ZnO as an efficient nucleating agent and morphology template for rapid, facile and scalable synthesis of MOF-46 and ZnO@MOF-46 with selective sensing properties and enhanced photocatalytic ability†
Maryam Rad and
Saeed Dehghanpour*
Department of Chemistry, Alzahra University, P.O. Box 1993891176, Tehran, Iran. E-mail: Dehghanpours@alzahra.ac.ir; Fax: +982188041344; Tel: +982188041344
First published on 22nd June 2016
Abstract
MOF-46 and a novel core–shell heterostructure containing ZnO@MOF-46 rods have been successfully synthesized via a simple, versatile and economic method. The products have been characterized by X-ray diffraction (XRD), FTIR spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDX). Time and solvent effects on the growth process of ZnO@MOF-46 were investigated. Luminescent properties of the activated sample showed distinct solvent-dependent photoluminescence emissions. The product also demonstrated unique sensing properties in the detection of aromatic compounds via a fluorescence quenching and enhancing mechanism. Aromatic compounds with electron-withdrawing substituents such as nitro act as fluorescence quenchers for ZnO@MOF-46 and aromatic compounds with electron-donating substituents such as CH3 were found to enhance the ZnO@MOF-46 fluorescence. The ZnO@MOF-46 showed excellent photodegradation of methylene blue. The degradation rates in the presence of various scavengers including benzoquinone, isopropyl alcohol and ammonium oxalate (O2˙−, OH˙ and h+ scavengers, respectively) were studied and the results showed that all of the species (O2˙−, OH˙ and h+) contribute to degradation but OH˙ is the main active species which plays a major role in this system and hence photocatalyst degradation was supposed to have a radical mechanism. Band structure parameters of ZnO@MOF-46 were determined using cyclic voltammetry and DRUV/VIS spectroscopy and the mechanism of photodegradation was then discussed.
Introduction
Metal–organic frameworks (MOFs) are composed of metal containing secondary building units (SBUs) connected by organic linkers, in which these units are repeated and held together by strong bonds (reticular synthesis) to create open crystalline frameworks with inherent porosity.1–3 Compared with conventional inorganic porous materials, MOFs possess higher pore volume, high surface areas, structural adaptivity and flexibility4,5 and have a wide range of potential applications such as adsorption, separation, drug delivery, sensing, catalysis and photocatalysis.6–12 MOFs have been generally synthesized via hydrothermal and solvothermal methods with reaction times ranging from several hours to days. Several efforts have been made to find alternative routes to prepare MOFs by environmentally-friendly and low-cost methods such as microwave-assisted, sonochemical, electrochemical and mechanochemical methods.13–16 However, finding new synthetic methods is still a challenge for researchers. Furthermore, various metal sources (for example, metal oxides, hydroxides, and acetates) have been used for MOF synthesis because of their low cost and safety.17 Majano's group reported the complete conversion of a water-insoluble hydroxide, copper(II) hydroxide, into a pure phase metal–organic framework, HKUST-1, in aqueous ethanolic media for the first time. The conversion of Cu(OH)2 into HKUST-1 could be carried out at room temperature and was suitable for large scale production.18 Li et al. successfully used three aluminum compounds, Al2O3, Al(OH)3, and boehmite, to hydrothermally synthesize MIL-53(Al).19 Falcaro's group used ZnO particles as heterogeneous nucleation agents for seeding, growing, and precisely positioning Zn-based metal–organic frameworks including MOF-5, IRMOF-3, IRMOF-8 and IRMOF-10. They presented a technique, which can be used for positioning MOF crystals on different supports, such as the channels of a microfluidic device and paper fibers at the interface of a lateral flow of seed and precursor solutions for the first time.20 In addition, other groups used ZnO nanoparticles with various morphologies as Zn2+ ions and/or growth template to synthesize ZnO@ZIF-8 core–shell structures.21–23Heterostructured MOFs in combination with other functional materials must show greater advantages compared to pure MOFs due to their synergistic effect. Coupling metal oxides with MOFs can be an alternative route to combine the advantages of both the metal oxides as cores (unique optical, electrical, magnetic, and catalytic properties) and the MOF as shells (structural adaptivity and flexibility, ordered crystalline pores, and multi-coordination sites).24–28 Zinc oxide is an n-type semiconductor with many potential applications and ZnO nanostructures have the richest morphologies including various nanorods, nanopillars, nanowires, nanodonats, nanodrums, nanopropellers, nanonails, and nanobridges.29–33 The variety of synthetic methods for ZnO preparation, such as vapor deposition, hydro/solvothermal methods, sol–gel process, precipitation from microemulsions and mechanochemical processes, makes it possible to obtain products with particles differing in shape, size and spatial structure.34–40 Because of these great properties, ZnO has been the most desirable metal oxide to supply the semiconductor@MOFs heterostructure. In this work, the aim was to develop a simple, versatile, scalable and rather rapid method, which facilitates the synthesis of MOF-46 and ZnO@MOF-46 core–shell heterostructure. An interesting alternative way, from safety and economic perspective, is using zinc oxide. Using ZnO as a precursor is waste free and avoids formation of by product anions such as Cl− and NO3−. Chlorides are corrosive in the mixture and nitrates can have a disturbing effect due to the exothermic nature of MOF synthesis. The hydrolysis of nitrate salts during the hydrothermal synthesis can generate large amounts of toxic and corrosive nitric acid. In addition, the removal of the salt from waste water is expensive.18–20 MOF-46 was synthesized by Yaghi and co-workers for the first time and no other synthetic method for preparation of MOF-46 has been reported since.41 In this work, the successful synthesis of MOF-46 and ZnO@MOF-46 with core–shell heterostructure is reported, in which ZnO is used as the source of Zn2+ ions for formation of MOF-46 by dissolving ZnO in solvent mixtures. To study the effect of time on formation of core–shell product, several experiments were carried out in different reaction times and the products showed different compositions. Furthermore, the effect of the solvent composition on growth process of ZnO@MOF-46 was studied and it was observed that changing the composition of reaction solvents affected the products. Solvothermal condition was also applied and different samples were synthesized. The prepared ZnO@MOF-46 core–shell was investigated for sensing small solvent molecules and detection of aromatic compounds, which demonstrated distinct solvent-dependent luminescent spectra as well as good sensing for the detection of aromatic compounds via a fluorescence quenching and enhancement mechanism. Moreover, the photocatalytic properties of ZnO@MOF-46 have been investigated. The prepared ZnO@MOF-46 showed excellent photodegradation of methylene blue.
