DOI:
10.1039/C6RA12121G
(Paper)
RSC Adv., 2016,
6, 59624-59632
Synthesis of microencapsulated benzyl benzoate with a CaCO3 shell and its application to the durable anti-mite finishing of nylon 6 fabric
Received
10th May 2016
, Accepted 6th June 2016
First published on 6th June 2016
Abstract
Microcapsules based on the benzyl benzoate core and CaCO3 shell were synthesized via an interfacial co-precipitation method and used to treat nylon 6 fabric to impart anti-mite activity. The results of FTIR, SEM and upright metallurgical microscopy confirmed that the benzyl benzoate was successfully encapsulated by the CaCO3 shell. The morphologies, particle size distribution, encapsulation ratio and encapsulation efficiency of the microcapsules were investigated systematically. The nylon 6 fabric was treated with the resultant microcapsules via a finishing process and the microcapsules were immobilized on the surface of the nylon 6 fabric with chemical bonds, by using a blocked waterborne PU adhesive. The morphology of the treated fabric was investigated by SEM and the benzyl benzoate content determined by UV-vis spectrophotometry. The mite repellent tests showed that the treated nylon 6 fabric exhibited excellent anti-mite properties and laundering durability, and the mite repellent rate was up to 82.3% and remained at 61.8% after being washed 20 times.
1 Introduction
Nylon 6, as one of the most important synthetic fibers, is widely used in home textiles such as clothing, socks, bedding bags, blankets and curtains, because it is less expensive than other nylon species, such as nylon 6,6. It also possesses several other favorable advantages, such as high strength, excellent resilience and abrasion resistance. However, the aforementioned home textiles breed dust mites very easily at certain temperatures and humidity. As we all known, dust mites are harmful microorganisms that can cause several forms of allergy related diseases, including hay fever, asthma, eczema and atopic dermatitis.1–3 Therefore, owing to the great concern for human health and safety, imparting textiles with anti-mite properties is clearly necessary and has attracted significant attention from researchers.4,5 To date, the anti-mite finishing of textiles is the most efficient approach to granting textiles anti-mite activity, due to its easy handling and versatility for both natural and synthetic fibers. The anti-mite agents used for the anti-mite finishing of textiles are mainly organic chemical compounds, such as benzyl benzoate,6–8 tannic acid,9 essential oils,10 clove oil,11 pyrethroid,12 etc. Benzyl benzoate in particular, is commonly applied because of several favorable advantages, such as high efficiency, low toxicity and no irritation. However, textiles treated directly with benzyl benzoate cannot sustain long-term anti-mite efficacy because the benzyl benzoate on the surface of the textiles is exposed to the environment and can be easily washed away. Moreover, it is also difficult to immobilize the benzyl benzoate on the surface of textiles since it is liquid. Fortunately, the microencapsulation technique, which is an effective method to protect the functional agents from surrounding substances and slow their release, has been widely applied to textile finishing to fabricate lasting anti-microbial, fragrance-free, fire-retardant textiles.13,14 The microencapsulation of benzyl benzoate may therefore be an effective way to prolong the anti-mite efficacy of textiles, and solidifying benzyl benzoate through the microencapsulation technique will make it easier to immobilize the benzyl benzoate on the surface of the textiles during the finishing process.
Over the past decades, some researchers15–18 have reported that the microcapsules used in textile finishing were mainly prepared with film-forming polymers such as chitosan, polyurethane, polyamide, and urea resin as wall material. Nevertheless, there exists a typical and deadly shortage of these polymer walls, in that they are easily broken during the textile finishing process and use, due to their low intensity, leading to the serious loss of core material. What is worse, some of the raw materials applied to synthesize these polymer walls, like acyl chloride and formaldehyde, are toxic to the human body, which also limits their practical application. Recently, calcium carbonate (CaCO3), a porous inorganic material, was used to encapsulate biomacromolecules owing to its low toxicity, biocompatibility, biodegradability and ability to enable sustained and controlled release.19–21 CaCO3 has also been reported as being used in encapsulating phase change material, since it can offer high chemical and mechanical stability.22 Moreover, the raw materials used to prepare the CaCO3 shell are abundant, non-toxic and extremely cheap. Considering the above merits, there is no doubt that CaCO3 is an appropriate choice for the encapsulation of benzyl benzoate as wall material for the anti-mite finishing of textiles. Amazingly, hitherto, there has been no report on treating the nylon 6 fabric with anti-mite microcapsules that use CaCO3 as the wall material.
