Pt76Cu24 BANTs. | ||
The high-angle annular dark-field scanning transmission electron microscopy-energy dispersive spectrometer (HAADF-STEM-EDS) mapping was used to analyze the element distribution in Pt76Cu24 BANTs, as shown in Fig. 2. The images reveal that Pt and Cu are uniformly distributed through the entire structure, which confirms the formation of homogeneous alloys in PtCu BANTs. Additionally, the cross-sectional compositional line profiles of Pt76Cu24 BANTs further confirm the uniform distribution of Pt and Cu.
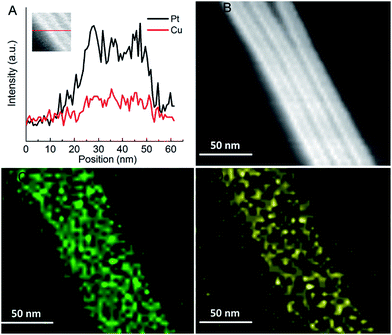 | ||
| Fig. 2 (A) Distribution of the components in Pt76Cu24 BANTs. (B) STEM image of Pt76Cu24 BANTs. EDS mapping analysis showing the elements Pt (C) and Cu (D). | ||
Fig. 3A and B show the representative XRD patterns of Pt63Cu37, Pt76Cu24, and Pt82Cu18 BANTs, respectively. Distinct diffraction peaks can be observed in each pattern, indicating the highly crystalline feature of PtCu BANTs. In the case of Pt76Cu24 BANTs, the diffraction peaks are located at 40.30, 46.25, 69.25, and 83.08°, which are between the peak positions of pure Pt and Cu, corresponding to (111), (200), (220), and (311) plane. Interestingly, the shift of diffraction angles was observed when the molar ratio between Pt and Cu was changed. These results demonstrate the alloy formation between Pt and Cu. The surface properties of Pt76Cu24 BANTs were further examined by XPS. As illustrated in Fig. 3C, the XPS spectra were dominated by Pt and Cu. In the Pt 4f spectrum (Fig. 3D), the peaks at the binding energy of 71.27 and 74.58 eV are assigned to Pt 4f7/2 and Pt 4f5/2. These two peaks can be further split into two pairs of peaks, which are located at 71.25, 72.21 and 74.57, 76.25 eV, indicating the presence of Pt, PtO and Pt(OH)2.36 Based on the peak intensity of XPS spectrum of Pt, Pt(0) is the dominant specie (∼77%), which is beneficial to ORR. Likewise, Cu 2p3/2 and Cu 2p1/2 could be observed at the binding energy around 932, 942, and 953 eV in Fig. S3,† demonstrating the formation of Cu(0), Cu(I), and Cu(II) with the amount of 65%, 29% and 6%, respectively.37,38
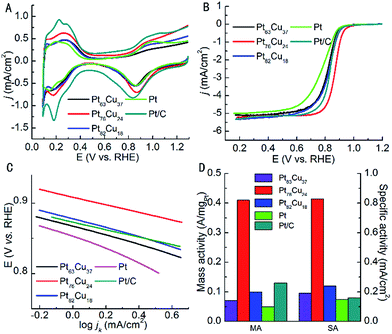
The electrochemical performances of PtCu BANTs with different composition were investigated, as shown in Fig. 4 and Table 1. The cyclic voltammetry (CV) curves (Fig. 4A) of PtCu BANTs, Pt NTs and commercial Pt/C catalysts were obtained in N2-saturated 0.1 M HClO4 solution with a scan rate of 50 mV s−1, which display characteristic hydrogen adsorption/desorption peaks for all the catalysts bellow 0.4 V. Based on the hydrogen adsorption/desorption peaks, the ECSA was calculated by assuming a value of 210 μC cm−2 for the adsorption of a hydrogen monolayer. The results show that the ECSAs of Pt63Cu37, Pt76Cu24, Pt82Cu18 BANTs, and Pt NTs are 39, 49, 42 and 35 m2 g−1, which are around half of commercial Pt/C catalyst (81 m2 g−1). Compared with monometallic Pt NTs, the ECSAs of PtCu BANTs increased due to the introduction of Cu. The electrocatalytic properties of BANTs for ORR were investigated in O2-saturated 0.1 M HClO4 solution. The linear sweep voltammetry (LSV) curves (Fig. 4B) of all the catalysts were obtained by RDE system, which clearly demonstrate the enhanced catalytic activity of Pt76Cu24 BANTs compared to commercial Pt/C catalyst. In particular, Pt76Cu24 BANTs show a positively shifted onset potential (0.925 V) and half-wave potential (0.871 V) compared with that of Pt/C (0.891 V and 0.829 V). Fig. 4C shows the Tafel plot of the catalysts. The decreased Tafel slope of Pt76Cu24 BANTs (53 mV per decade) compared to Pt NTs (95 mV per decade) indicates the superior ORR activity of Pt76Cu24 BANTs. Fig. 4D illustrates the MA and SA of each sample, which were obtained by normalizing the kinetic currents with Pt loading and ECSA, respectively. The MA and SA of Pt76Cu24 BANTs reach 0.41 A mgPt−1 and 0.83 mA cm−2, which are about 3 and 5 times higher than that of Pt/C catalyst (0.13 A mgPt−1 and 0.16 mA cm−2). The better electrocatalytic activity of Pt76Cu24 BANTs compared with other PtCu BANTs should be attributed to the optimized composition (Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu is around 3
Cu is around 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which is consistent with other works.39,40
1), which is consistent with other works.39,40