Experimental
Reagents and chemicals
The zinc nitrate hexahydrate (Zn(NO3)2·6H2O, 99%), sodium hydroxide (NaOH, 96%), ethanol (C2H5OH, absolute), ethylenediamine (EDA, 99%), N,N-dimethylformamide (DMF, 99.8%), 2-aminoterephthalic acid (BDC-NH2, 99%), methylene blue (82%) and the solvent for sensing studies were obtained from commercial suppliers (Sigma-Aldrich and Merck Chemical Companies) and used without further purification. ZnO precursors were synthesized via a solvothermal route previously reported by Liu's group.42 An alkali solution of zinc was prepared by dissolving 2.97 g (10 mmol) of zinc nitrate (Zn(NO3)2·6H2O) and 8.00 g (200 mmol) of NaOH in deionized water to form a 20.0 mL solution ([Zn2+] = 0.50 M, [OH−] = 10.00 M; molar ratio of Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OH− = 1
OH− = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). 3.0 mL of alkali Zn2+ solution were then mixed with 5.0 mL of deionized water and 25.0 mL of pure alcohol (C2H5OH), followed by adding 5.0 mL of ethylenediamine (EDA). Before transferring the reaction mixture to a Teflon-lined autoclave, it was pretreated in an ultrasonic water bath for 40 min. The autoclave was maintained at 180 °C for 20 h in an electric oven. Finally, the white crystalline products were harvested by centrifugation redispersion cycles with each successive supernatant being decanted and replaced with deionized water.
20). 3.0 mL of alkali Zn2+ solution were then mixed with 5.0 mL of deionized water and 25.0 mL of pure alcohol (C2H5OH), followed by adding 5.0 mL of ethylenediamine (EDA). Before transferring the reaction mixture to a Teflon-lined autoclave, it was pretreated in an ultrasonic water bath for 40 min. The autoclave was maintained at 180 °C for 20 h in an electric oven. Finally, the white crystalline products were harvested by centrifugation redispersion cycles with each successive supernatant being decanted and replaced with deionized water.
Characterization
Infrared spectra were recorded on a Bruker Tensor 27 spectrophotometer. Emission spectra were obtained using a Varian Cary Eclipse fluorescence spectrophotometer at room temperature. UV-vis spectra were obtained with a Shimadzu UV-260 spectrophotometer. Scanning electron microscopy (SEM) images were obtained on a Philips XL-30ESEM equipped with an X-ray energy dispersive detector. X-ray powder diffraction (XRD) patterns of the samples were recorded on a PHILIPS PW 1800 X-ray powder diffractometer with Cu Kα (λ = 1.5406 Å) radiation. Electrochemical measurements were carried out using an Autolab PGSTAT30 digital potentiostat/galvanostat in a conventional three-electrode system comprised of glassy carbon as the working electrode, platinum wire as the counter electrode, and Ag/AgCl (1 N KCl) as the reference electrode. 3 mg of MOF-46 were suspended in 0.5 mL of H2O to make a slurry solution. Then, an equal volume of this solution was added dropwise on the surface of glassy carbon using a micropipette. The prepared electrode was dried in air at room temperature and the electrochemical measurements were then performed.Synthesis of MOF-46
MOF-46 was synthesized via slow diffusion method reported by Yaghi's group.41 In this method, MOF-46 is synthesized via a simple and scalable route from ZnO as Zn2+ source for the first time. 2-Aminoterephthalic acid (181 mg, 1 mmol) and ZnO rods (82 mg, 1 mmol) were added to a glass vial containing a mixed solvent of DMF/H2O (48 mL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of v/v). The mixture was then stirred for 24 h at room temperature. After 24 h, the cream colored product was collected by filtration and washed four times with DMF and two times with ethanol. In a typical large scale reaction, 2-aminoterephthalic acid (9.05 g, 50 mmol) and ZnO (4.1 g, 50 mmol) were added to a glass vial containing a mixed solvent of DMF/H2O (600 mL, 1
2 of v/v). The mixture was then stirred for 24 h at room temperature. After 24 h, the cream colored product was collected by filtration and washed four times with DMF and two times with ethanol. In a typical large scale reaction, 2-aminoterephthalic acid (9.05 g, 50 mmol) and ZnO (4.1 g, 50 mmol) were added to a glass vial containing a mixed solvent of DMF/H2O (600 mL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of v/v). The procedure was same as above and after 24 h, the cream colored MOF-46 product was obtained and filtered (crystallographic data for the MOF-46 is given in Table S1†).
2 of v/v). The procedure was same as above and after 24 h, the cream colored MOF-46 product was obtained and filtered (crystallographic data for the MOF-46 is given in Table S1†).
General procedure for the synthesis of ZnO@MOF-46
Before using ZnO, it was calcinated at 500 °C in a muffle furnace to remove the probably adsorbed material on the surface. In a typical synthesis, 2-aminoterephthalic acid (181 mg, 1 mmol) and ZnO (82.00 mg, 1 mmol) were added to a glass vial containing a mixed solvent of DMF/H2O (48 mL, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of v/v). The mixture was sonicated for 10 min and then left at room temperature. After 48 h, the cream colored product was collected by centrifugation and washed five times with DMF and two times with ethanol. The reaction was performed for 6, 12 and 24 h under the same conditions (Scheme 1). In addition, some experiments were carried out in different solvent compositions with same synthetic method and molar ratio during 48 h.
