Laser irradiation durability of photorefractive ferroelectric liquid crystal blends containing terthiophene photoconductive chiral dopants†
Takeo Sasaki*a,
Masanori Yoshinoa,
Yumiko Nakaa,
Khoa V. Lea and
Takafumi Sassab
aDepartment of Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: sasaki@rs.kagu.tus.ac.jp
bAdvanced Device Laboratory, RIKEN (The Institute of Physical and Chemical Research), Cooperation Center, South Area, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan
First published on 20th July 2016
Abstract
Ferroelectric liquid crystal blends composed of a smectic liquid crystalline mixture, a photoconductive chiral dopant, and an electron trap reagent exhibit significant photorefractivity together with rapid responses. As such, they allow the dynamic amplification of moving optical signals. In the present work, the photochemical durability of a photorefractive ferroelectric liquid crystal blend sandwiched between two transparent electrodes was investigated. A series of photoconductive chiral dopants was prepared and the durability of blends incorporating these dopants during laser irradiation was examined. The photorefractivity of the liquid crystal blend with application of a DC electric field was found to decay on increasing the laser irradiation time. However, no evidence for a photochemical reaction of the photoconductive chiral dopant was obtained. The effect of the conduction of photogenerated ionic species on the photorefractivity decay was clarified. It was concluded that the observed decay of the photorefractivity was caused by the adsorption of photogenerated ionic species on the electrode.
Introduction
The dynamic amplification of optical signals by photorefractive ferroelectric liquid crystal (FLC) blends has been investigated,1 and FLCs doped with photoconductive compounds have been shown to exhibit a large photorefractive effect.1,2 This phenomenon is able to generate a refractive index grating (or hologram) within a medium,3–5 based on a change in the refractive index of the medium resulting from photoinduced electric field and electrooptic effects. The mechanism by which the photorefractive effect occurs in FLCs doped with photoconductive compounds is shown in Fig. 1. When laser beams undergo interference within a mixture of an FLC and a photoconductive compound, charge separation occurs between the bright and dark regions and internal electric fields are produced. These fields alter the direction of spontaneous polarization in the spaces between the bright and dark regions of the interference fringes, which in turn induces periodic changes in the orientations of the FLC molecules. The switching of the FLC molecules is based on a bulk polarization response and is thus extremely fast.6,7 One of the challenges associated with FLC materials is that it is difficult to obtain thick FLC films; these films must typically be 2 μm thick because the ferroelectric state (or surface stabilized (SS) state) of the FLC material is induced by the interaction between the FLC molecules and the surfaces of the substrates that sandwich the FLC material. Even though these films are quite thin, the change in apparent refractive index induced by the internal electric field is large since these materials exhibit significant birefringence. The photorefractive effect promotes asymmetric energy exchange, in which the energy of one of the interfering laser beams is transferred to the other. Since asymmetric energy exchange is essentially the amplification of one beam by another, it can be utilized in optical signal amplification.8,9 Recently, the properties of photorefractive FLC blends containing photoconductive chiral dopants (Fig. 2) have been reported.1,10,11 In such cases, the dopant molecule contains a photoconductive moiety and has a chiral structure, and dopants with terthiophene photoconductive moieties have been found to exhibit high miscibility with smectic liquid crystals. The gain coefficient of a photorefractive FLC blend was determined to be in excess of 1200 cm−1, with a response time less than 1 ms.1 Using the asymmetric energy exchange properties of this material, an optical image moving at 30 fps was successfully amplified.1 | ||
Fig. 2 Structures of the smectic LCs (8PP8 and 8PP10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture), photoconductive chiral dopants, and the electron trap reagent (TNF). 1 mixture), photoconductive chiral dopants, and the electron trap reagent (TNF). | ||
One problem associated with the use of organic materials in laser beam manipulation is the low durability of these compounds. In the present study, therefore, a series of photoconductive chiral terthiophene dopants was prepared and the photochemical stability of these compounds was investigated. The photorefractive effect of FLC blends with terthiophene dopants was found to decay with irradiation time, and the associated decay mechanism was elucidated and improvements in the durability of the photorefractivity were explored.