| ECSA (m2 g−1) | Onset potential (V) | Half-wave potential (V) | MA (A mgPt−1) | SA (mA cm−2) | Tafel slope (mV per decade) | |
|---|---|---|---|---|---|---|
| Pt63Cu37 BANTs | 39 | 0.894 | 0.814 | 0.07 | 0.19 | 62 |
| Pt76Cu24 BANTs | 49 | 0.925 | 0.872 | 0.41 | 0.83 | 53 |
| Pt82Cu18 BANTs | 42 | 0.902 | 0.827 | 0.1 | 0.24 | 71 |
| Pt NTs | 35 | 0.887 | 0.776 | 0.05 | 0.15 | 95 |
| Pt/C | 81 | 0.891 | 0.829 | 0.13 | 0.16 | 53 |
To investigate the long-term stability of PtCu BANTs, the accelerated durability tests (ADT) of the catalysts were conducted by potential cycling between 0.6 and 1.2 V vs. RHE in O2-saturated 0.1 M HClO4 solution at room temperature with scan rate of 100 mV s−1. After 5000 cycles, Pt76Cu24 BANTs lost 9.1% of initial ECSA (Fig. 5A) and presented a degradation of 24 mV in the half-wave potential (Fig. 5C). In contrast, commercial Pt/C catalyst exhibited 45% loss of the initial ECSA (Fig. 5B) and a large decrease of 33 mV in the half-wave potential (Fig. 5D). The morphology changes of Pt76Cu24 BANTs and Pt/C catalysts further confirm the better durability of Pt76Cu24 BANTs. As shown in Fig. S4,† the 1D porous structure of Pt76Cu24 BANTs with the diameter ∼15 nm were well maintained after ADT, while severe aggregation was observed for Pt/C catalyst. For the 1D Pt76Cu24 BANTs, we can still clearly see the dendritic structure on the surface. However the hollow structure was lost after the long-term stability test, which might be the major cause of the ECSA loss, diameter decrease as well as the reduced activity. We also analysed the composition of Pt76Cu24 BANTs after ADT using XPS technique (Fig. S5†). The results show that the atomic ratio between Pt and Cu is ∼81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19. For Pt element, the concentration of Pt(II) species (25%) is almost same as that in the as-prepared catalyst (23%). However, the concentration of oxidized Cu decreased from 35% to 21%. The higher Pt/Cu ratio and lower concentration of oxidized Cu might be attributed to the electrochemical etching during the long-term stability test.
19. For Pt element, the concentration of Pt(II) species (25%) is almost same as that in the as-prepared catalyst (23%). However, the concentration of oxidized Cu decreased from 35% to 21%. The higher Pt/Cu ratio and lower concentration of oxidized Cu might be attributed to the electrochemical etching during the long-term stability test.
The enhanced electrocatalytic activity and good durability of PtCu BANTs could be ascribed to the following reasons: (1) the change of geometry (1D vs. 0D) since the 1D shape would facilitate the reaction kinetics, electron transport and the diffusion of O2 to Pt surface; (2) the porous and hollow structure, which not only provide large surface area and porosity for high Pt utilization, but also improve mass transport and gas diffusion; (3) the elimination of carbon support, which would enable a thinner electrode catalyst layer and improve mass transport and Pt utilization within the catalyst layer due to the direct contact between the catalyst layer and the gas diffusion layer; (4) the incorporation of a nonprecious metal into the Pt lattice to form an alloys, which could not only decrease the cost but improve the catalytic performance owing to the synergistic effect; (5) the self-supported 1D nanostructures are less vulnerable to Ostwald ripening, Pt detachment and aggregation, which could significantly minimizing the loss of ECSA.41
Conclusions
In summary, we proposed a facile approach to synthesize PtCu BANTs with porous surface and controllable composition using Te NWs as template in aqueous solution. The electrochemical experiments showed that the MA and SA of as-prepared Pt76Cu24 BANTs are 0.41 A mgPt−1 and 0.83 mA cm−2, which are higher than that of commercial Pt/C catalysts by a factor of 3 and 5. This template-based method provides a novel approach to prepare electrocatalysts with desired composition and morphology, which is expected to present potential applications in other fields beyond fuel cells.Acknowledgements
This work was supported by Washington State University Startup Funding. We thank Franceschi Microscopy & Image Center at Washington State University for TEM measurements.Notes and references
- S. J. Guo, S. Zhang and S. H. Sun, Angew. Chem., Int. Ed., 2013, 52, 8526 CrossRef CAS PubMed.