1 of v/v). The mixture was sonicated for 10 min and then left at room temperature. After 48 h, the cream colored product was collected by centrifugation and washed five times with DMF and two times with ethanol. The reaction was performed for 6, 12 and 24 h under the same conditions (Scheme 1). In addition, some experiments were carried out in different solvent compositions with same synthetic method and molar ratio during 48 h.
 | ||
| Scheme 1 Schematic illustration of synthetic procedure of ZnO@MOF-46 core–shell heterostructure formation. | ||
Fluorescence measurements
The fluorescence spectrum of the activated ZnO@MOF-46 was collected and monitored before exposing to the different solvents and aromatic compounds. To activate the ZnO@MOF-46 sample, it was immersed in chloroform for 3 days. Fresh chloroform was supplied every 12 hours. The chloroform exchanged crystals of ZnO@MOF-46 were collected by filtration and evacuated at 60 °C for 2 h. The luminescence spectra were recorded upon excitation at 325 nm.Photocatalytic activity
70 mg of ZnO@MOF-46 powder was added into a 150 mL aqueous solution of methylene blue (5 ppm) and the pH was adjusted to 10 by adding NaOH aqueous solution. The mixture was then magnetically stirred in the dark for 1 h to ensure the establishment of an adsorption/desorption equilibrium. The solution was then exposed to UV irradiation of a 50 W mercury lamp at a distance of 10 cm between the liquid surface and the lamp under O2 flow. The solution was stirred with the aid of a magnetic stirrer during the irradiation process. At 20 min intervals, 3 mL samples were taken out from the vessel and analyzed by UV-visible spectroscopy. The characteristic absorption of methylene blue, 664 nm, was chosen to monitor the photocatalytic degradation process.Results and discussion
Morphology and composition of products
The morphology and size of the prepared ZnO were examined by SEM (Fig. S1†). Edged hexagonal shapes were observed at the tips of rods, thus clearly showing high crystallinity. Indexing the diffraction pattern (Fig. S1b†) shows that the ZnO crystals are of wurtzite-structure (JCPDS no. 36-1451).42 MOF-46 has been prepared by Yaghi and co-workers,41 via slow diffusion method and single crystals were obtained during 4–7 days. In this study, the quantitative conversion of zinc oxide into MOF-46 at room temperature was successfully performed. The morphology and size of MOF-46 was determined by SEM images. Fig. 1a shows the microcrystalline and rhombus shape of MOF-46. The XRD results reveal that the obtained sample is in good agreement with the simulated XRD pattern of MOF-46 with a monoclinic space group (C2/m) according to the published crystal structure data (Fig. 1b).41 This result shows that ZnO rods are desirable and versatile precursors for the heterogeneous nucleation of Zn-based MOFs such as MOF-46. Furthermore, the successful synthesis of MOF-46 was performed by increasing the amount of precursors by 50 times. Hence, this method can be used as a scalable route for MOF-46 synthesis.As mentioned above, Falcaro's group used ZnO particles as heterogeneous nucleation agents for seeding, growing, and precisely positioning IRMOF-3, which has the same 2-aminoterephthalic organic linker and Zn2+ metal centers in comparison with MOF-46.20 They also used ZnO nanoparticles as seeds for the heterogeneous nucleation of IRMOF-3, which they synthesized by using Zn(NO3)2·6H2O as Zn2+ source and combination of the conventional precursors for preparation of IRMOF-3 including metal salt and organic ligand with ZnO. They were able to control the positioning of IRMOF-3, for instance on paper substrate. However, ZnO has been used as Zn2+ ion source instead of zinc salt in synthesis of MOF-46 and ZnO@MOF-46 heterostructure and morphology template in preparation of ZnO@MOF-46 and no foreign Zn2+ ions source was used in the present work.
Fig. 2 shows the SEM images of the synthesized ZnO@MOF-46 heterostructures during different times. It can be observed that the product is thicker than that obtained using hexagonal ZnO rods as precursors. Products were also studied using SEM/EDX to determine the elemental information on the particle surfaces (Fig. S2†). The results of SEM/EDX characterization show the percentages of the elements in the surface of the products are different from those of pure MOF-46 or ZnO. Considering the fact that N and C are two main elements of 2-aminoterephthalic acid, which only exists in MOF-46 as shell, the formation of ZnO@MOF-46 heterostructure will be confirmed. The XRD result of ZnO@MOF-46 (48 h, Fig. 3) reveal that there are two distinct characteristic diffraction peaks including ZnO. Moreover, the pattern of MOF-46 crystals agree well with the simulated XRD pattern of MOF-46 with a monoclinic space group (C2/m) according to the published crystal structure data.41
 | ||
| Fig. 3 XRD patterns of ZnO@MOF-46 rods obtained at room temperature (48 h) in compare with pure MOF-46 and ZnO. | ||
Fig. 4 shows a TEM image of prepared ZnO@MOF-46 heterostructure during 48 h. In this image, the core–shell structure of the product can be clearly observed. The image shows that the thickness of the outer shell with light contrast is about 100 ± 20 nm, while the diameter of the inner ZnO rods with dark contrast is about 230 ± 20 nm. It can be observed that the ZnO cores are thinner than the precursor ZnO rods, which are 470 ± 100 nm in diameter before being used in the reaction. Also ZnO nanorods as core with length about 1.5 μm are shorter than precursor ZnO rods with length about 4.7 ± 2 μm. The IR spectrum of the pure ZnO shows the characteristic band of ZnO stretching vibration at 557 cm−1. The broad absorption bands at 3385 and 1637 cm−1 are attributed to the O–H stretching vibrations of adsorbed water on the ZnO surface. The characteristic bands of stretching vibration of ZnO in ZnO@MOF appear at 521, 521, 523 and 521 cm−1 in prepared samples at 6, 12, 24 and 48 h, respectively. In the IR spectrum of pure MOF-46, the characteristic bands of stretching vibrations of NH2 appear at 3120 and 3318 cm−1. The characteristic bands of NH2 vibrations are gradually recognizable and appear in the IR spectra of prepared samples at 6, 12, 24 and 48 h, respectively. In addition, the characteristic IR bands of 2-aminoterephthalic acid carboxylate groups gradually appear around 1600 and 1400 cm−1 at 6, 12, 24 and 48 h (Fig. S3†). All the above results confirm that ZnO@MOF-46 has been successfully synthesized with a core–shell structure.