Experimental
Materials
The structures of the LC compounds, the electron acceptor trinitrofluorenone (TNF) and the photoconductive chiral compounds used in this study are all shown in Fig. 2. The synthesis of the photoconductive chiral dopant 3T-2MB is described in our previous paper.10 The synthetic route for 3-Me-3T-2MB is shown in Scheme 1. TNF was obtained from the Tokyo Kasei Co. The base LC used in this study was a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 5-octyl-2-(4-octyloxyphenyl) pyrimidine (8PP8) and 2-(4-decyloxyphenyl)-5-octyl pyrimidine (8PP10), both obtained from the Wako Chemicals Co. and purified by recrystallization from a mixture of methanol and ethyl acetate. To prepare samples, the base LC, TNF, and one of the photoconductive chiral compounds were dissolved in dichloroethane, after which the solvent was evaporated. The mixture was then dried under vacuum at room temperature for one week. The samples were subsequently injected into a 10 μm gap glass cell equipped with 1 cm2 ITO electrodes and a polyimide alignment layer (LX-1400, Hitachi Chemicals Co.) in order to perform the measurements. For spontaneous polarization measurement, 2 μm gap cell was used.
1 mixture of 5-octyl-2-(4-octyloxyphenyl) pyrimidine (8PP8) and 2-(4-decyloxyphenyl)-5-octyl pyrimidine (8PP10), both obtained from the Wako Chemicals Co. and purified by recrystallization from a mixture of methanol and ethyl acetate. To prepare samples, the base LC, TNF, and one of the photoconductive chiral compounds were dissolved in dichloroethane, after which the solvent was evaporated. The mixture was then dried under vacuum at room temperature for one week. The samples were subsequently injected into a 10 μm gap glass cell equipped with 1 cm2 ITO electrodes and a polyimide alignment layer (LX-1400, Hitachi Chemicals Co.) in order to perform the measurements. For spontaneous polarization measurement, 2 μm gap cell was used.
Synthesis of the photoconductive chiral dopant 3-Me-3T-2MB
1H NMR (CDCl3) δ 0.88 (t, 3H, CH3), 1.23–1.42 (m, 10H, –(CH2)5–), 1.62 (m, 2H, –CH2–), 2.15 (s, 3H, Ar–CH3), 2.68 (t, 2H, Ar–CH2–), 6.77 (d, 1H, Ar–H), 6.99 (d, 1H, Ar–H).
1H NMR (CDCl3) δ 0.88 (t, 3H, CH3), 1.23–1.42 (m, 10H, –(CH2)5–), 1.55 (m, 2H, –CH2–), 2.08 (s, 3H, Ar–CH3), 2.63 (t, 2H, Ar–CH2–), 6.71 (s, 1H, Ar–H).
1H NMR (CDCl3) δ 0.88 (t, 3H, CH3), 1.23–1.42 (m, 10H, –(CH2)5–), 1.63 (m, 2H, –CH2–), 2.12 (s, 3H, Ar–CH3), 2.68 (t, 2H, Ar–CH2–), 6.71 (s, 1H, Ar–H), 6.96 (t, 1H, Ar–H), 7.06 (d, 1H, Ar–H), 7.13 (d, 1H, Ar–H).
1H NMR (CDCl3) δ 0.88 (t, 3H, CH3), 1.22–1.43 (m, 10H, –(CH2)5–), 1.62 (m, 1H, –CH2–), 2.12 (s, 3H, Ar–CH3), 2.80 (t, 2H, Ar–CH2–), 6.92 (s, 1H, Ar–H), 7.13 (d, 1H, Ar–H), 7.48 (d, 1H, Ar–H).
1H NMR (CDCl3) δ 0.96 (t, 3H, CH3), 1.00 (d, 3H, CH3), 1.27–1.44 (m, 2H, –(CH2)–), 1.82 (m, 1H, –COO–CH–(CH2)–),4.10 (dd, 1H, –COO–CH2–), 7.06 (d, 1H, Ar–H), 7.54 (d, 1H, Ar–H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of hexane and chloroform). The product was obtained as a yellow liquid (1.52 g, yield: 62.8%).
