PEGylated graphene oxide-based nanocomposite-grafted chitosan/polyvinyl alcohol nanofiber as an advanced antibacterial wound dressing†
Poornima Dubeya and
P. Gopinath*ab
aNanobiotechnology Laboratory, Centre for Nanotechnology, Indian Institute of Technology Roorkee, Roorkee, Uttarakhand-247667, India
bDepartment of Biotechnology, Indian Institute of Technology Roorkee, Roorkee, Uttarakhand-247667, India. E-mail: pgopifnt@iitr.ernet.in; genegopi@gmail.com; Fax: +91-1332-273560; Tel: +91-1332-285650
First published on 4th July 2016
Abstract
Designing composite nanomaterials that display multiple antibacterial mechanisms offers new prototype against bacterial resistance. This study presents a multi-component composite-based nanofiber embodying the antibacterial and physiochemical properties of silver nanoparticles (Ag NPs), graphene oxide (GO), chitosan (CS), and curcumin (CUR). Physiologically stable PEGylated GO–Ag NP–CUR nanocomposites were synthesized, with the PEGylated GO serving as the template. The as-synthesized nanocomposite was incorporated into the CS/polyvinyl alcohol (PVA) nanofiber. The successful formation and stability of the PEGylated-GO–Ag NP–CUR composite nanofiber were characterized by various techniques. The antibacterial potential of the PEGylated-GO–Ag NP–CUR composite nanofiber was evaluated and showed an enhanced antibacterial effect compared to various nanoformulations. The plausible antibacterial mechanism of the PEGylated-GO–Ag NP–CUR nanofiber was determined and depicted herein. The presence of GO in the composite nanofiber enhances its mechanical properties compared to CS/PVA nanofiber, with an ultimate tensile strength (UTS) of 25 MPa compared to 7.2 MPa and a Young's modulus (E) of 363.7 MPa compared to 73 MPa. The biocompatibility of the nanofiber mat was confirmed by in vitro cell viability assay. Therefore a facile approach for the design of a biocompatible wound dressing with enhanced mechanical and antibacterial property was explored and detailed herein.
1 Introduction
Skin refers to the soft tissue that constitutes the primary line of defense against infections by foreign invaders and holds the capacity of self-wound healing.1 However, several chronic wounds show delayed response that necessitates medicinal intervention.2 Antimicrobial wound dressing serves as a coating material to protect wounds from the entry of pathogens and to aid in the regeneration process for healing to take place. However, microbial biofouling is a serious problem associated with biomedical coating materials and can lead to severe infections at a wound site.3 The surface colonization by microbes and their ability to cause fouling of a coating material can hamper the material's effectiveness. Among various bacterial infections, colonization by Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) onto the skin is common. As a promising alternative, nanofiber-based composite nanomaterials can serve as scaffolds for tissue regeneration, as they closely imitate the diameter of elongated collagen fibrils in the natural extracellular matrix.4 Nanofibrous scaffolds offer an enormous opportunity for the development of numerous types of novel drug-delivery systems (DDS) due to their simple and efficient top-down fabrication procedure.5 Furthermore, nanofibers have a high surface area, microporosity, surface roughness, and hydrophilicity and thus aid in fibroblasts cell adhesion, migration, and proliferation at a wound infection site.4 Among the various different polymers used for nanofibers production, chitosan (CS) has a number of valuable characteristics, such as biocompatibility, biodegradability, and the potential for improved wound healing.6 However, its unique chemical structure limits pure CS nanofiber formation. Thus, scientists have explored blending it with suitable synthetic biodegradable polymers, among which PVA presents several advantageous features for biomedical applications.7Among the antimicrobial agents, Ag NPs have been widely used owing to their antimicrobial potential against a broad spectrum of microorganisms.4,9 They have been used in numerous biomedical applications,8 as well as with burns, wound dressings, and other microbial infections, in augmentation devices, and with antimicrobial filters, etc.9 Nevertheless, the effectiveness of Ag NPs is hampered by their agglomeration in biological systems. To address this issue, GO-based films and support matrices to increase stability and to simultaneously aid in the synthesis and antimicrobial properties of Ag NPs have been recently explored.10 The high concentration of oxygenated groups confers GO sheets with the versatility to bind to solid surfaces via covalent and non-covalent interactions, thus providing multiple opportunities to develop multifunctional materials.11 Recently, the antibacterial potential of Ag NPs assembled on graphene oxide sheets (GO–Ag) as novel antibacterial systems was explored.11 However, GO is poorly stable at high ionic strength and in serum medium, which hampers its biomedical application. Thus, researchers have explored methods for GO functionalization utilizing various stabilizing agents, such as poly(ethylene glycol) (PEG), etc.12
Among the natural antibacterial materials, CUR, a natural preservative and common ingredient in many food items, possesses remarkable antimicrobial potential,13 albeit its effectiveness is hampered due to its water insolubility. However, its potency could be increased by enhancing its water solubility. As GO is amphiphilic in nature, thus it allows hydrophobic drug loading by a non-covalent physiosorption method via the π–π stacking interaction of aromatic compounds.12 Thus herein, we have exploited the potential of GO to serve as a drug carrier for water insoluble drugs. The PEGylated GO was synthesized to enhance its physiological stability and also to act as a template for the synthesis of PEGylated GO–Ag NP composite, with CUR then loaded onto the composite to obtain the PEGylated GO–Ag NP–CUR to further enhance the antimicrobial property. Additionally, for the development of an advanced antimicrobial wound dressing mat, the as-prepared composite was further incorporated into a CS/PVA-based nanofiber mat. To the best of our knowledge, this is the first report on the synthesis of an antimicrobial wound dressing nanofiber mat based on PEGylated GO–Ag NP–CUR nanocomposite with enhanced antimicrobial potential.
2 Experimental section
2.1 Materials
Silver nitrate (AgNO3, 99%), chitosan, (m. wt. 190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–240
000–240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da), and PVA (m. wt. 1
000 Da), and PVA (m. wt. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) were obtained from Sigma Aldrich (St. Louis, MO, USA). The graphite fine powder (98% extra pure) was procured from LOBA Chemie. E. coli and S. aureus were used as model microorganisms. E. coli (DH5α) and S. aureus (MTCC 737) were obtained from Merck (India) and IMTECH (India), respectively. The bacterial strains culture media Luria–Bertani broth (LB) and nutrient broth (NB) were purchased from Merck (Germany) and Himedia (India). The 7-dichlorofluorescin diacetate (DCFH-DA) was procured from Sigma Aldrich (USA).