Growth process of ZnO@MOF-46
Several factors such as reaction time, solvent, molar ratio and synthetic method affect the core–shell formation.43,44 To investigate the time effect on the growth process of ZnO@MOF-46, the reaction was performed at various time intervals. The TEM images (Fig. 5) show that increasing reaction time increases the thickness of MOF-46 as shell and decreases the diameter of the ZnO as core. Therefore, the ratio of the thickness of MOF-46 to that of ZnO cores has increased (0.07, 0.26, 0.37 and 0.50 for 6, 12, 24 and 48 h, respectively) (Fig. 5e). Furthermore, the changes in composition and structure of the obtained ZnO@MOF-46 affected by reaction time are revealed in the XRD patterns (Fig. 6). As the time increases, the corresponding diffraction peaks of MOF-46 become more obvious (ratio of the most intense peak of MOF-46 at 2 theta of 16.66° to the most intense peak of ZnO at 2 theta of 36.22° increases with time: 0.17, 0.32, 0.45, and 0.53 for 6, 12, 24 and 48 h, respectively). It can be concluded that the 2-aminoterephthalic acid ligands penetrate through the pores of the MOF-46 and coordinate to the Zn2+ ions released on the Zn surface of the ZnO core and form MOF-46. At the same time, Zn2+ ions diffuse to the outside and coordinate to ligands on the surface of MOF-46 shell. | ||
| Fig. 6 XRD patterns of ZnO@MOF-46 obtained at 6, 12, 24 and 48 h at different reaction time in compare with pure MOF-46. | ||
In these experiments, foreign Zn source was not used and the Zn2+ ions required for the formation of MOF-46 originate directly from ZnO while they dissolve in the solvent mixture. Moreover, to study the solvent effect on the formation of ZnO@MOF-46 core–shell rods, the reaction was performed using different ratios of reaction solvents by mixing 2-aminoterephthalic acid and ZnO with molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 during 48 h. When only DMF was used as the solvent, ZnO was unable to dissolve and supply the needed Zn2+ ions to form MOF-46 and the absence of MOF-46 can be observed from XRD results (Fig. S4†). However, when H2O was added into DMF with a certain ratio, the ZnO rods could be dissolved and MOF-46 can grow on the surface of ZnO rods. When the ratio of H2O to DMF was 2
1 during 48 h. When only DMF was used as the solvent, ZnO was unable to dissolve and supply the needed Zn2+ ions to form MOF-46 and the absence of MOF-46 can be observed from XRD results (Fig. S4†). However, when H2O was added into DMF with a certain ratio, the ZnO rods could be dissolved and MOF-46 can grow on the surface of ZnO rods. When the ratio of H2O to DMF was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, most of the ZnO rods were converted to rhombus micro sized MOF-46 particles (Fig. S4†). Since 2-aminoterephthalic acid is slightly soluble in H2O, the reaction could not be performed when only H2O was used as the solvent. Due to amphoteric nature of oxides, ZnO can be easily dissolved in acidic or basic aqueous solutions and releases Zn2+ ions.45–47 In this reaction, 2-aminoterephthalic acid has two important roles: (1) dissolving ZnO to release Zn2+ ions and (2) as a ligand to coordinate with Zn2+ ions and form MOF-46. When only DMF is used as the solvent, the etching ability of 2-aminoterephthalic acid is weak and it cannot dissolve enough of ZnO to release the Zn2+ ions for formation of MOF-46.43 In fact, the balance between the rate of release of Zn2+ ions and their coordination rate with ligand is important to have a good yield of ZnO@MOF-46 heterostructure. Therefore, when the ratio of H2O to DMF is 2
1, most of the ZnO rods were converted to rhombus micro sized MOF-46 particles (Fig. S4†). Since 2-aminoterephthalic acid is slightly soluble in H2O, the reaction could not be performed when only H2O was used as the solvent. Due to amphoteric nature of oxides, ZnO can be easily dissolved in acidic or basic aqueous solutions and releases Zn2+ ions.45–47 In this reaction, 2-aminoterephthalic acid has two important roles: (1) dissolving ZnO to release Zn2+ ions and (2) as a ligand to coordinate with Zn2+ ions and form MOF-46. When only DMF is used as the solvent, the etching ability of 2-aminoterephthalic acid is weak and it cannot dissolve enough of ZnO to release the Zn2+ ions for formation of MOF-46.43 In fact, the balance between the rate of release of Zn2+ ions and their coordination rate with ligand is important to have a good yield of ZnO@MOF-46 heterostructure. Therefore, when the ratio of H2O to DMF is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the rate of dissolution and release of Zn2+ ions is faster than the coordination rate and free standing MOF-46 will be formed. Due to the ability of DMF to slow down the rate of release of Zn2+ ions, the mixture of DMF and H2O at an appropriate ratio (3
1, the rate of dissolution and release of Zn2+ ions is faster than the coordination rate and free standing MOF-46 will be formed. Due to the ability of DMF to slow down the rate of release of Zn2+ ions, the mixture of DMF and H2O at an appropriate ratio (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was used to make a balance between rate of release of Zn2+ ions and their coordination with the ligand.
1) was used to make a balance between rate of release of Zn2+ ions and their coordination with the ligand.