1 mixture of hexane and chloroform). The product was obtained as a yellow liquid (1.52 g, yield: 62.8%).1H NMR (CDCl3) δ 0.93 (t, 3H, –CH3), 1.00 (d, 3H, –CH3), 1.25–1.29 (m, 10H, –(CH2)5–), 1.69 (m, 2H, Ar–CH2–CH2–), 1.87 (m, 1H, –CH–), 2.38 (t, 3H, Ar–CH3), 2.80 (t, 2H, Ar–CH2–), 4.10 (dd, 1H, –COO–CH2–), 4.18 (dd, 2H, –COO–CH2–), 6.73 (d, 1H, Ar–H), 6.96 (d, 1H, Ar–H), 7.05 (s, 1H, Ar–H), 7.08 (d, 1H, Ar–H), 7.66 (d, 1H, Ar–H).
Measurements
Phase transition temperatures were determined using differential scanning calorimetry (DSC; DSC822, Mettler) and microscopic observations (FP-80, FP-82, Mettler; DM2700 polarizing microscope, Leica). Spontaneous polarization (Ps) was measured by the triangular waveform voltage method (10 Vp–p, 100 Hz). The photorefractive effect was examined via a two-beam coupling experiment. The experimental set-up is shown in Fig. S1.† In these trials, a linearly polarized beam from a diode-pumped solid state laser (DPSS laser, 488 nm, continuous wave) was divided in two by a beam splitter, and these two beams underwent interference in the sample film. 488 nm wavelength was chosen because the photoconductive chiral dopants used in this study show alight absorbance at this wavelength. A p-polarized beam was used in the majority of the experiments in this study. The laser intensity was 2 mW for each beam and each had a 0.5 mm diameter. The beam angles incident to the glass plane were 30° and 50° and the interference fringe interval was 1.87 μm. Throughout measurements, the sample was heated to 30 °C using a thermo-controller (DB1000, Chino Co.). An electric field ranging from 0 to 10 V μm−1 was applied to the sample from a regulated DC power supply (Kenwood DW36-1) while the change in the transmitted beam intensity was monitored by photodiodes (ET-2040, Electro-Optics Technology, Inc.) and the data recorded on a computer. The time required to form the refractive index grating in the FLC was ascertained based on the simplest single-carrier model of photorefractivity, in which the gain transient is exponential.5 The rising signal of the diffracted beam was fitted using a single exponential function:| γ(t) − 1 = (γ − 1)[1 − exp(−t/τ)]2, | (1) |
Results and discussion
FLC blend phase transition temperatures and textures observed under polarizing microscope
The phase transition temperatures of the photorefractive FLC blends incorporating 3T-2MB, 3-Me-3T-2MB, 3′-Me-3T-2MB, 3′′-Me-3T-2MB, and 3′,3′′-Me-3T-2MB are presented in Fig. 3. The phase transition temperatures were ascertained by DSC and polarizing microscope. All the samples exhibited a SmC* phase, which is the ferroelectric phase. As the concentration of the dopant was increased, the phase transitions were found to move to lower temperatures.The textures of the samples as observed under a polarizing microscope are shown in Fig. 4. The extent of defects in the samples was found to be greater when employing the photoconductive dopants with methyl substituents, and the dopant with two methyl substituents (3′,3′′-Me-3T-2MB) gave a highly defective LC phase. For this reason, the highest allowable concentration of the dopant in the base LC was lower when using dopants with methyl substituents.
Ferroelectricity and photoconductivity of FLC blends
The spontaneous polarizations of FLC blends containing 3T-2MB, 3-Me-3T-2MB, 3′-Me-3T-2MB, 3′′-Me-3T-2MB, and 3′,3′′-Me-3T-2MB were assessed in 2 μm gap glass cells incorporating an ITO electrode coated with a polyimide alignment layer. Unfortunately, the spontaneous polarization of these samples could not be measured in this apparatus, although it is believed to be less than 1 nC cm−2. Zig-zag defects and a striped texture were both observed in samples held in 10 μm gap cells under a polarizing microscope, demonstrating that helical structures were formed in the samples in the wide gap cell. All samples exhibited electro-optical switching and the associated response times in the 2 μm gap cells were found to be shorter than 2 ms. These results indicate that these FLC blends all form an SmC* phase.The UV-vis spectra of samples in the 10 μm gap cell are shown in Fig. 5. In these specimens, the ratio of the base LC, photoconductive chiral dopant and TNF was held constant at 93.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.0
6.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1. It was confirmed that the samples absorbed at 488 nm, which was the wavelength of the writing laser used in this study. The photo-conductivities of the FLC blends were subsequently measured. As shown in Fig. 6, the samples were good insulators in the dark. When the samples were irradiated with a 488 nm laser beam, clear photocurrents were observed, with magnitudes in the range of 250–350 nA cm−2. The observed differences in photocurrent density between samples were attributed to variations in the miscibility of the photoconductive chiral dopant with the base LC. In fact, the formation of defects and the carrier mobility are both dominated by the miscibility of the dopant and the base LC, since light scattering and low mobility of the carrier lead to lower photoconductivity.