The fixation of microcapsules to the textiles is another key factor to make the textile resistant to washing. Binders that immobilize microcapsules on the surface of the textiles through only weak van der Waals' forces provide unsatisfactory bonding effects. As a result, the microcapsule may still be washed off the textiles after washing several times. To solve this problem, a blocked waterborne PU adhesive, which was developed in our own laboratory, was chosen as a binder to fix the microcapsules onto the surface of the textiles. The blocked waterborne PU adhesive fixed the microcapsules to the textiles with chemical bonds that would greatly improve the textiles laundering durability.
In this paper, anti-mite microcapsules based on a benzyl benzoate core and CaCO3 shell were synthesized through an interfacial co-precipitation method, using the raw materials of benzyl benzoate, sodium dodecyl benzene sulfonate, CaCl2 and Na2CO3. The prepared anti-mite microcapsules, together with the blocked waterborne PU adhesive, were then applied to the finishing process to fabricate durable anti-mite nylon 6 fabric. The morphology, particle size distribution, encapsulation ratio and encapsulation efficiency of the microcapsules were studied using FT-IR, SEM, an upright metallurgical microscope, a dynamic light scattering particle size analyzer and TG. The anti-mite properties and laundering durability of the nylon 6 fabric treated with the microcapsules were also investigated.
2 Materials and methods
2.1 Material
Benzyl benzoate (BABE) with a purity of 99 wt% was purchased from TCI Chemical Co. Ltd., Japan. CaCl2 and Na2CO3 were commercially supplied by Tianjin Fuchen Chemical Reagent Co. Ltd., China. Sodium dodecyl benzene sulfonate (SDBS), used as a surfactant, was commercially obtained from Tianjin Fuyu Fine Chemical Co. Ltd, China. Blocked waterborne polyurethane adhesive was developed in our laboratory. Nylon 6 fabric was kindly supplied by Guangdong Meida Polyamide Fiber Co. Ltd.
2.2 Synthesis of microencapsulated benzyl benzoate with CaCO3 shell
The synthesis of microencapsulated benzyl benzoate with a CaCO3 shell was performed in an oil/water emulsion with SDBS as the surfactant. Microcapsules were obtained at different concentrations of surfactant, emulsifying speeds, core–shell mass ratios and temperatures. A typical synthetic procedure is as follows. In a beaker, 5.5 g CaCl2 were first dissolved in 100 mL deionized water. Then, 5.5 g of benzyl benzoate were added dropwise into 100 mL of aqueous solution containing 1.3 g SDBS in a three-necked round-bottomed flask, with vigorous agitation for 20 min at 45 °C. The pre-prepared CaCl2 aqueous solution was added dropwise into the mixture of surfactant and benzyl benzoate, then the solution was continually stirred at 1000 rpm for 3 h to form a stable emulsion. Subsequently, 5.3 g Na2CO3 were dissolved in 100 mL of deionized water and the resultant solution was rapidly poured into the pre-prepared benzyl benzoate emulsion with a constant agitation speed of 500 rpm. Finally, the microencapsulated benzyl benzoate with CaCO3 shell was obtained as a white powder by filtration, and was washed three times, with ethanol and deionized water, respectively, to remove the unencapsulated benzyl benzoate. The powder was then dried at 60 °C under vacuum for 24 h.
2.3 Treatment of nylon 6 fabric with CaCO3 encapsulating benzyl benzoate microcapsules
Before the finishing process, the nylon 6 fabric was cleaned with ethanol to eliminate the stains on the surface. The CaCO3 encapsulated benzyl benzoate microcapsules, sodium dodecyl benzene sulfonate (used as dispersant) and blocked waterborne polyurethane adhesive were dispersed in water by ultrasonication to prepare the finishing solution. The pretreated nylon 6 fabric was immersed into the above finishing solution for 30 min, and then dried at 100 °C for 10 min. Then, the nylon 6 fabric was treated with the finishing solution with a fixed concentration of benzyl benzoate microcapsules (20 g L−1), sodium dodecyl benzene sulfonate (2 g L−1) and different concentrations of blocked waterborne polyurethane adhesive (0 g L−1, 10 g L−1, 30 g L−1, 50 g L−1, 70 g L−1).
2.4 Characterization
2.4.1 Fourier transform infrared spectroscopy (FTIR). The FTIR spectrum of the microcapsules was obtained using a Bruker Vector 33 IR spectrophotometer. The sample, in the form of a KBr pellet, was scanned from 4000–400 cm−1, with a scanning number of 32.