000 Da) were obtained from Sigma Aldrich (St. Louis, MO, USA). The graphite fine powder (98% extra pure) was procured from LOBA Chemie. E. coli and S. aureus were used as model microorganisms. E. coli (DH5α) and S. aureus (MTCC 737) were obtained from Merck (India) and IMTECH (India), respectively. The bacterial strains culture media Luria–Bertani broth (LB) and nutrient broth (NB) were purchased from Merck (Germany) and Himedia (India). The 7-dichlorofluorescin diacetate (DCFH-DA) was procured from Sigma Aldrich (USA).
2.2 Synthesis of GO
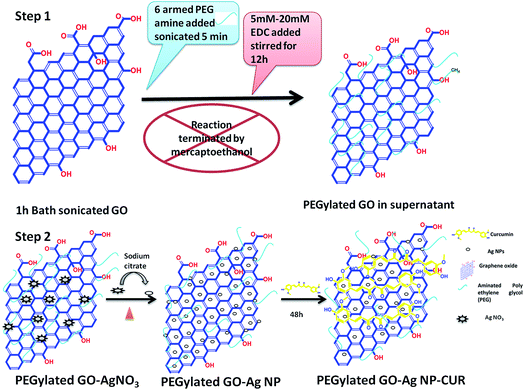
GO was prepared through a modified Hummer's method by using graphite powder as a precursor.14 Here, concentrated H2SO4 (69 mL) was added to a solution mixture of graphite powder (3.0 g, 1 wt equiv.) and NaNO3 (1.5 g, 0.5 wt equiv.), and the solution was cooled by keeping it in an ice bath at 0 °C. Further, KMnO4 (9.0 g, 3 wt equiv.) was slowly added in small portions to keep the reaction temperature below 20 °C, and it turns a green color. The reaction was warmed to 35 °C and stirred for 1 h at 100 rpm. After 20 min, the solution turns to a brownish gray suspension once the oxidation reaction is completed. The suspension was then diluted with deionized water (DI) (46.0 mL) and the suspension was mixed by stirring. Then, the temperature of the suspension was raised to 98 °C and a violent effervesce was observed. The diluted suspension was maintained at 98 °C for a further 15 min. Then, the solution was further diluted to 140 mL with warm water and left for an additional 2 h at room temperature. Then, the dispersion was treated with 3 mL of H2O2 (30%), resulting in the formation of a bright yellow color. The solution was allowed to rest for 2 days. The mixture was then purified following the filtration and centrifugation, with multiple washes followed by freeze drying, which occurred as follows. The precipitate was recovered by centrifugation and washed with HCl (10% v/v) and DI water to remove chemical residues and to neutralize the pH. Finally, the resulting material was resuspended in DI water and additionally purified by dialysis for 3–4 days. The resulting dark brown graphite oxide suspension was dried by lyophilization and stored in a sealed Falcon tube. Finally, the obtained graphite oxide was exfoliated in an ultrasonic bath for 3 h into the GO aqueous dispersion (0.1 mg mL−1). The obtained supernatant was stable for up to several months.2.3 Synthesis of PEG-functionalized GO (PEGylated GO)
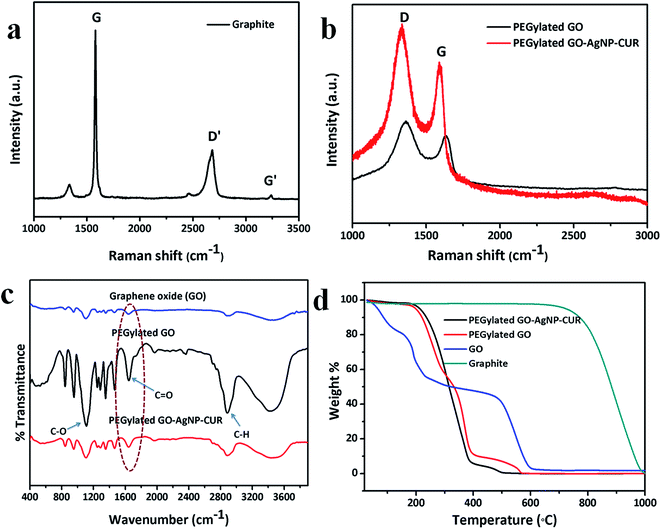
For the PEGylation of GO, the introduction of carboxylic acid functional groups is important. Thus, 10 mL of GO aqueous solution (∼5 mg mL−1) obtained by bath-sonication for 1 h was mixed with 10 mL of 3 M NaOH solution and bath-sonicated further for 3 h. It was found that the PEG-amine was able to bind to the GO after sonication with N-3-dimethylaminopropyl-N′-ethylcarbodiimide hydrochloride (EDC) in a very basic medium. Sonication is required for hydrolysis of the esters on the GO to carboxyl groups after base treatment. Subsequently, HCl was added to neutralize the solution and the solution was filtered and washed, and the resulting product was GO modified with carboxylic acid (GO-COOH). The carboxylic-group-modified GO was diluted with water to obtain ∼1 mg mL−1. Afterwards, 10 mg mL−1 of 6-arm polyethylene glycol-amine (PEG-amine) was added to the solution and the mixture was bath-sonicated for 5 min. To the solution mixture, EDC was added (5–20 mM) and the solution was bath-sonicated for another 40 min, followed by stirring for 12 h (Fig. 1). The reaction was completed by adding mercaptoethanol. The obtained solution was then centrifuged in 1× phosphate buffered saline (PBS) buffer, the obtained supernatant was stored. The obtained supernatant was PEGylated-GO. The PEGylation was established by the Fourier transform infrared (FTIR) spectral analysis of PEGylated-GO by confirming the amidation and presence of distinct C–H and C–O vibrational frequency bands of the PEG chains (Fig. 3c). The obtained PEGylated-GO was stable in serum medium (DMEM) without aggregation for up to several months, which further confirmed the successful PEGylation of GO sheet.122.4 Synthesis of PEGylated GO–Ag NP and PEGylated GO–Ag NP–CUR nanocomposite
Ag NP-decorated PEGylated GO nanocomposite was prepared by a slight modification of the Turkevich method using sodium citrate as the stabilizing agent.11,15 The procedure involves the reduction of AgNO3 by sodium citrate in the presence of PEGylated GO suspension. In brief, 1.3 mL of PEGylated-GO suspension (1.35 mg mL−1) was added into 98.7 mL of deionized water. The desired amount (18, 36, or 54 mg) of AgNO3 salt was added to the PEGylated-GO solution. After the reaction solution started boiling, the desired amount (20, 40, or 60 mg) of sodium citrate powder was quickly added to the reaction mixture and was further refluxed for 1 h (Fig. 1). The obtained PEGylated-GO–Ag NP nanocomposite was purified by centrifugation, filtration, and washing with deionized water. The final pellet was redispersed in deionized water. The concentration of Ag NP in the PEGylated GO–Ag NP nanocomposites was measured by atomic absorption spectroscopy (AAS in the graphite furnace mode (Avanta M, GBC)). To synthesis the PEGylated GO–Ag NP–CUR nanocomposite, CUR solution (5 mg mL−1) in ethanol was mixed with already prepared PEGylated GO–Ag NP solution and stirred for 48 h at room temperature. The unbound CUR was removed by centrifugation followed by dialysis.2.5 Physicochemical characterization of various nanocomposites alone and with the composite nanofiber
Ultraviolet-visible (UV-vis) spectral measurements were done to confirm the synthesis of the various nanocomposites using an Hitachi UV-vis spectrophotometer.The synthesized Ag NPs' size and the morphology of the composite nanofiber were observed with a TEM instrument (FEI Technai G2) operated at an accelerating voltage of 200 kV. The aqueous dispersion was drop-cast on carbon-coated copper TEM grids and dried at room temperature, followed by sample analysis under the TEM microscope. For the nanofibers sample preparation, the sample was deposited on non-carbon-coated copper TEM grids over the aluminum foil in the electrospinning chamber.