0.1. It was confirmed that the samples absorbed at 488 nm, which was the wavelength of the writing laser used in this study. The photo-conductivities of the FLC blends were subsequently measured. As shown in Fig. 6, the samples were good insulators in the dark. When the samples were irradiated with a 488 nm laser beam, clear photocurrents were observed, with magnitudes in the range of 250–350 nA cm−2. The observed differences in photocurrent density between samples were attributed to variations in the miscibility of the photoconductive chiral dopant with the base LC. In fact, the formation of defects and the carrier mobility are both dominated by the miscibility of the dopant and the base LC, since light scattering and low mobility of the carrier lead to lower photoconductivity.
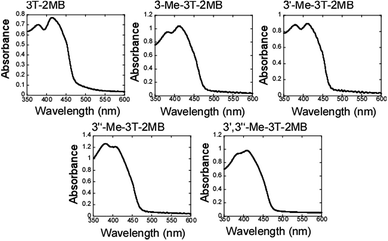 | ||
| Fig. 5 UV-vis spectra of FLC blends with photoconductive chiral dopants. The concentrations of the photoconductive chiral dopants and of TNF were held constant at 6 and 0.1 wt% in all samples. | ||
Asymmetric energy exchange in photorefractive FLC blends
Two-beam coupling experiments were conducted on the photorefractive FLC blends. Fig. 7 presents a typical example of the asymmetric energy exchange observed in an FLC blend with 3T-2MB. When laser beams underwent interference in the FLC sample, the transmitted intensity of one beam increased while that of the other decreased, resulting in the amplification of one beam by the other. Thus, the transmittance characteristic of the amplified beam can be described as| I = I0eΓL, | (2) |
 | (3) |
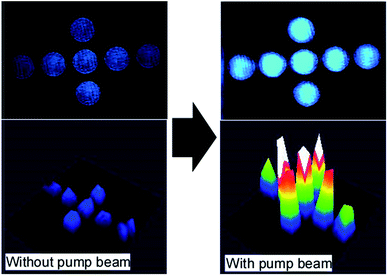
Optical image amplification was demonstrated using a 3T-2MB sample as shown in Fig. 8. A computer-generated image was displayed on a spatial light modulator (SLM) and a 473 nm DPSS laser beam was irradiated. The laser beam containing the image (signal beam) was transmitted through the FLC sample and monitored by a CCD camera. When the pump beam (a beam divided from the signal beam before SLM) was incident to the FLC sample and interfered with the signal beam, the signal was amplified through asymmetric energy exchange. The intensity of the signal beam was amplified 6-fold compared to that without the pump beam. A computer-generated animation was displayed on the SLM. The frame rate was 30 fps. A 473 nm beam was irradiated on the SLM and the reflected beam was incident on the FLC sample. A pump beam interfered with the beam from the SLM in the FLC sample. A laser beam containing the moving image of the animation was amplified by the incident pump beam (Fig. 9 and ESI video†). This result shows that the response of the photorefractive FLC was fast enough to amplify the optical image in real time. If a photorefractive material with a response time of ∼100 ms is used in place of the FLC sample, the amplification would not occur. In that case, although a still image can be amplified, the intensity of the image would be weakened to the original magnitude when the image starts to move at a video rate.
 | ||
| Fig. 9 Optical image amplification experiment. A computer-generated animation was displayed on the SLM. The reflected beam form the SLM (473 nm) was irradiated on the FLC sample and interfered with the reference beam. The image transmitted through the FLC sample (3T-2MB, 10 wt%) was monitored by a CCD camera (see the ESI video†). | ||
The magnitude of the gain coefficient of the FLC blend with 3T-2MB is plotted as a function of the laser irradiation time in Fig. 10(a). It is evident that the gain coefficient decayed rapidly to 20% of the initial value within 90 min. The texture of the sample was observed under polarizing microscope, as shown in Fig. 10(b), and a spot where the alignment of the LC molecules was disturbed was observed at the irradiated site.