Also with the change of molar ratio of ZnO to 2-aminoterephthalic acid, different products were obtained. When the molar ratio of ZnO to 2-aminoterephthalic acid was as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, compared to obtained sample in 1
1, compared to obtained sample in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (reaction time of 48 h), the thickness of ZnO core was greater (300–400 nm) (Fig. S5a†). On the other hand, with molar ratio 1
1 molar ratio (reaction time of 48 h), the thickness of ZnO core was greater (300–400 nm) (Fig. S5a†). On the other hand, with molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (48 h) a greater amount of ZnO nanorods dissolved and Zn2+ ions concentration in solution increases and thus reduce the thickness of ZnO core (100–130 nm) (Fig. S5b†).
2, (48 h) a greater amount of ZnO nanorods dissolved and Zn2+ ions concentration in solution increases and thus reduce the thickness of ZnO core (100–130 nm) (Fig. S5b†).
In addition, the method of synthesis plays a key role in the successful formation of core–shell rods. Under solvothermal conditions, in different time intervals (6, 12, 24 and 48 h), all the ZnO was converted to a crystalline material with unknown crystal phase and ZnO@MOF-46 core–shell structure couldn't obtained (Fig. S6†). However, on using the precipitation in solution method by mixing the precursors and leaving them at room temperature, the growth process of MOF-46 occurred on the surface of ZnO rods and ZnO@MOF-46 core–shell rods were formed.
Solvent dependent luminescence of ZnO@MOF-46 and its selective sensitivities for detecting aromatic compounds
The PL spectra of the activated MOF-46 and ZnO@MOF-46 samples were recorded at room temperature and it was found that MOF-46 and ZnO@MOF-46 emitted nearly the same luminescence spectrum upon excitation at 325 nm with emission bands located at around 418 and 416 nm for MOF-46 and ZnO@MOF-46, respectively. These fluorescent emissions of MOF-46 and ZnO@MOF-46 can be assigned to the intraligand transition of the 2-aminoterephthalic acid ligand modified by metal coordination because similar emission occurs at 425 nm for the free ligand (Fig. 7). The PL spectrum of ZnO@MOF-46 shows a blue shift in emission, accompanying a reduction in intensity in comparison with pure MOF-46. This phenomenon has been previously reported in the literature. Zhang group synthesized ZnO@MOF-5 hybrid films via growing MOF-5 crystals onto ordered ZnO nanorod arrays and reported a significant reduction in the intensity of PL emission of ZnO@MOF-5 compared with MOF-5. They related this reduction to fluorescence resonance energy transfer between ZnO and MOF-5.48 Lee et al. used MOFs as sensitizers of TiO2 and observed that the intensity of PL emission of MOFs was reduced in the presence of TiO2 and explained that the reduction happened because the excited electrons of MOFs can be transferred into the TiO2 film.49Since the thickness of MOF-46 shell to that of ZnO core in the prepared sample during 48 h has a bigger ratio, this sample was chosen for investigation. To study the potential luminescence sensing properties of ZnO@MOF-46, 0.01 g of the activated and fine grinding sample was immersed in 6 mL of different solvents ranging in polarity from water to chloroform (chloroform, methanol, ethanol, water, dimethylformamide, tetrahydrofuran, acetone and acetonitrile), treated by ultrasonication for 30 min and then aging for 3 days. The solvents were exchanged with fresh solvents every 12 hours. Fluorescence spectra were then recorded and compared to fluorescence spectra before exposing and immersing in different solvents (Fig. 8 and S7†). PL spectra show great dependence on the solvent molecules, especially in the case of methanol and acetone, which exhibit the most enhancing or quenching behavior, showing high sensitivity to solvent guests.
To investigate the sensitivity of ZnO@MOF-46 toward aromatic compounds, a suspension of 0.01 g of ZnO@MOF-46 dispersed in 6 mL of DMF, the aromatic content of which was gradually increased, was prepared to monitor the fluorescence response. In a typical experiment, various concentrations of aromatic compound solution (0–600 ppm) in DMF were gradually (50 ppm values) added to ZnO@MOF-46 suspension in DMF and the fluorescence spectra were recorded each time shortly after adding the aromatics. Detectable changes in emission response of ZnO@MOF-46 were observed after adding 100 ppm of aromatic compound solution and emission was almost quenched at 600 ppm concentration of nitrobenzene. The sensing study was mainly based on two different categories of aromatic compounds, namely compounds with electron-withdrawing substituents such as nitro (group A) and those with electron-donating substituents such as CH3 (group B). It was observed that aromatic compounds with electron-withdrawing groups act as fluorescence quenchers for ZnO@MOF-46. Among these, the most effective quencher is nitrobenzene (NB). In contrast with the quenching behavior shown by compounds with electron-withdrawing substituents, those having electron-donating substituents (or at least lacking nitro substituents) were found to enhance the ZnO@MOF-46 fluorescence, with enhancement increasing in the order bromobenzene (BrB) < iodobenzene (IB) < toluene (TO) (Fig. 9 and S8†). Therefore, it can be concluded that ZnO@MOF-46 acts as an electron-donor in the case of electron-withdrawing aromatics and an electron acceptor in the case of electron-donating aromatics. To explain the observed enhancing and quenching fluorescence behavior, a donor–acceptor electron-transfer mechanism can be used. In the case of aromatics containing electron-withdrawing substituents, the lowest unoccupied molecular orbital (LUMO) is a π*-type orbital with low-energy, which is stabilized by the electron-withdrawing substituents through conjugation. Thus, MOFs, especially those with d10 metal ions, can be regarded as giant “molecules” and their valence and conduction bands can be treated in a fashion similar to molecular orbitals (MOs). The conduction band of a ZnO@MOF-46 lies at a higher energy level than the lowest unoccupied MO of aromatic analyte and thus creates a driving force for electron transfer from the conduction band of ZnO@MOF-46 to the LUMO electron-deficient analytes, leading to a quenching effect.50–53 In the case of aromatics containing electron-donating substituents, the lowest unoccupied Molecular Orbital (MO) (LUMO) is a π* type orbital with high-energy, which lies above the conduction band of ZnO@MOF-46. Therefore, the excited electrons transfer from LUMO to the conduction band of ZnO@MOF-46 and lead to fluorescence enhancement (Scheme 2).54