The surface morphology of the as-synthesized various nanocomposites incorporated Ag NPs were further investigated with an FE-SEM instrument (FEI Quanta 200 F, the Netherlands) operated with an accelerating voltage of 5 kV and a working distance of 20 mm. The presence of GO and GO–Ag nanocomposite sheets over the CS/PVA nanofiber was also verified by FE-SEM.
XRD patterns for the Ag NP loaded, bare GO, and PEGylated-GO sheet were obtained with a Bruker AXS D8 Advance powder X-ray diffractometer (Cu Kα radiation, λ = 1.5406 Å) in the range of 5–90 °C at a scan speed of 0.05° min−1.
Raman measurements were carried out on an InVia Renishaw spectrometer equipped with a 514 nm argon ion laser, CCD detector, and a 50× objective lens. The spectra were recorded from 500 to 4500 cm−1. All the samples were deposited on glass slides in powder form without using any solvent.
The chemical structure of the various nanocomposites was confirmed by FTIR spectral analysis. FTIR analysis was performed for the GO alone, for the PEGylated GO, and for the PEGylated-GO-based composite powder and nanofiber samples. The FTIR spectra were obtained using a Thermo-Nicolet spectrometer with KBr pellets in the range 400–4000 cm−1.
For the various as-synthesized nanocomposites and composite-incorporated nanofibers, compositional analysis was carried out by Thermogravimetric analysis (TGA). Here, about 10 mg of powder sample was heated from 30 °C to 800 °C at a constant rate of 10 °C min−1 in an EXSTAR TG/DTA 6300. The nitrogen atmosphere was maintained during the TG analysis of all of the samples. The weight loss of various phases in the thermogram was correlated with the degradation of specific components of the GO and PEG components present on the sheets. The samples were analyzed in perforated and covered aluminum pans under nitrogen-purging conditions.
2.6 Fabrication of various formulations of CS/PVA blended nanofibers
CS 2 wt% (0.2 g) was added to 10 mL of 2% v/v acetic acid solution and the polymer suspension was incubated at 90 °C for 1 h and then mixed overnight using a magnetic stirrer. The CS/PVA blend was prepared by adding 1 g of PVA into the previously prepared CS solution. The final ratio of PVA in the blend was 10 wt% (relative to the CS wt%). EDTA was added to the above solution, thus the final formulation contains EDTA along with CS/PVA for the nanofiber formation. After incubation, the CS/PVA solution was loaded into a 2.5 mL plastic syringe fitted with a metal 21-gage needle. The syringe was placed vertically on the syringe pump and a voltage supply was attached to the needle tip and to the collector plate using alligator clips. The needle-to-collector distance was 14 cm, and a rectangular glass plate was used to collect the fibers. The flow rate and the applied voltage were 0.8 mL h−1 and 25 kV, respectively. Electrospinning was conducted under controlled conditions of temperature (30.0 ± 5 °C) and relative humidity (40–60%). This protocol was used for the synthesis of the base polymeric nanofiber. The composite nanofibers were made by adding the as-prepared nanocomposite solutions into the above-synthesized blended polymeric solutions, such as by adding the PEGylated GO water dispersion to the CS/PVA blended solution and stirring for 24 h at room temperature and then electrospinning at an optimal voltage of 25 kV and flow rate of 0.4 mL h−1. Likewise, various formulations of composite nanofiber were made with a small change in voltage and flow rate. For the PEGylated GO–Ag NP composite nanofiber, the flow rate was 0.3 mL h−1 and the voltage was 20 kV, while for the PEGylated GO–Ag NP–CUR composite nanofiber, the flow rate was 0.6 mL h−1 and the voltage was 25 kV.2.7 Contact angle measurement
The hydrophilicity of the nanofibers surface was investigated by measuring the static contact angles of water on the CS/PVA blended nanofiber alone and on various composite nanofiber surfaces with a Drop Shape Analysis System-DSA30 (Kruss, Hamburg, Germany). A volume of 30 μL of ultrapure water was dropped onto the dried nanofibers at 37 °C, and the contact angle was calculated after 40 s of incubation time to prevent inconsistency in the contact angle values due to location and time. Bare CS/PVA nanofibers, PEGylated GO composite nanofibers, PEGylated GO–Ag NP composite nanofibers, and PEGylated GO–Ag NP–CUR composite nanofibers were studied to understand the effect of surface roughness and the surface topology of the nanofibers on the contact angle due to the incorporation of a hydrophobic drug and the effects of the nanoparticles on the nanofiber wettability.42.8 Mechanical properties
The stress–strain curves of a lone CS/PVA blended nanofiber mat and the PEGylated-GO-incorporated CS/PVA nanofiber mat were measured according to ASTM D882-12 (2012)16 using a low-load universal testing machine (Bose ElectroForce® 3200 series III test instrument) from Bose-ElectroForce Systems Group (USA). The specimens were carefully peeled off from aluminum foil and the film strips were cut into specific dimensions (0.5 × 5 cm) and held between two clamps. The samples were mounted into the grips and stretched with a strain rate of 10 mm min−1 until breakage. Three samples of each formulation were tested and E, UTS, and the elongation at break (εb) were evaluated from the stress–strain curves.2.9 In vitro release study
To quantify the release profile of the Ag NPs and CUR from the nanocomposite-incorporated nanofibers, the nanofibers (10 mg) were immersed in PBS buffer (pH 7.4) at physiological temperature, 37 °C, under shaking conditions at 100 rpm. The solution was collected at different time points. The Ag+ ions release behavior from the PEGylated GO–Ag NP–CUR-loaded composite nanofiber mats was determined with AAS using a standard of pure Ag (2, 3, 4 μg mL−1). CUR release was estimated from UV-vis spectrophotometry analysis. The calibration curve of the known concentration of CUR was plotted to determine the unknown concentrations.2.10 Evaluation of the antibacterial activity of the composite nanofibers
Two representative bacterial strains, namely E. coli and S. aureus, were taken as the model for Gram negative and Gram positive bacteria, respectively. The bacterial cells were incubated overnight in a shaken incubation at 220 rpm and at 37 °C. The obtained culture was cultivated to log phase by dilution of the overnight culture (1 mL) into a fresh LB medium (9 mL) and by growing for 2 h until reaching an optical density of 1.0 at 600 nm (OD 600 nm). The same culture was used for the various assays to test the antibacterial potential of the control and the various composite nanofibers.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min) at 4 °C and washed with PBS three times. The supernatant was discarded and the cell pellet rinsed and resuspended in PBS (1 mL). The fluorescent dye PI was added to give a final concentration of 2 μg mL−1. The samples were mounted onto the glass slide as a thin smear and then air-dried. The fluorescence was observed with a fluorescent microscope (a Nikon Eclipse LV100 microscope) at an excitation wavelength of 535 nm and an emission wavelength of 625 nm.