Photochemical reactivity of the photoconductive chiral dopant 3T-2MB
The observed decay of the photorefractivity could be caused by the photochemical reaction of the photoconductive chiral dopant 3T-2MB, since the terthiophene chromophore may undergo photoinduced dimerization or polymerization reactions.15–17 If these reactions occur, the products would be insoluble in the base LC and the photorefractivity would therefore be reduced. These reactions, however, could be prevented by the introduction of steric hindrance to the dopant molecule. The photoconductive chiral dopants introduced with methyl substituents were prepared (Fig. 2). The change in magnitude of the gain coefficients of the FLC blends are shown as functions of time in Fig. 11. Despite the introduction of steric hindrance in some dopants, no changes were observed in the decay profiles, even though the methyl substituent is expected to shield the reaction site and also hinder the approach to the terthiophene moieties. These results indicate that the photorefractivity decay does not result from photochemical dimerization, nor from polymerization. 1H NMR spectra of a 3T-2MB sample before and after irradiation with 488 nm light are shown in Fig. 12(a). As can be seen, no changes were observed in the NMR spectrum after laser irradiation. The UV-vis spectra and the IR spectra of a 3T-2MB sample before and after irradiation with 488 nm light are also presented in Fig. 12(b) and (c), and there are no evident changes in either spectra. It therefore appears that the terthiophene moiety does not undergo photochemical dimerization, polymerization, or decomposition, and hence the decay of the photorefractive effect is caused by other factors.Thermal recovery of the photorefractive effect
The photorefractive gain with DC field application evidently decays with increasing laser irradiation time and, in the present study, a two-beam coupling experiment was conducted on a decayed sample after keeping the sample in the dark. Fig. 13 shows the two-beam coupling gain signal as initially acquired, after irradiation by the laser beams for 60 min and after the sample was held in darkness without application of an electric field for 12 h. The decayed signal was found to recover to 85% of the initial value, indicating that the decay in the photorefractive effect is caused by a reversible change in the properties of the sample. One possible mechanism for this reversible gain decay is the adsorption of photogenerated ionic species onto the ITO electrode. It is known that image sticking in LC display is often caused by accumulation of ionic species at interface between LC and the alignment layer.18 When 488 nm laser is irradiated, charge transfer occurs between terthiophene and TNF generating a terthiophene cation and a TNF anion. The generated terthiophene cations and TNF anions at the irradiated site may move to the electrode under the application of an electric field. The ionic species generated at the irradiated site, such as terthiophene cations and TNF anions, may move to the electrode under the application of an electric field. The adsorbed ionic species are thus removed from the LC phase and the conductivity of the LC phase is consequently reduced. The adsorption of ionic species on the surface of the polyimide alignment layer coated over top of the ITO electrode also disturbs the alignment of the LC molecules, leading to the appearance of a spot at the irradiated site, as shown in Fig. 10(b). | ||
| Fig. 13 The two-beam coupling signal observed in an FLC blend with 3T-2MB as measured initially, after laser beam irradiation (250 mW cm−2 each) for 60 min and after being held in darkness for 12 h. | ||
Improvement of the duration of the photorefractive effect in FLC blends using a bipolar field