2.4.2 Scanning electron microscopy (SEM). The morphologies of the synthesized microcapsules and the treated nylon 6 fabric were observed using a Hitachi S-3700N scanning electron microscope. The specimen was made electrically conductive by sputter coating with a thin layer of gold–palladium alloy. The micrographs were taken in high vacuum mode with acceleration voltage of 20 kV and a medium spot size.
2.4.3 Upright metallurgical microscopy observation. The synthesized microcapsules were dispersed in water by ultrasonication and the resultant suspension was dropped on a glass slide. The microstructures of the microcapsules were then determined using an 8XB-PC upright metallurgical microscope.
2.4.4 Particle diameter and size distribution. The mean diameter and size distribution of the microcapsule samples were determined on a Horiba LA-960 dynamic light scattering particle size analyzer.
2.4.5 The encapsulation ratio and encapsulation efficiency of the CaCO3 encapsulating benzyl benzoate microcapsules. The encapsulation ratio and encapsulation efficiency of CaCO3 encapsulating benzyl benzoate microcapsules were measured by TGA, using a NETZSCH TG209F1 thermal gravimetric analyzer under nitrogen atmosphere. The specimen with a mass of 5–10 mg was placed in an aluminum crucible and ramped from room temperature up to 600 °C at a heating rate of 10 °C min−1. When the microcapsules were heated to 600 °C, all of the encapsulated benzyl benzoate evaporated, and only the CaCO3 shells remained. Therefore, the encapsulation ratio was obtained via TGA by the weight loss.The encapsulation ratio (R) was calculated using eqn (1).
| |
 | (1) |
where
Wc is the weight of the encapsulated core material,
Ws is the weight of the shell material.
The encapsulation efficiency (E) was calculated using eqn (2).
| |
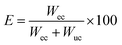 | (2) |
where
Wec is the encapsulated core material and
Wuc is the unencapsulated core material.
2.4.6 UV-vis spectroscopy. The benzyl benzoate content on the treated nylon 6 fabric will affect the anti-mite properties fabric, so the benzyl benzoate on the treated fabric was extracted by ethanol and its content was determined via UV-vis spectrophotometry. All samples were measured using a UV-4802 double-beam UV-vis spectrophotometer at a 300 nm min−1 scanning rate from 300 to 700 nm.Six standards were prepared, with concentrations of 22.60 mg L−1, 18.08 mg L−1, 11.30 mg L−1, 5.65 mg L−1, 2.83 mg L−1 and 1.41 mg L−1 of benzyl benzoate in ethanol. The standards were scanned using the UV-vis spectrophotometer to obtain the absorbances at 241 nm (the maximum wavelength). A standard curve was developed by a linear fitting of the absorbances vs. concentrations of the standards. The benzyl benzoate on the treated fabric was extracted three times with ethanol, the resultant ethanol solution was collected and the concentration of benzyl benzoate in the ethanol solution was quantified by the standard curve. The content of benzyl benzoate on the treated nylon 6 fabric was calculated using the following eqn (3):
| |
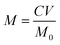 | (3) |
where
M (mg g
−1) is the benzyl benzoate content per gram of nylon 6 fabric treated with microcapsules,
C (mg L
−1) is the concentration of benzyl benzoate in the ethanol solution,
V (L) is the volume of ethanol solution,
M0 (g) is the weight of the treated nylon 6 fabric.
2.4.7 Laundering durability of the treated nylon 6 fabric. The laundering durability of the treated nylon 6 fabric was evaluated by the benzyl benzoate content remaining on the fabric after being washed. The treated nylon 6 fabric was washed according to the following procedure: the samples were put into a beaker containing 1000 mL of 0.5% (w/v) detergent solution, with agitation of 300 rpm at 40 °C for 10 min, then the samples were rinsed with flowing tap water for 5 min and dried at ambient temperature. The main components of the detergent were surfactant, non-phosphorus softener and stain precipitant. The above procedure was repeated 1, 5, 10, 15, and 20 times, and samples were obtained based on the different washing times. The benzyl benzoate on the obtained sample was extracted by ethanol three times and the resulting solutions were investigated by UV-vis spectrophotometry to determine the benzyl benzoate content.