000 rpm for 5 min) at 4 °C and washed with PBS three times. The supernatant was discarded and the cell pellet rinsed and resuspended in PBS (1 mL). The fluorescent dye PI was added to give a final concentration of 2 μg mL−1. The samples were mounted onto the glass slide as a thin smear and then air-dried. The fluorescence was observed with a fluorescent microscope (a Nikon Eclipse LV100 microscope) at an excitation wavelength of 535 nm and an emission wavelength of 625 nm.2.11 Cell culture
NIH 3T3 (mouse embryonic fibroblast cells) cells were procured from the National Centre for Cell Sciences (NCCS) Pune, India, and cultured in a DMEM medium containing 10% (v/v) fetal bovine serum (FBS, Gibco Life Technologies) and 1% (v/v) penicillin–streptomycin (Sigma Aldrich, USA), and incubated at 37 °C in a 5% CO2 incubator at 90% humidity.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well for the cell viability assay. MTT test was performed in 24-well plates (tissue culture grade, 24 wells, flat bottom, Corning). The NIH-3T3 cells were seeded at sub-confluence onto the various nanofibers, namely the alone CS/PVA nanofiber and the various PEGylated-GO-based composite nanofibers, and incubated in a humidified atmosphere (37 °C, 5.0% CO2). After incubation for 96 h, the DMEM medium was removed and the cells grown over nanofibers were washed with PBS. After the PBS wash, MTT (5 mg mL−1) was added with the medium to each well. The plate was again incubated inside the incubator. After 3–4 h of incubation with MTT, the supernatant was removed, and 150 mL of DMSO was added in each well and the plate was kept for mild shaking for 10 min for solubilization of the formazan crystals. When the purple formazan crystals were dissolved completely, a microtiter plate reader was used for the absorbance measurement at 570 nm wavelength. The cell viability was calculated by the following formula:
000 cells per well for the cell viability assay. MTT test was performed in 24-well plates (tissue culture grade, 24 wells, flat bottom, Corning). The NIH-3T3 cells were seeded at sub-confluence onto the various nanofibers, namely the alone CS/PVA nanofiber and the various PEGylated-GO-based composite nanofibers, and incubated in a humidified atmosphere (37 °C, 5.0% CO2). After incubation for 96 h, the DMEM medium was removed and the cells grown over nanofibers were washed with PBS. After the PBS wash, MTT (5 mg mL−1) was added with the medium to each well. The plate was again incubated inside the incubator. After 3–4 h of incubation with MTT, the supernatant was removed, and 150 mL of DMSO was added in each well and the plate was kept for mild shaking for 10 min for solubilization of the formazan crystals. When the purple formazan crystals were dissolved completely, a microtiter plate reader was used for the absorbance measurement at 570 nm wavelength. The cell viability was calculated by the following formula:| % cell viability = [A570 treated/A570 control] × 100 |
2.12 Statistical analysis
The data were expressed as the mean or the standard deviation of one or more individual experiments, wherever applicable. Analysis of the data was performed with the Student's test with GraphPad Prism 6.0, and Origin Pro 8.0 for various statistical analyses.3 Result and discussion
3.1 Physicochemical characterization of the various nanocomposites
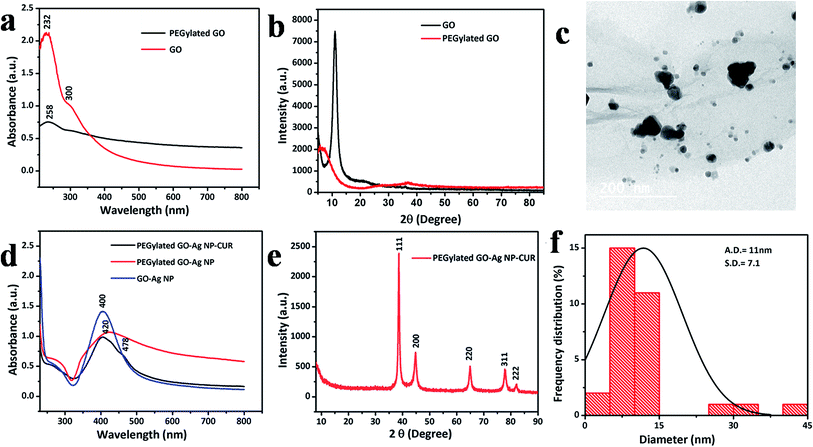
Graphite oxide was employed as the starting functional precursor based on its excellent properties for covalent modification owing to the reactive sites of oxygen-bearing groups on its surface. The aqueous dispersion of graphite oxide was then exfoliated by ultrasonication to afford GO. The stability of the GO was tested in aqueous and physiological medium (DMEM). GO was found to be stable in water for 30 days without precipitation (Fig. S1†). Nevertheless, it becomes aggregated and is precipitated in DMEM medium (Fig. S1†), which clearly indicates a requisite for improving the stability of GO under physiological conditions for further biomedical applications. Therefore, the PEGylation of GO with a well-defined 6-armed PEG–NH2 was done as PEG is biocompatible in nature. The PEG–NH2, with its higher reactivity, readily forms an amide bond with carboxyl on the surface of GO sheets, with the amidation process validated by FTIR analysis. Before the amidation reaction, a sharp peak at 1732 cm−1 was present in the spectrum of GO, but after amidation, a new peak originating from an amide group (–NH–CO) appeared at 1652 cm−1 in the FTIR spectrum (Fig. 3c). This evidence distinctly demonstrates that the initial carboxyls on the surface of GO sheets are entirely converted to amide groups.12 Hence, the biocompatible PEG was linked onto the surface of GO sheets via a stable covalent modification and was found to be physiologically stable in DMEM medium (Fig. S1†).Further, PEGylated GO was used as the precursor for various nanocomposites syntheses. The PEGylated GO serves as a template for Ag NP synthesis, as GO has sufficient electron donation groups even after PEGylation. The dark brown-blackish solution of PEGylated GO turned dark blackish red after reduction of the Ag ions by the PEGylated GO sheet to give PEGylated GO–Ag NP. The UV-vis spectrum of the GO alone was attributed to an absorption band at 230 nm associated with the electronic π–π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic bonds and a shoulder peak at 305 nm assigned to the n–π* transitions of C