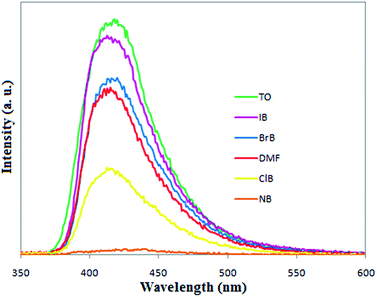 | ||
| Fig. 9 Emission spectra of ZnO@MOF-46 in DMF (concentration of aromatic compounds = 600 ppm, TO = toluene, IB = iodobenzene, BrB = bromobenzene, ClB = chlorobenzene and NB = nitrobenzene). | ||
Photocatalytic activity
Methylene blue (MB) was selected as a model pollutant in aqueous media for evaluating the photocatalytic effectiveness of ZnO@MOF-46. As illustrated in Fig. 10, the absorption peaks of MB clearly decrease under UV irradiation in the presence of ZnO@MOF-46. Furthermore, control experiments on photodegradation of MB were performed and the degradation of MB under the following reaction conditions was monitored (a) without catalyst; (b) in the presence of pure ZnO and (c) in the presence of pure MOF-46. The results are displayed as changes in the C/C0 plot of MB solution versus irradiation time (Fig. S9†) and it can be observed that the photocatalytic activity increases from 12.6% (without catalyst), 41.5% (in the presence of pure ZnO) and 51% (in the presence of pure MOF-46) to 81% for ZnO@MOF-46 after 3 h of irradiation. These results indicate that ZnO@MOF-46 is a good candidate for photocatalytic degradation of MB. | ||
| Fig. 10 Absorption spectra of the MB solution during the decomposition reaction with ZnO@MOF-46 as photocatalyst. | ||
In order to evaluate the stability of the photocatalysts, recycling reaction was carried out for the photodegradation of MB over ZnO@MOF-46. At the end of the first test, the photocatalyst was washed with ethanol and dried at 75 °C and reused under the same conditions. The absorption peak of MB showed a decrease of about 70% after 3 h, which shows that ZnO@MOF-46 is a stable and recyclable photocatalyst (Fig. S10†).
In order to find a photocatalytic mechanism, reactive species detection experiments were used to detect the main oxidative species in the photocatalytic process.55 Benzoquinone, isopropyl alcohol and ammonium oxalate (O2˙−, OH˙ and h+ scavengers) were separately added to MB solution at a known concentration (1 mM). The photocatalytic degradation of MB was apparently restrained after injection of 1 mM isopropyl alcohol (Fig. S11†). A decrease in MB degradation efficiency was also observed by the addition of benzoquinone as O2˙− scavenger and ammonium oxalate as h+ scavenger, respectively. However, isopropyl alcohol was found to be the most effective scavenger showing a drastic reduction in MB photodegradation. This suggests that all of O2˙−, OH˙ and h+ contribute to degradation, but OH˙ is the main active species and plays a major role in this system.
The diffuse reflectance spectra of ZnO and MOF-46 were recorded. The band gap of these samples could be calculated by the (αhν)2 = K(hν − Eg) equation, where hν is the photon energy (eV), α is the absorption coefficient, K is a constant, and Eg is the band gap (Fig. S12†).56 The band gaps of ZnO, MOF-46 and ZnO@MOF-46 were estimated by extrapolating the linear region in the plot of (αhν)2 versus photon energy as 3.23, 2.72 and 2.44 eV, respectively. On the hand, the chemical potentials of MOF-46 were calculated by cyclic voltammetry measurements. The oxidation and reduction peaks are apparent at 1.16 and −1.36 V, respectively (Fig. S13†). These potentials are normalized to NHE as 0.9 V for oxidation and −1.60 for reduction peak. The peak-to-peak separation between oxidation and reduction peaks is 2.50 V and these peaks can be regarded as valence and conduction bands, respectively.57
For ZnO@MOF-46, the enhancement of the photocatalytic activity can be explained by the interaction and synergistic effect of ZnO and MOF-46. A possible mechanism for photocatalytic degradation of MB over ZnO@MOF-46 is shown in Scheme 1. During the irradiation, MOF-46 electrons (e−) can be excited from the valence band (VB) to the conduction band (CB) of MOF-46. These photogenerated electrons in the CB of MOF-46 can then migrate to the CB of ZnO.58 At the same time, positively charged holes on the VB of ZnO could be injected to the VB orbital of MOF-46 (Scheme 3).59 These processes lead to more effective separation of electron–hole pairs and suppress their recombination.
Furthermore, combining absorbed O2 on the photocatalyst surfaces with electrons (e−) on the CB will create the oxygen radicals (O2˙), which will convert to hydroxyl radicals (OH˙). The adsorbed hydroxyl (OH−) on the surfaces of MOF-46 will interact with the hole (h+) on the VB and make hydroxyl radicals (OH˙). Hydroxyl radicals (OH˙) could effectively degrade the MB dye.60,61
The effect of pH on photocatalytic degradation was investigated at different pH and the results are given in Fig. S14.† The degradation of MB was monitored without adding NaOH, pH = 9 and pH = 10. The rate of photocatalytic degradation of dye increases with increase in pH from 35% (without NaOH), 60% (pH = 9) to 81% (pH = 10). This observation can be attributed to an increase in OH− ion concentration that generates more OH˙ radical.
