Photoelectrochemical performance of g-C3N4/Au/BiPO4 Z-scheme composites to improve the mineralization property under solar light
Abstract
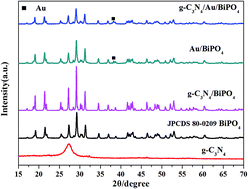
g-C3N4/Au/BiPO4 as a hierarchical Z-scheme system was prepared through three steps at different reaction temperatures, using thiourea as a precursor to synthesize g-C3N4. Their photoelectric activities were also investigated via the degradation of 10 mg L−1 methyl orange (MO). g-C3N4/Au/BiPO4 exhibited remarkably promoted photocatalytic activity and photoelectrochemical property compared with g-C3N4, BiPO4 and g-C3N4/BiPO4. The electrochemical impedance spectra and photocurrent result also proved that efficient charge separation and a better electron transport property were achieved by g-C3N4/Au/BiPO4. Such excellent performances can be attributed to the Z-scheme system which improved the separation efficiency of photo-induced carriers and suppressed the recombination of electron–holes. It is worth pointing out that the metallic Au species not only acted as a solid state electron mediator, but also could absorb photons from incident light and present a surface plasmon resonance effect in this hybridization system.

 Please wait while we load your content...
Please wait while we load your content...