C aromatic bonds and a shoulder peak at 305 nm assigned to the n–π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds; whereas the PEGylated GO showed a red shift of the plasma peak to 258 nm which further confirms the synthesis of reduced GO (Fig. 2a).17 The characteristic surface plasmon band of Ag NP present in GO–Ag NP and PEGylated GO–Ag NP was observed at 400 nm and 420 nm (Fig. 2d), respectively, due to the size difference of synthesized Ag NPs, which was validated by TEM analysis (Fig. 2c and f). The synthesized PEGylated GO–Ag NP–CUR was confirmed further by the UV-visible spectrum of the composite showing a shoulder peak corresponding to the CUR peak at 478 nm (Fig. 2d).
O bonds; whereas the PEGylated GO showed a red shift of the plasma peak to 258 nm which further confirms the synthesis of reduced GO (Fig. 2a).17 The characteristic surface plasmon band of Ag NP present in GO–Ag NP and PEGylated GO–Ag NP was observed at 400 nm and 420 nm (Fig. 2d), respectively, due to the size difference of synthesized Ag NPs, which was validated by TEM analysis (Fig. 2c and f). The synthesized PEGylated GO–Ag NP–CUR was confirmed further by the UV-visible spectrum of the composite showing a shoulder peak corresponding to the CUR peak at 478 nm (Fig. 2d).
The structural characteristics and crystallinity of the GO, PEGylated GO and Ag NP, and CUR-incorporated GO in CS/PVA composite nanofibers were investigated by the powder XRD method. The representative peak of GO was present at 2θ = 11°, whereas the PEGylated GO showed a reduced intensity broader peak, which also confirms the reduction of the GO sheet into reduced GO (Fig. 2b). The interlayer distance of GO was enhanced compared to the pristine graphite. This trend was attributed to the introduction of oxygen-bearing functional groups into the carbon lattice after the oxidation. On the other hand, after reduction, the peak at 11° vanished and a new boarder peak appeared. This could be ascribed to the exclusion of functional groups and may indicate to a certain degree the deoxygenation of GO and exfoliation of graphene. The PEGylated GO–Ag NP–CUR composite was typified with the characteristic elemental peaks of Ag, as shown at 2θ = 37.99°, 44.2°, 64.53°, and 77.28°, corresponding to Ag(111), Ag(200), Ag(220), and Ag(311), respectively and the above four miller indices diffraction peaks were in agreement with JCPDS 040783 (Fig. 2e).
Raman spectroscopy is an essential tool sensitive to the electronic structure and has been utilized for the characterization of GO with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds contributing to high Raman intensities. The Raman spectra of graphite showed two main characteristics peaks: the G-peak, which arises from first-order scattering of the E2g photons from sp2 hybridized carbon atoms (1565 cm−1), and the D-peak (1365 cm−1) (Fig. 3a), which arises from the breathing mode of κ-point photons of an A1g symmetry.18 On the other hand, the Raman spectrum of GO shows the presence of the characteristic intense D band at 1320 cm−1 and the G band at 1570 cm−1 (Fig. 3b). The functionalization of GO with Ag NP and CUR leads to an increase in the Raman intensities of both the D and G bands. The increase in the Raman scattering can be attributed to the surface-enhanced Raman scattering (SERS), as previously described.19 This increase in intensities indicates a reduction in the sp2 domain as a consequence of the attachment of Ag NPs onto the GO surface.
C double bonds contributing to high Raman intensities. The Raman spectra of graphite showed two main characteristics peaks: the G-peak, which arises from first-order scattering of the E2g photons from sp2 hybridized carbon atoms (1565 cm−1), and the D-peak (1365 cm−1) (Fig. 3a), which arises from the breathing mode of κ-point photons of an A1g symmetry.18 On the other hand, the Raman spectrum of GO shows the presence of the characteristic intense D band at 1320 cm−1 and the G band at 1570 cm−1 (Fig. 3b). The functionalization of GO with Ag NP and CUR leads to an increase in the Raman intensities of both the D and G bands. The increase in the Raman scattering can be attributed to the surface-enhanced Raman scattering (SERS), as previously described.19 This increase in intensities indicates a reduction in the sp2 domain as a consequence of the attachment of Ag NPs onto the GO surface.