Conclusions
In this work, MOF-46 and a novel core–shell heterostructure, ZnO@MOF-46, were successfully synthesized by using ZnO rods as the growth temple of the product and Zn2+ ions source via a simple method. The proposed synthetic method for the preparation of MOF-46 showed advantages such as short processing time, low cost and room temperature conditions. The effects of solvent composition, time of the reaction and synthesis method on the formation of ZnO@MOF-46 were all found to be important with regards to a successful and manageable synthesis of ZnO@MOF-46. Solvent-dependent luminescence properties of ZnO@MOF-46 were considered and the results showed that ZnO@MOF-46 could serve as a potential luminescent sensor in detecting small molecules. Moreover, the core–shell sample showed unique fluorescence quenching and enhancement behavior upon exposure to the aromatic compounds. The quenching mechanism toward the compounds with electron-withdrawing groups is attributed to electron transfer from conduction band of ZnO@MOF-46 to the LUMO electron-deficient aromatic compound and the enhancing mechanism toward the compounds with electron-donating groups is attributed to electron transfer from LUMO of aromatic compound to the conduction band of ZnO@MOF-46. Furthermore, the photocatalytic behavior of ZnO@MOF-46 was studied and it was proven to be a good photocatalyst for the decolorization of MB.Acknowledgements
Financial support of the project by the Alzahra University is gratefully acknowledged.References
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed
.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974–986 CrossRef CAS PubMed
.
- H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. O. Yazaydin, R. Q. Snurr, M. O'Keeffe, J. Kim and O. M. Yaghi, Science, 2010, 329, 424–428 CrossRef CAS PubMed
.
- M. Muller, S. Turner, O. I. Lebedev, Y. Wang, G. V. Tendeloo and R. A. Fischer, Eur. J. Inorg. Chem., 2011, 1876–1887 CrossRef
.
- Z.-J. Lin, J. Lu, M. Hong and R. Cao, Chem. Soc. Rev., 2016, 6, 41369–41375 Search PubMed
.
- W. Y. Hong, S. P. Perera and A. D. Burrows, Microporous Mesoporous Mater., 2015, 214, 149–155 CrossRef CAS
.
- S. R. Venna and M. A. Carreon, J. Am. Chem. Soc., 2010, 132, 76–78 CrossRef CAS PubMed
.
- S. Qiu, M. Xue and G. Zhu, Chem. Soc. Rev., 2014, 43, 6116–6140 RSC
.
- E. N. Koukaras, T. Montagnon, P. Trikalitis, D. Bikiaris, A. D. Zdetsis and G. E. Froudakis, J. Phys. Chem. C, 2014, 118, 8885–8890 CrossRef CAS
.
- M. Rad, S. Dehghanpour, S. Fatehfard, C. Gholamrezazadeh and A. Mahmoudi, Polyhedron, 2016, 106, 10–17 CrossRef CAS
.
- F. Song, C. Wang, J. M. Falkowski, L. Ma and W. Lin, J. Am. Chem. Soc., 2010, 132, 15390–15398 CrossRef CAS PubMed
.
- T. Wen, D.-X. Zhang and J. Zhang, Inorg. Chem., 2013, 52, 12–14 CrossRef CAS PubMed
.
- X. Wu, Z. Bao, B. Yuan, J. Wang, Y. Sun, H. Luo and S. Deng, Microporous Mesoporous Mater., 2013, 180, 114–122 CrossRef CAS
.
- C. G. Carson, A. J. Brown, D. S. Sholl and S. Nair, Cryst. Growth Des., 2011, 11, 4505–4510 Search PubMed
.
- H. Al-Kutubi, J. Gascon, E. J. R. Sudhclter and L. Rassaei, ChemElectroChem, 2015, 2, 447–459 CrossRef CAS
.
- M. Klimakow, P. Klobes, A. F. Thunemann, K. Rademann and F. Emmerling, Chem. Mater., 2010, 22, 5216–5221 CrossRef CAS
.
- J.-B. Lin, R.-B. Lin, X.-N. Cheng, J.-P. Zhang and X.-M. Chen, Chem. Commun., 2011, 47, 9185–9187 RSC
.
- G. Majano and J. P. Ramirez, Adv. Mater., 2013, 25, 1052–1057 CrossRef CAS PubMed
.
- Z. Li, Y.-N. Wu, J. Li, Y. Zhang, X. Zou and F. Li, Chem.–Eur. J., 2015, 21, 1–9 CrossRef
.
- E. Zanchetta, L. Malfatti, R. Ricco, M. J. Styles, F. Lisi, C. J. Coghlan, C. J. Doonan, A. J. Hill, G. Brusatin and P. Falcaro, Chem. Mater., 2014, 27, 690–699 CrossRef
.
- Y. Liu, S. Li, X. Zhang, H. Liu, J. Qiu, Y. Li and K. L. Yeung, Inorg. Chem. Commun., 2014, 48, 77–80 CrossRef CAS
.
- Y. Yue, Z.-A. Qiao, X. Li, A. J. Binder, E. Formo, Z. Pan, C. Tian, Z. Bi and S. Dai, Cryst. Growth Des., 2013, 13, 1002–1005 Search PubMed
.
- I. Stassen, N. Campangol, J. Fransaer, P. Vereechen, D. D. Vos and R. Ameloot, CrystEngComm, 2013, 15, 9308–9311 RSC
.
- M. Meilikhov, K. Yuesenko, D. Esken, S. Turner, G. V. Tendeloo and R. A. Fischer, Eur. J. Inorg. Chem., 2010, 3701–3714 CrossRef CAS
.
- H. R. Moon, D.-W. Lim and M. P. Suh, Chem. Soc. Rev., 2013, 42, 1807–1824 RSC
.
- Y. Liu and Z. Tang, Adv. Mater., 2013, 25, 5819–5825 CrossRef CAS PubMed
.
- P. Falcaroa, R. Ricco, A. Yazdi, I. Imaz, S. Furukawa, D. Maspoch, R. Ameloot, J.-D. Evans and C.-J. Doonan, Coord. Chem. Rev., 2016, 307, 237–254 CrossRef
.
- L. Lin, T. Zhang, H. Liu, J. Qiu and X. Zhang, Nanoscale, 2015, 7, 7615–7623 RSC
.