2.4.8 Anti-mite property of treated nylon 6 fabric. The anti-mite activity of treated nylon 6 fabric was evaluated according to the Chinese National standard GB/T 24253-2009. Seven identical Petri dishes were prepared; one was placed in the center and the others were placed around the center dish in the form of petals. The Petri dishes were bridged by sellotape pasted on the edge of each Petri dish and 2000 ± 200 dust mites were placed in the center Petri dish. Three controls and three samples were placed into the surrounding six Petri dishes alternately and 0.05 g of feed for the mites were placed on the three samples. The seven Petri dishes were then placed into a container with a lid and the container was placed in the OMWS Oumai incubator. After 24 hours, the mites on the controls and samples were counted. The mite repellent rate was obtained by eqn (4).| |
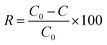 | (4) |
where R is the mite repellent rate, C0 is the number of mites from the controls, and C is the number of mites from the treated nylon 6 fabrics. The anti-mite properties of the treated fabric were assessed based on the mite repellent rate.
3 Results and discussion
3.1 Synthesis of microencapsulated benzyl benzoate with CaCO3 shell
Microcapsules with a CaCO3 shell were obtained as a result of the precipitation of Ca2+ and CO32− onto the surface of benzyl benzoate droplets through an interfacial co-precipitation process in an oil-in-water emulsion. Sodium dodecyl benzene sulfonate was used as an anionic surfactant in the oil-in-water emulsion. The schematic formation mechanism23,24 for the above mentioned microcapsules is illustrated in Fig. 1. The oily benzyl benzoate was first added to the aqueous solution, which contained the sodium dodecyl benzene sulfonate anionic surfactants, to create a stable oil-in-water emulsion during the synthetic process. At this stage, the sodium dodecyl benzene sulfonate anionic surfactant molecules covered the surface of the benzyl benzoate droplets with the hydrophobic chains oriented in the oil phase, while the hydrophilic groups, the benzenesulfonic acid ions, were associated with the water molecules. After a surfactant layer was formed, the CaCl2 aqueous solution was added dropwise into the emulsion system to cover the surfaces of the benzyl benzoate droplet. The added Ca2+ could be assembled on the surface of the benzyl benzoate micelles through electrostatic interactions between the Ca2+ and benzenesulfonic acid ions of the surfactants. Ultimately, the Na2CO3 aqueous solution was quickly poured into the above solution and the CaCO3 shell was formed, encapsulating the benzyl benzoate droplets by the precipitation reaction between Ca2+ and CO32−.
 |
| | Fig. 1 Scheme of formation mechanism for the microencapsulated benzyl benzoate with the CaCO3 shell, via an interfacial co-precipitation method. | |
3.2 FTIR analysis of benzyl benzoate microcapsules
The FTIR spectra of benzyl benzoate, CaCO3 and the synthesized benzyl benzoate microcapsules are presented in Fig. 2. Fig. 2(a) displays the spectrum of CaCO3, the presence of a broad band at 1418 cm−1 represents the asymmetric stretching of carbonate ions, indicating the existence of CO32−. Moreover, the characteristic absorption peak at 834 cm−1 and 731 cm−1 correspond to the out-of-plane bending vibration and in-plane bending vibration of O–C–O in CaCO3, respectively. Fig. 2(b) shows the spectrum of benzyl benzoate; the presence of two absorption peaks at 3064 cm−1 and 3033 cm−1 are attributed to the asymmetric stretching of C–H in the benzene ring of benzyl benzoate, while the sharp and strong peak at 1722 cm−1 is attributed to the asymmetric stretch of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in benzyl benzoate. The absorption peak at 1264 cm−1 in Fig. 2(b) corresponds to the asymmetric stretching of C–O in benzyl benzoate. Fig. 2(c) shows the spectrum of the synthesized benzyl benzoate microcapsule. The characteristic absorption peaks of CaCO3 at 1481 cm−1, 834 cm−1 and 731 cm−1 were also observed in the spectrum of the microcapsule, which indicates the formation of the CaCO3 shell. The characteristic absorption peaks of benzyl benzoate at 3064 cm−1, 3033 cm−1, 1722 cm−1 and 1264 cm−1 could also be observed in microcapsules' spectrum. This result indicates that there was no chemical interaction between benzyl benzoate and CaCO3; the benzyl benzoate is easily encapsulated by the CaCO3 shell through an interfacial co-precipitation method.