The TG analysis of pristine graphite, GO, PEGylated GO, and PEGylated GO–Ag NP–CUR was performed (Fig. 3d). It was observed that graphite was thermally stable, whereas GO was found to be thermally unstable and very soon started to lose mass due to evaporation of the stored water in the π-stacked structure.12 Remarkably, the mass loss speeded up in the range of 150 to 230 °C due to pyrolysis of the labile oxygenated functional groups on the GO surface. The second major weight loss was around 500–600 °C and was attributed to the thermal decomposition of the graphitic layer.20 The PEG polymeric coatings appeared to enhance the thermal stability of the GO sheets. The PEGylated GO sheet started thermal degradation around 300 °C and the same trend was seen with all the PEGylated-GO-based nanocomposites.12 Contrarily to the nanocomposites, TG analysis of the composite nanofibers (Fig. 7b) showed a decrease in the thermal stability of the composite-nanofiber-incorporated PEGylated-GO-based composites compared to the CS/PVA nanofibers alone. On the carbon TEM grids, GO displayed a normal trend to unfold and present a flake-like and wrinkled morphology (Fig. 2c). On the other hand, the TEM micrographs confirmed the formation of GO sheets decorated with Ag NPs (Fig. 2c) with an even distribution of spherical-shaped Ag NPs (average size of 11 nm), while no particles were found to be detached from the GO sheet surface (Fig. 2c). The nucleation of the Ag NPs onto surface of GO could be ascribed to the interaction of Ag+ ions with various negatively charged groups present on the surface of PEGylated GO, such as carboxyl groups, hydroxyl, or amide groups on PEGylated GO sheets, for the attachment and growth of the nanoparticles.11 In the PEGylated GO–Ag NP–CUR composite nanofiber, the size of the Ag NPs was observed to be around 60 nm, with variations in the shape of the nanoparticles (Fig. 6a and b).
3.2 Fabrication and characterization of the various formulations of nanofibers
A schematic of the procedure for the fabrication of CS/PVA blended nanofibers with a self-assembled PEGylated GO sheet is depicted in Fig. 4. Various PEGylated-GO-based composite nanofibers were optimized to obtain bead-free nanofibers, as shown in Fig. S2.† PEGylated-GO-incorporated CS/PVA composite nanofibers were successfully electrospun. The parameters followed for the synthesis were a 15 cm tip to collector distance, 25 kV applied voltage, and a 0.6 mL h−1 flow rate under 50–60% humidity at 35 °C temperature conditions.3.3 Physicochemical characterization of the various composite nanofiber mats
The FE-SEM micrograph showed the successful fabrication of various compositions of the composite nanofibers (Fig. 5). Alone CS/PVA blended nanofiber showed a uniform bead-free nanofiber formation with a diameter distribution around 147 nm (Fig. 5a). PEGylated-GO-incorporated CS/PVA composite nanofiber showed a significantly flat structure of a self-assembled GO sheet on the surface of the nanofiber. Though the PEGylated GO sheet attained the micron size, the expanded GO single sheet (Fig. 5b–d) was clearly embedded in the nanofibers at the edges of the sheet (Fig. S2c and d†).3 The nanofiber showed a stretching out of continuous nanofibers through the edges of GO sheet, with the sheet retaining its flat structure, which was in agreement with the previously done study.21 The diameter distribution of PEGylated-GO-incorporated nanofibers showed a narrow distribution up to 150 nm. The average diameter of the GO-incorporated nanofibers was found to be 134 nm (Fig. 5b), whereas the PEGylated-GO–Ag NP-incorporated composite nanofibers showed a diameter of 128 nm (Fig. 5d). The reason for this decrease in diameter could be due to the increase in conductivity by the electrospinning solution based on the presence of Ag NP incorporation, leading to the synthesis of nanofibers with smaller diameters.4 The CUR-incorporated nanofiber was found to be around 140 nm in diameter, whereas PEGylated GO–Ag NP–CUR showed an average diameter of 133 nm (Fig. 5e). The chemical structure of the nanocomposite incorporated into the nanofiber was studied by FTIR analysis (Fig. S3†).3.4 Analysis of the hydrophilicity and mechanical properties of the various composite nanofibers
The results of the water contact angle measurements of the different nanoformulations of CS/PVA composite nanofibers are shown in Fig. 7a. A contact angle of 27° was observed for the bare CS/PVA composite nanofiber, whereas the incorporation of PEGylated GO into the nanofiber increased the contact angle increased up to 58°.21 The increase in the contact angle was attributed to the amphiphilic nature of GO, as the hydrophobic sheet of GO leads to an increase in the nanofiber hydrophobicity. Furthermore, the CUR composite nanofiber showed a very high contact angle of 88°, which was attributed to the extreme hydrophobicity.13 The final nanocomposite nanofiber showed an increase in contact angle from the base nanofiber of CS/PVA alone of 27° to 51° (Fig. 7a). Though the contact angle of the composite nanofiber was increased from the bare nanofiber, it was still less compared to CUR and PEGylated GO nanofiber alone. The reason for this decrease was due to the addition of Ag NP in the composite nanofiber, as was also evident from our previous study, which showed that the presence of Ag leads to the formation of a Ag+ ion oxidation layer, which then leads to a further increase in the hydrophilicity of the nanofiber.4 Among various other advantageous features, including hydrophilicity, biocompatibility, and biodegradability, the mechanical strength of a nanofiber wound dressing is equally important. The mechanical properties of CS/PVA alone and PEGylated-GO-incorporated CS/PVA blended composite nanofiber are shown in Fig. 6c and d. GO is well known among carbon-based materials for possessing good mechanical properties21 (Fig. 6c and d). It was observed that, contrarily to CS/PVA blended nanofiber, the PEGylated-GO-incorporated nanofiber showed an enhanced UTS and Young's modulus, which is shown in Table S1.†3.5 In vitro release study of drugs from the nanocomposite-incorporated nanofibers
The in vitro CUR and silver release profile are shown in Fig. S4.† A distinct biphasic release profile was observed for drug and Ag NPs release from the nanocomposite nanofiber after contact with hydrophilic milieu. CUR and silver both showed an initial burst release of around 4% and 10%, respectively, which was due to the outside retained particles on the nanofiber surface. The initial rapid phase was followed by a slow controlled release of nanofibers of around 80% for CUR and 90% for Ag NPs in 48 h and 36 h, respectively (Fig. S4†).3.6 Enhanced antibacterial activity by the nanocomposite-incorporated nanofibers