If the decay of the photorefractive effect in FLC blends is caused by adsorption of the photogenerated ionic species, the decay could potentially be suppressed by the application of a bipolar electric field. The photogenerated 3T-2MB cations and TNF anions drift to the electrode under the application of a DC field and so, in order to prevent ions adsorbing on the electrode surface, the direction of the electric field should be reversed. The frequency of the applied bipolar field must be chosen in accordance with the mobility of the ionic species and the strength of the field must be optimized. Fig. 14 shows the gain coefficient decay profiles measured under a bipolar field. The decay of the gain coefficient stopped at 80% of the initial value under the bipolar field condition. The data provide evidence that the decay of the photorefractive effect in FLC blends is caused by the drift of ionic species generated by photoirradiation. Since the 3T-2MB cation and the TNF anion are different sizes, the mobilities of those ions are also different. There is thus room to optimize the shape and the strength of the bipolar field. It should be noted that the electric fields used in LC displays are not DC fields but rather complicated bipolar fields, since these are necessary to prevent image sticking caused by the adsorption of LC molecules and ionic impurities. The application of bipolar fields is thus already an intrinsic aspect of LC devices used for photorefractive devices. | ||
| Fig. 14 Gain coefficient of an FLC blend with 3T-2MB with the application of a bipolar electric field as a function of time. The 3T-2MB and TNF concentrations in the sample were 6 and 0.1 wt%. | ||
Conclusion
The decay of the photorefractive effect in FLC blends containing terthiophene photoconductive chiral dopants was investigated, focusing on the possibility of photochemical reactions of the components. However, no evidence for photochemical reactions in the blends prepared in this study was observed. In contrast, it is believed that the adsorption of ionic species generated at the photoirradiated spot impedes the photorefractive effect. A bipolar electric field was applied during two-beam coupling trials and it was found that the decay of the gain coefficient was suppressed by the application of this field.Acknowledgements
This work was supported by the program for Strategic Promotion of Innovative Research and Development (S-Innovation), Japan Science and Technology Agency (JST).References
- T. Sasaki, S. Kajikawa and Y. Naka, Faraday Discuss., 2014, 174, 203 CAS.
- T. Sasaki and Y. Naka, Opt. Rev., 2014, 21, 99 CrossRef CAS.
- B. Lynn, P. A. Blanche and N. Peyghambarian, J. Polym. Sci., Part B: Polym. Phys., 2014, 52, 193 CrossRef CAS.
- S. Kober, M. Salvador and K. Meerholtz, Adv. Mater., 2011, 23, 4725 CrossRef.
- O. Ostroverkhova and W. E. Moerner, Chem. Rev., 2004, 104, 3267 CrossRef CAS PubMed.
- K. Skarp and A. A. Handschy, Mol. Cryst. Liq. Cryst., 1998, 165, 439 Search PubMed.
- P. Oswald and P. Pieranski, Smectic and Columnar Liquid Crystals, Taylor & Francis, New York, 2006 Search PubMed.
- A. Goonesekera, D. Wright and W. E. Moerner, Appl. Phys. Lett., 2000, 76, 3358 CrossRef CAS.
- N. Koukourakis, T. Abdelwahab, M. Y. Li, H. Höpfner, Y. W. Lai, E. Darakis, C. Brenner, N. C. Gerhardt and M. R. Hofmann, Opt. Express, 2011, 19, 22004 CrossRef PubMed.
- T. Sasaki, D. Miyazaki, K. Akaike, M. Ikegami and Y. Naka, J. Mater. Chem., 2011, 21, 8678 RSC.
- T. Sasaki, M. Ikegami, T. Abe, D. Miyazaki, S. Kajikawa and Y. Naka, Appl. Phys. Lett., 2013, 102, 063306 Search PubMed.
- P. Gawrys, D. Boudinet, A. Kornet, D. Djurado, S. Pouget, J. M. Verilhac, M. Zagorska and A. Pron, J. Mater. Chem., 2010, 20, 1913 RSC.
- H. B. Ammar, X. Miao, C. Fischmiester, L. Toupet and P. H. Dixneuf, Organometallics, 2010, 29, 4213 CrossRef.
- S. A. Ponomarenko, S. Kirchmeyer, A. Elschner, N. M. Alpatova, M. Halik, H. Klaul, U. Zschieschang and G. Schmid, Chem. Mater., 2006, 18, 579 CrossRef CAS.
- M. Fujitsuka, T. Sato, H. Segawa and T. Shimizu, Synth. Met., 1995, 69, 309 CrossRef CAS.
- N. Jayasuriya and J. Kagan, J. Org. Chem., 1989, 54, 4203 CrossRef CAS.
- T. Liu, L. Ding, G. He, Y. Yang, W. Wang and Y. Fang, ACS Appl. Mater. Interfaces, 2011, 3, 1245 CAS.
- D. Xu, F. Peng, H. Chen, J. Yuan, S. T. Wu, M. C. Li, S. L. Lee and W. C. Tsai, J. Appl. Phys., 2014, 116, 193102 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12231k |
| This journal is © The Royal Society of Chemistry 2016 |