- S. Zhang, Y. Y. Shao, G. P. Yin and Y. H. Lin, J. Mater. Chem. A, 2013, 1, 4631 CAS.
- Y. Y. Shao, S. Zhang, C. M. Wang, Z. M. Nie, J. Liu, Y. Wang and Y. H. Lin, J. Power Sources, 2010, 195, 4600 CrossRef CAS.
- C. Z. Zhu, D. Du, A. Eychmuller and Y. H. Lin, Chem. Rev., 2015, 115, 8896 CrossRef CAS PubMed.
- C. Z. Zhu, H. Li, S. F. Fu, D. Du and Y. H. Lin, Chem. Soc. Rev., 2016, 45, 517 RSC.
- S. J. Guo, D. G. Li, H. Y. Zhu, S. Zhang, N. M. Markovic, V. R. Stamenkovic and S. H. Sun, Angew. Chem., Int. Ed., 2013, 52, 3465 CrossRef CAS PubMed.
- H. Y. Zhu, S. Zhang, S. J. Guo, D. Su and S. H. Sun, J. Am. Chem. Soc., 2013, 135, 7130 CrossRef CAS PubMed.
- B. Y. Xia, H. B. Wu, N. Li, Y. Yan, X. W. Lou and X. Wang, Angew. Chem., Int. Ed., 2015, 54, 3797 CrossRef CAS PubMed.
- L. Su, S. Shrestha, Z. H. Zhang, W. Mustain and Y. Lei, J. Mater. Chem. A, 2013, 1, 12293 CAS.
- H. W. Liang, X. A. Cao, F. Zhou, C. H. Cui, W. J. Zhang and S. H. Yu, Adv. Mater., 2011, 23, 1467 CrossRef CAS PubMed.
- S. F. Fu, C. Z. Zhu, D. Du and Y. H. Lin, ACS Appl. Mater. Interfaces, 2015, 7, 13842 CAS.
- B. Y. Xia, H. B. Wu, Y. Yan, X. W. Lou and X. Wang, J. Am. Chem. Soc., 2013, 135, 9480 CrossRef CAS PubMed.
- L. Ma, C. M. Wang, B. Y. Xia, K. K. Mao, J. W. He, X. J. Wu, Y. J. Xiong and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 5666 CrossRef CAS PubMed.
- B. Chen, D. J. Cheng and J. Q. Zhu, J. Power Sources, 2014, 267, 380 CrossRef CAS.
- B. Y. Xia, H. B. Wu, X. Wang and X. W. Lou, Angew. Chem., Int. Ed., 2013, 52, 12337 CrossRef CAS PubMed.
- Z. Y. Huang, H. H. Zhou, F. F. Sun, C. P. Fu, F. Y. Zeng, T. Q. Li and Y. F. Kuang, Chem. Eur. J., 2013, 19, 13720 CrossRef CAS PubMed.
- J. W. Hong, S. W. Kang, B. S. Choi, D. Kim, S. B. Lee and S. W. Han, ACS Nano, 2012, 6, 2410 CrossRef CAS PubMed.
- Z. C. Pan, Y. H. Xiao, Z. G. Fu, G. H. Zhan, S. K. Wu, C. M. Xiao, G. H. Hu and Z. G. Wei, J. Mater. Chem. A, 2014, 2, 13966 CAS.
- G. X. Zhang, S. H. Sun, M. Cai, Y. Zhang, R. Y. Li and X. L. Sun, Sci. Rep., 2013, 3, 1526 Search PubMed.
- W. Liu, P. Rodriguez, L. Borchardt, A. Foelske, J. P. Yuan, A. K. Herrmann, D. Geiger, Z. K. Zheng, S. Kaskel, N. Gaponik, R. Kotz, T. J. Schmidt and A. Eychmuller, Angew. Chem., Int. Ed., 2013, 52, 9849 CrossRef CAS PubMed.