O in benzyl benzoate. The absorption peak at 1264 cm−1 in Fig. 2(b) corresponds to the asymmetric stretching of C–O in benzyl benzoate. Fig. 2(c) shows the spectrum of the synthesized benzyl benzoate microcapsule. The characteristic absorption peaks of CaCO3 at 1481 cm−1, 834 cm−1 and 731 cm−1 were also observed in the spectrum of the microcapsule, which indicates the formation of the CaCO3 shell. The characteristic absorption peaks of benzyl benzoate at 3064 cm−1, 3033 cm−1, 1722 cm−1 and 1264 cm−1 could also be observed in microcapsules' spectrum. This result indicates that there was no chemical interaction between benzyl benzoate and CaCO3; the benzyl benzoate is easily encapsulated by the CaCO3 shell through an interfacial co-precipitation method.
 |
| | Fig. 2 FTIR spectra of (a) CaCO3, (b) benzyl benzoate and (c) synthesized benzyl benzoate microcapsules. | |
3.3 Morphologies and microstructure
Fig. 3 shows the morphologies of the microencapsulated benzyl benzoate with the CaCO3 shell. It was clearly observed that the microcapsules were compact and had a rough surface and the shells were porous. During the precipitation of CaCO3, the amorphous nanoprecipitates were firstly formed upon the mixing of CaCl2 and Na2CO3, and then the nanoprecipitates aggregated to form microparticles with spherical morphology and porous internal structure.25–27 Moreover, the precipitated surfactants could also lead to the porosity of the CaCO3 shell after being dissolved in water. Fig. 3(c) shows the micrograph of the partly damaged microcapsules, it can be concluded from the micrograph that typical core–shell structured microcapsules were prepared in this research.
 |
| | Fig. 3 SEM micrographs of the benzyl benzoate microcapsules, (a) ×1000 (b) ×20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (c) broken microcapsules. 000 (c) broken microcapsules. | |
Fig. 4 shows the upright metallurgical microscope photos of CaCO3 encapsulating benzyl benzoate microcapsules. The different intensities of the benzyl benzoate core and CaCO3 shell confirmed the core–shell structure of the microcapsules.
 |
| | Fig. 4 Upright metallurgical microscope images of CaCO3 encapsulating the benzyl benzoate microcapsules. | |
3.4 Particle size distribution
Since the CaCO3 encapsulated benzyl benzoate microcapsules are to be used for the anti-mite finishing of nylon 6 fabric, their particle size should be as small as possible for the fabric to be comfortable. The particle size distribution of the microcapsules synthesized with different concentrations of surfactants and different emulsifying speeds were determined using a dynamic light scattering particle size analyzer, and the results are presented in Fig. 5. In Fig. 5(a), it can be clearly observed that with the same stirring rate, increasing the concentration of SDBS in the emulsion system would decrease the particle size of the microcapsules as well as narrow the size distribution. The mean diameter of the synthesized microcapsules decreased sharply from 13 μm to 6 μm with the SDBS concentration varying from 1 mmol L−1 to 5 mmol L−1, and then decreased slowly to 3 μm as the concentration of SDBS increased to 15 mmol L−1. The surfactant affected the size of the microcapsules by controlling the size of the benzyl benzoate droplets in emulsion, which were the templates for the formation of the final microcapsules. When the benzyl benzoate was turned into small droplets by vigorous agitation in the emulsion stage, the existence of surfactants prevented the formed benzyl benzoate droplets from aggregating, and thus, smaller and more stable benzyl benzoate droplet templates could be obtained, leading to the smaller particle size of the microcapsules. Therefore, the particle size of the microcapsules in this research decreased with increasing concentration of SDBS, until the amount of SDBS was enough to sustain a stable oil/water system. It can be seen from Fig. 5(b) that the size distribution of the microcapsules was significantly affected by the emulsifying speed. When the emulsifying speed increased from 400 rpm to 1000 rpm, the mean diameter of the microcapsules sharply decreased from 35 μm to 5 μm. For a given concentration of surfactant, it would be possible to obtain a much smaller particle size and narrower size distribution by increasing the emulsifying speed, since high emulsifying speed would benefit the formation of small and stable benzyl benzoate droplets in the emulsion stage.