Among carbon-based materials, GO has been recently explored for its antibacterial potential. The first study on the antibacterial property of GO–Ag NP-incorporated PLGA/CS nanofibers was done by de Faria and co-workers.3 Taking this into consideration, we designed a simple and cost-effective biocompatible nanofiber wound dressing mat with enhanced mechanical and antibacterial potential. Herein, the PEGylated-GO-based nanocomposite-incorporated nanofiber mat was explored as a wound dressing for the first time. Two representative bacterial strains, the Gram negative (E. coli) and Gram positive (S. aureus) bacteria, were selected for exploration of the antibacterial efficacy because they are the most commonly present strains in nosocomial infections and the primary cause of infection at wound sites.22The agar-based disk diffusion process (Kirby–Bauer) is a simplistic and rapid semi-quantitative approach for determination of the antibacterial activity of diffusible antimicrobial agents from a variety of antibacterial drug-delivery systems.4 The disc diffusion tests demonstrate an exclusion area around nanofiber disks having an antibacterial property, whereas the control nanofibers are devoid of any antibacterial agent and show no such exclusion area. Primarily, the antibacterial efficacy of various nanoformulations of the composite nanofibers was accessed based on disk diffusion assay. The comparison of the largest exclusion area based on the size of the zone of inhibition for various nanoformulations corresponding to their antibacterial effect is shown by histogram in Fig. 8a. The antibacterial efficacy was checked against both Gram positive and Gram negative bacteria and it was found that the nanofibers affect both strains differently. A bigger zone of inhibition was seen against S. aureus by the CS/PVA nanofiber alone, and for various composite nanofibers. The average diameter of the zone of inhibition against S. aureus was 0.2 mm, 0.35 mm, 4.0 mm, 6.0 mm, 4.0 mm, and 11.0 mm for the CS/PVA nanofiber, PEGylated GO alone, Ag NP alone, PEGylated GO–CUR, PEGylated GO–Ag NP, and PEGylated GO–Ag NP–CUR composite nanofiber, respectively. The average zone of inhibition against E. coli was 0 mm, 0.3 mm, 3.0 mm, 5.2 mm, 3.2 mm, and 7 mm for CS/PVA nanofiber, PEGylated GO alone, PEGylated GO–CUR, PEGylated GO–Ag NP, and PEGylated GO–Ag NP–CUR composite nanofiber, respectively (Fig. 8a). The probable reason for the distinct antibacterial activity could be the presence of chitosan in the nanofiber. Furthermore, the minimum inhibitory concentration (MIC) was investigated for various nanoformulations, and is shown in Table S2.† Further investigation with various amounts (mg) of PEGylated GO–Ag NP–CUR composite nanofiber showed enhanced antibacterial potential when compared to the same amount of PEGylated GO–Ag NP composite nanofiber alone (Fig. 10a and b). The reason for this difference could be the incorporation of CUR onto the PEGylated GO nanocomposite, which further enhances the antibacterial potential of the nanocomposite membrane.23 CUR is renowned for its antimicrobial effect. Moreover, the immobilization of biological agents onto the surface of nanomaterials has been shown to further enhance the action at the bio-nano interface. Nevertheless, the antimicrobial activity of nanomaterials strongly depends on their surface structure. Thus, in the above fabricated nanofiber, GO also provided a high surface area along with the nanofiber for drug release, mass transfer, and for fast interaction, which complemented the action of the wound dressing nanofiber mat and increases the effect.21
3.7 Bacterial cell integrity disruption induced by the composite nanofiber
PI has been used for investigation of the cell membrane integrity of bacterial cells treated with nanofibers. PI is basically a DNA-binding fluorescent intercalating agent with fluorescence excitation at 535 nm and emission at 617 nm.24 Cells with a damaged cell membrane allow PI to enter into the cell and bind to nucleic acids. It was observed that the PEGylated GO–Ag NP–CUR composite nanofiber treated cells showed enhanced red fluorescence emitting cells, whereas the control cells showed negligible red fluorescence (Fig. 9B(a–f)), which indirectly highlights cells with lost membrane integrity. The probable reason for the loss of cell integrity is cell deformation due to the binding of released Ag NPs from the composite. The bacterial cell deformation by the composite nanofibers was further confirmed by SEM images of the treated bacteria, as shown in Fig. S5B.†3.8 PEGylated GO–Ag NP–CUR composite nanofiber induced bacterial cell death via ROS
The induction of oxidative stress through the generation of ROS by nanoparticles is a well-recognized mechanism of antibacterial activity.21 All three components, including Ag NPs, GO, and CUR, are known to induce oxidative stress in bacterial cells.23 Thus, a study was performed to evaluate the potential of PEGylated GO–Ag NP–CUR composite nanofibers for the induction of oxidative stress. It was found that the amount of ROS production was more for cells treated with PEGylated GO–Ag NP–CUR composite nanofiber than for cells treated with PEGylated GO–Ag NP composite nanofiber alone (Fig. 9A(a and b)†). The induction of oxidative stress due to ROS generation was found to be more with S. aureus bacteria than with E. coli, which is in agreement with the previous studies (Fig. 9 and 11). | ||
| Fig. 11 Schematic of a plausible antibacterial mechanism of the PEGylated GO–Ag NP–CUR composite nanofiber-based wound dressing mat. | ||
3.9 Plausible antibacterial mechanism of PEGylated GO–Ag NP–CUR composite nanofibers
The antibacterial effects of GO, Ag NPs, and CUR have been explored individually in various bacterial systems; however, very little is known about their combined mode of action at a molecular level. Therefore, a conceivable antibacterial mechanism of the composite nanofiber was depicted here based on our study and considering the previous findings (Fig. 11). There could be three plausible ways of the antibacterial activity of the composite nanofiber leading to bacterial cell death.(1) The release of Ag NPs and CUR from the composite nanofiber through diffusion and dissolution of the polymeric components. The released Ag NPs are converted into Ag+ ions upon surface oxidation, and the electrostatic interactions between the released ions and the negatively charged bacterial cell wall ultimately leads to the death of the bacterial cell (as supported by our AAS study).9
(2) Bacterial cell membrane disruption (as supported by FE-SEM and PI assay) is the other probable mode of action of the composite nanofiber as reported in previous studies.9 The leakage of intracellular cellular materials due to membrane disruption further causes a shrinkage of the cytoplasmic membrane, ultimately leading to cellular lysis.