- C. Li, O. Dag, T. D. Dao, T. Nagao, Y. Sakamoto, T. Kimura, O. Terasaki and Y. Yamauchi, Nat. Commun., 2015, 6, 6608 CrossRef CAS PubMed.
- B. Cai, D. Wen, W. Liu, A. K. Herrmann, A. Benad and A. Eychmuller, Angew. Chem., Int. Ed., 2015, 53, 13101 CrossRef PubMed.
- C. Zhu, D. Wen, M. Oschatz, M. Holzschuh, W. Liu, A. K. Herrmann, F. Simon, S. Kaskel and A. Eychmuller, Small, 2015, 11, 1430 CrossRef CAS PubMed.
- C. L. Li, T. Sato and Y. Yamauchi, Angew. Chem., Int. Ed., 2013, 52, 8050 CrossRef CAS PubMed.
- H. A. E. Victor Malgras, W. Hongjing, J. Bo, L. Cuiling, C.-W. Kevin Wu, K. Jung Ho and Y. Yusuke, Adv. Mater., 2016, 28, 993 CrossRef PubMed.
- C. Z. Zhu, S. J. Guo and S. J. Dong, Adv. Mater., 2012, 24, 2326 CrossRef CAS PubMed.
- C. Z. Zhu, S. J. Guo and S. J. Dong, J. Mater. Chem., 2012, 22, 14851 RSC.
- S. M. Alia, G. Zhang, D. Kisailus, D. S. Li, S. Gu, K. Jensen and Y. S. Yan, Adv. Funct. Mater., 2010, 20, 3742 CrossRef CAS.
- Y. W. Lee, M. A. Lim, S. W. Kang, I. Park and S. W. Han, Chem. Commun., 2011, 47, 6299 RSC.
- B. Mayers, X. C. Jiang, D. Sunderland, B. Cattle and Y. N. Xia, J. Am. Chem. Soc., 2003, 125, 13364 CrossRef CAS PubMed.
- W. Hong, J. Wang and E. K. Wang, Small, 2014, 10, 3262 CrossRef CAS PubMed.
- H. H. Li, S. Y. Ma, Q. Q. Fu, X. J. Liu, L. Wu and S. H. Yu, J. Am. Chem. Soc., 2015, 137, 7862 CrossRef CAS PubMed.
- H. W. Liang, S. Liu, J. Y. Gong, S. B. Wang, L. Wang and S. H. Yu, Adv. Mater., 2009, 21, 1850 CrossRef CAS.
- W. Zhang, Z. Y. Wu, H. L. Jiang and S. H. Yu, J. Am. Chem. Soc., 2014, 136, 14385 CrossRef CAS PubMed.
- L. Wang, C. P. Hu, Y. Nemoto, Y. Tateyama and Y. Yamauchi, Cryst. Growth Des., 2010, 10, 3454 CAS.
- P. Song, J. J. Feng, S. X. Zhong, S. S. Huang, J. R. Chen and A. J. Wang, RSC Adv., 2015, 5, 35551 RSC.
- N. Pollock, G. Fowler, L. J. Twyman and S. L. McArthur, Chem. Commun., 2007, 2482 RSC.
- Y. I. Kuznetsov, L. P. Kazansky, I. A. Seljaninov, D. Starosvetsky and Y. Ein-Eli, Solid State Commun., 2009, 12, C21 CAS.
- D. L. Wang, H. L. L. Xin, R. Hovden, H. S. Wang, Y. C. Yu, D. A. Muller, F. J. DiSalvo and H. D. Abruna, Nat. Mater., 2013, 12, 81 CrossRef CAS PubMed.
- Y. C. Tseng, H. S. Chen, C. W. Liu, T. H. Yeh and K. W. Wang, J. Mater. Chem. A, 2014, 2, 4270 CAS.
- Z. W. Chen, M. Waje, W. Z. Li and Y. S. Yan, Angew. Chem., Int. Ed., 2007, 46, 4060 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: EDX spectra of Pt63Cu37, Pt76Cu24, and Pt82Cu18 BANTs, TEM images of Pt63Cu37, Pt82Cu18 BANTs, and Pt NTs, digital pictures of PtCu BANTs dispersed in water and ethanol, high resolution XPS spectrum of Cu in Pt76Cu24 BANTs, TEM images of Pt76Cu24 BANTs before and after ADT, TEM images of commercial Pt/C catalyst before and after ADT. XPS analysis of Pt76Cu24 BANTs after ADT. See DOI: 10.1039/c6ra12415a |
| This journal is © The Royal Society of Chemistry 2016 |