Kinetic study of the alkaline degradation of imidapril hydrochloride using a validated stability indicating HPLC method
Abstract
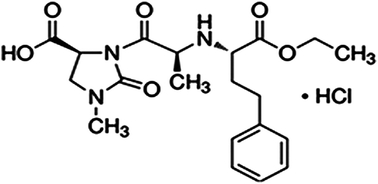
An aqueous alkaline degradation study was performed for imidapril hydrochloride (IMD) drug in the presence of its degradation products and an isocratic stability indicating method was presented using a HPLC technique. The separations were performed using an ACE Generix 5C8, 150 × 4.6 mm column and a mobile phase consisting of buffer solution (0.1 M potassium dihydrogen phosphate and 0.02 M tetra-N-butyl ammonium hydrogen sulphate of pH = 4.5 with 1 N HCl) and acetonitrile 60 : 40 (v/v). The wavelength of the detector was adjusted at 210 nm. The method showed high sensitivity concerning accuracy, precision, linearity and specificity within the acceptable range from 0.1 to 100 μg mL−1 and the limit of quantification was found to be 0.0211 μg mL−1 for IMD. The proposed method was used to determine the drug in its pharmaceutical formulation and to investigate the degradation kinetics of the drug's alkaline-stressed sample. The reactions were found to follow a first-order reaction. The activation energy could also be estimated. The optimized stability indicating HPLC method was validated according to ICH guidelines.

 Please wait while we load your content...
Please wait while we load your content...