- L. Xu, Y.-L. Hu, C. Pelligra, C.-H. Chen, L. Jin, H. Huang, S. Sithambaram, M. Aindow, R. Joesten and S. L. Suib, Chem. Mater., 2009, 21, 2875–2885 CrossRef CAS
.
- A. K. Radzimska and T. Jesionowski, Materials, 2014, 7, 2833–2881 CrossRef PubMed
.
- S. G. Kumar and K. S. R. K. Rao, RSC Adv., 2015, 5, 3306–3351 RSC
.
- K.-M. Lee, C.-W. Lai, K.-S. Ngai and J.-C. Juan, Water Res., 2016, 88, 428–448 CrossRef CAS PubMed
.
- S.-M. Lam, J.-C. Sin, A.-Z. Abdullah and A.-R. Mohamed, Desalin. Water Treat., 2012, 41, 131–169 CrossRef CAS
.
- A. Stankovic, Lj. Veselinovic, S. D. Skapin, S. Markovic and D. Uskokovic, J. Mater. Sci., 2011, 46, 3716–3724 CrossRef CAS
.
- P.-C. Chang, Z. Fan, D. Wang, W.-Y. Tseng, W.-A. Chiou, J. Hong and J. G. Lu, Chem. Mater., 2004, 16, 5133–5137 CrossRef CAS
.
- P. Li, Y. Wei, H. Liu and X.-K. Wang, J. Solid State Chem., 2005, 178, 855–860 CrossRef CAS
.
- M. Ristic, S. Music, M. Ivanda and S. Popovic, J. Alloys Compd., 2005, 397, L1–L4 CrossRef CAS
.
- S. Music, D. Dragevic, S. Popovic and M. Ivanda, Mater. Lett., 2005, 59, 2388–2393 CrossRef CAS
.
- S. A. Vorobyova, A. I. Lesnikovich and V. V. Mushinskii, Mater. Lett., 2004, 58, 863–866 CrossRef CAS
.
- F. G. Petzold, J. Jasinski, E. L. Clark, J. H. Kim, J. Absher, H. Toufar and M. K. Sunkara, Catal. Today, 2012, 198, 219–227 CrossRef CAS
.
- M. E. Braun, C. D. Steffek, J. Kim, P. G. Rasmussen and O. M. Yaghi, Chem. Commun., 2001, 2532–2533 RSC
.
- B. Liu and H. C. Zeng, J. Am. Chem. Soc., 2003, 125, 4430–4431 CrossRef CAS PubMed
.
- W.-W. Zhan, Q. Kuang, J.-Z. Zhou, X.-J. Kong, Z.-X. Xie and L.-S. Zheng, J. Am. Chem. Soc., 2013, 135, 1926–1933 CrossRef CAS PubMed
.
- H. Al-Kutubi, A. Dikhtiarenko, H.-R. Zafarani, E.-J.-R. Sudholter, J. Gascon and L. Rassaei, CrystEngComm, 2015, 17, 5360–5364 RSC
.
- Q. Kuang, T. Xu, Z.-X. Xie, S.-C. Lin, R.-B. Huang and L.-S. Zheng, J. Mater. Chem., 2009, 19, 1019–1023 RSC
.
- R. Remias, A. Kukovecz, M. Daranyi, G. Kozma, S. Varga, Z. Konya and I. Kiricsi, Eur. J. Inorg. Chem., 2009, 3622–3627 CrossRef CAS
.
- X. Wang, J. Liu, S. Leong, X. Lin, J. Wei, B. Kong, Y. Xu, Z.-X. Low, J. Yao and H. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 9080–9087 Search PubMed
.
- Y. Zhang, D. Lan, Y. Wang, H. Cao and H. Jiang, Phys. E, 2011, 43, 1219–1223 CrossRef CAS
.
- D. Y. Lee, C. Y. Shin, S. J. Yoon, H. Y. Lee, W. Lee, N. K. Shrestha, J. K. Lee and S.-H. Han, Sci. Rep., 2014, 4, 3930–3934 CrossRef PubMed
.
- J.-S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864–11873 CrossRef CAS
.
- S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871–2883 RSC
.
- M. E. Germain, T. R. Vargo, P. G. Khalifah and M. J. Knapp, Inorg. Chem., 2007, 46, 4422–4429 CrossRef CAS PubMed
.
- H. Sohn, M. J. Sailor, D. Magde and W. C. Trogler, J. Am. Chem. Soc., 2003, 125, 3821–3830 CrossRef CAS PubMed
.
- M. Guo and Z.-M. Sun, J. Mater. Chem., 2012, 22, 15939–15946 RSC
.
- H. Wang, X. Yuan, Y. Wu, G. Zeng, X. Chen, L. Leng and H. Li, Appl. Catal., B, 2015, 174, 445–454 CrossRef
.
- C.-K. Lin, D. Zhao, W.-Y. Gao, Z. Yang, J. Ye, T. Xu, Q. Ge, S. Ma and D.-J. Liu, Inorg. Chem., 2012, 51, 9039–9044 CrossRef CAS PubMed
.
- S. K. Haram, B. M. Quinn and A. J. Bard, J. Am. Chem. Soc., 2001, 123, 8860–8861 CrossRef CAS PubMed
.
- S. Khanchandani, P. K. Srivastava, S. Kumar, S. Ghosh and A. K. Ganguli, Inorg. Chem., 2014, 53, 8902–8912 CrossRef CAS PubMed
.
- X.-X. Xu, Z.-P. Cui, J. Qi and X.-X. Liu, Dalton Trans., 2013, 42, 4031–4039 RSC
.
- W. Meng, Z. Xu, J. Ding, D. Wu, X. Han, H. Hou and Y. Fan, Cryst. Growth Des., 2014, 14, 730–738 Search PubMed
.
- L. Liu, J. Ding, C. Huang, M. Li, H. Hou and Y. Fan, Cryst. Growth
Des., 2014, 14, 3035–3043 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12410k |
| This journal is © The Royal Society of Chemistry 2016 |