(3) At the cellular level, the induction of ROS generation by the Ag+ ions released leads to an inhibition of plasmid or chromosomal DNA replication and an alteration in enzyme activity, including the ribosomal subunit 30S, in cells which in turn leads to bacterial cell lysis (as supported by DCF–DA assay).9
3.10 Cytocompatibility of the various composite nanofibers
In addition to the antibacterial activity, the cytocompatibility of the composite nanofiber is also an important feature of a wound dressing mat. CS and PVA are known to be biocompatible, non-toxic, and biodegradable polymers. Thus, various GO-based nanocomposites incorporated into CS/PVA nanofiber was evaluated for cytocompatibility assessment. The mouse embryonic fibroblast cells were used for the study as fibroblast cells are the primary element in the wound repair process. The control and various composite nanofibers were assessed by cell viability assay, and showed negligible toxicity when compared to control cells (Fig. 8b). Thus our study suggests the biocompatible nature of the wound dressing mat.3.11 Cell morphology analysis by FE-SEM
Chitosan possesses a cationic surface charge, which aids in cell attachment and the adhesion of a negatively charged cell membrane. FE-SEM analysis was done to understand the role of nanofibers in cell attachment and proliferation. The FE-SEM micrograph clearly shows that the scaffold supports cell adhesion and cell proliferation (Fig. S5A†).4 Conclusion
In summary, we successfully described the applicability of a multi-component-composite-incorporated nanofiber-based potential wound dressing material based on its enhanced antibacterial effect. The potential of each component was exploited fully in the advanced nanocomposite design. GO was chosen as a starting material for nanocomposite formation based on its amphiphilicity, mechanical property, and ample oxygen-bearing groups. Additionally, the PEGylation of GO further enhanced the physiological stability of GO. Furthermore, the CS-based polymeric blended nanofiber provides an additional advantage to the coating material by increasing the biocompatibility and antibacterial potential. The nanofiber and GO both present a high surface area for fibroblast attachment, spreading, and drug release. Our finding suggests an enhanced antibacterial mechanism against both Gram positive and Gram negative bacteria with negligible cytotoxicity against fibroblast cells. Further exploration of the antibacterial mechanism revealed the cell membrane disruption by the release of Ag+ ions and CUR along with the direct contact of GO and the nanofiber to bacterial cells. At the molecular level, the generation of enhanced ROS led to a disruption of the cellular machinery and functional pathways, which ultimately culminate in cell death. Thus, this novel PEGylated GO–Ag NP–CUR composite nanofibers mat was revealed to have good potential to serve as an ideal wound dressing material based on its good mechanical property, biocompatibility, hydrophilicity, and enhanced antibacterial potential against a broad antibacterial community.Acknowledgements
This study was supported by the Science and Engineering Research Board (no. SR/FT/LS-57/2012) and the Department of Biotechnology (no. BT/PR6804/GBD/27/486/2012), Government of India. PD is thankful to the Ministry of Human Resource Development, Government of India, for the fellowship. Sincere thanks to Department of Chemistry and Institute Instrumentation Centre, IIT Roorkee for the various analytical facilities provided.References
- R. Jennemann, M. Rabionet, K. Gorgas, S. Epstein, A. Dalpke, U. Rothermel, A. Bayerle, F. van der Hoeven, S. Imgrund, J. Kirsch, W. Nickel, K. Willecke, H. Riezman, H.-J. Gröne and R. Sandhoff, Hum. Mol. Genet., 2012, 21, 586–608 CrossRef CAS PubMed.
- S. P. Zhong, Y. Z. Zhang and C. T. Lim, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 510–525 CrossRef CAS PubMed.
- A. F. de Faria, F. Perreault, E. Shaulsky, L. Hoover Arias Chavez and M. Elimelech, ACS Appl. Mater. Interfaces, 2015, 7, 12751–12759 CAS.
- P. Dubey, B. Bhushan, A. Sachdev, I. Matai, U. S. Kumar and P. Gopinath, J. Appl. Polym. Sci., 2015, 132, 1–12 CrossRef.
- P. Dubey and P. Gopinath, J. Mater. Chem. B, 2016, 4, 726–742 RSC.
- H. Ueno, T. Mori and T. Fujinaga, Adv. Drug Delivery Rev., 2001, 52, 105–115 CrossRef CAS PubMed.
- Y. O. Kang, I.-S. Yoon, S. Y. Lee, D.-D. Kim, S. J. Lee, W. H. Park and S. M. Hudson, J. Biomed. Mater. Res., Part B, 2010, 92, 568–576 Search PubMed.
- P. Gopinath, S. K. Gogoi, A. Chattopadhyay and S. S. Ghosh, Nanotechnology, 2008, 19, 075104 CrossRef CAS PubMed.
- P. Dubey, I. Matai, U. S. Kumar, A. Sachdev, B. Bhushana and P. Gopinath, Adv. Colloid Interface Sci., 2015, 221, 4–21 CrossRef CAS PubMed.
- W. Shao, X. Liu, H. Min, G. Dong, Q. Feng and S. Zuo, ACS Appl. Mater. Interfaces, 2015, 7, 6966–6973 CAS.
- X. Chen, X. Huang, C. Zheng, Y. Liu, T. Xua and J. J. Liu, J. Mater. Chem. B, 2015, 3, 7020 RSC.
- Z. Xu, S. Wang, Y. Li, M. Wang, P. Shi and X. Huang, ACS Appl. Mater. Interfaces, 2014, 6, 17268–17276 CAS.
- G. Yakub, A. Toncheva, N. Manolova, I. Rashkov, V. Kussovski and D. Danchev, J. Bioact. Compat. Polym., 2014, 29, 607–627 CrossRef.
- C. D. Marcano, V. D. Kosynkin, M. J. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef PubMed.
- M. Koosha, H. Mirzadeh, A. M. Shokrgozarb and M. Farokhi, RSC Adv., 2015, 5, 10479 RSC.
- J. Chen, X. Wang and T. Chen, Nanoscale Res. Lett., 2014, 9, 86 CrossRef PubMed.
- J. Turkevich, C. P. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- R. S. Sahu, M. M. Devi, P. Mukherjee, P. Sen and K. Biswas, J. Nanomater., 2013, 2013, 1–6 CrossRef.
- W. Fan, H. Y. Lee, S. Pedireddy, Q. Zhang, T. Liu and Y. X. Ling, Nanoscale, 2014, 6, 4843 RSC.
- K.-H. Jeong, P. Y. Lee, H. M. Jin, S. E. Kim, J. J. Bae and H. Y. Lee, Chem. Phys. Lett., 2009, 470, 255–258 CrossRef.
- Y. Liu, M. Park, K. H. Shin, B. Pant, J. Choi, W. Y. Park, Y. J. Lee, J.-S. Park and Y.-H. Kim, J. Ind. Eng. Chem., 2014, 20, 4415–4420 CrossRef CAS.
- G. P. Bowler, I. B. Duerden and G. D. Armstrong, Clin. Microbiol. Rev., 2001, 2, 244–269 CrossRef PubMed.
- S. Barua, P. Chattopadhyay, M. M. Phukan, K. B. Konwar, J. Islamb and N. Karak, RSC Adv., 2014, 4, 47797 RSC.
- L. C. J. Romero, G. H. Ríos, A. Borges and M. Simoes, J. Evidence-Based Complementary Altern. Med., 2015, 2015, 1–9 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12192f |
| This journal is © The Royal Society of Chemistry 2016 |