 |
| | Fig. 5 Particle size distribution of microcapsules synthesized at different concentrations of SDBS (a) and different stirring speeds (b). | |
3.5 Encapsulation ratio and encapsulation efficiency
Encapsulation ratio and encapsulation efficiency are also essential parameters for the anti-mite microcapsules. It is desirable for the microcapsules to have the encapsulation ratio and encapsulation efficiency as high as possible. The encapsulation ratio and encapsulation efficiency of microcapsules synthesized at different concentrations of SDBS, core–shell ratios and temperatures were investigated, and the results are provided in Table 1. It is noteworthy that with the concentration of surfactant varying from 5 mmol L−1 to 15 mmol L−1, the encapsulation ratio increased from 19.4% to 26.5%, and encapsulation efficiency increased from 17.5% to 23.9%, since the surfactant could keep the benzyl benzoate droplets stable and they did not aggregate after the droplets were formed under vigorous agitation. In addition, the SDBS assembled the Ca2+ on the surface of the benzyl benzoate droplets to encapsulate the droplets after the addition of CO32−. To some extent, the droplets would be encapsulated more easily if more surfactant was available in the emulsion system. However, there was a limitation in its level, due to the stabilization of the emulsion system. It also could be found that when the mass ratio of benzyl benzoate and CaCl2 increased from 0.2 to 1, the encapsulation ratio increased from 10.8% to 26.5%, and the encapsulation efficiency decreased from 48.6% to 23.9%. This is because the more benzyl benzoate droplets are in the emulsion system, the greater the chance for the CaCO3 shell to encapsulate the benzyl benzoate droplets when they were precipitated. However, compared to the encapsulated benzyl benzoate, there were still lots of unencapsulated benzyl benzoate, leading to the decrease in encapsulation efficiency. It can also be seen from Table 1 that the effect of temperature on the encapsulation ratio and encapsulation efficiency was not significant.
Table 1 Effect of the concentration of SDBS, core–shell ratio and temperature on the encapsulation ratio and encapsulation efficiency of the microcapsules
| Sample code |
Concentration of SDBS (mmol L−1) |
mBABE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mCaCl2 (g g−1) mCaCl2 (g g−1) |
Temperature (°C) |
Encapsulation ratio (%) |
Encapsulation efficiency (%) |
| 1 |
5 |
1 |
45 |
19.4 |
17.5 |
| 2 |
10 |
1 |
45 |
22.0 |
19.8 |
| 3 |
15 |
1 |
45 |
26.5 |
23.9 |
| 4 |
15 |
0.2 |
45 |
10.8 |
48.6 |
| 5 |
15 |
0.6 |
45 |
19.8 |
29.7 |
| 6 |
15 |
1 |
35 |
22.7 |
20.5 |
| 7 |
15 |
1 |
40 |
25.3 |
22.8 |
3.6 Morphology of nylon 6 fabric treated with microcapsules
The microscopic surface morphologies of nylon 6 fabric treated with the microcapsules were investigated by SEM. Fig. 6 exhibits the SEM images of the control nylon 6 fabric, and the microcapsule treated nylon 6 fabric with different concentrations of blocked waterborne PU adhesives in the finishing solution. The micrographs indicate that the microcapsules were successfully attached onto the surface of the nylon 6 fabric. With the increasing concentration of blocked waterborne PU adhesive, the SEM micrographs exhibited some differences. As shown in Fig. 6(a), the surface of the control nylon 6 fiber was smooth. After being treated with microcapsules without using blocked waterborne PU adhesive, the surface of nylon 6 was still glossy, with a small amount of microcapsules distributed between the fibers (Fig. 6(b)). However, after adding blocked waterborne PU adhesive to the finishing solution, the surface of the treated nylon 6 fiber became coarser with a layer of blocked waterborne PU adhesive coating. The covered area of the blocked waterborne PU adhesive coating on the fabric increased with the increase of blocked waterborne PU adhesive concentration, and the amount of attached microcapsules on the surface of the fabric also increased. More agglomerating microcapsules could be found in the fabric treated with finishing solution containing a higher concentration of blocked waterborne PU adhesive, due to the blocked waterborne PU adhesive causing the microcapsules to adhere to each other. Therefore, the blocked waterborne PU adhesive plays an important role in fixing the microcapsules onto the fibers. As illustrated by the scheme in Fig. 7, on heating, the blocked waterborne PU adhesive deblocked and released active –NCO, which could react with the active hydroxyl groups existing on the surface of microcapsules, and the active hydroxyl or amino groups existing on the surface of nylon 6 fibers, and bind the microcapsules and fibers together with chemical bonds to achieve laundering durability for the anti-mite nylon 6 fabric.
 |
| | Fig. 6 SEM of nylon 6 fabric (a) control and treated nylon 6 fabric prepared at the blocked waterborne PU adhesive concentrations of (b) 0 g L−1, (c) 10 g L−1, (d) 30 g L−1, (e) 50 g L−1, and (f) 70 g L−1. | |
 |
| | Fig. 7 Scheme of the bonding mechanism of the blocked waterborne polyurethane adhesives. | |
3.7 Benzyl benzoate content of the treated nylon 6 fabric
The benzyl benzoate content of the treated nylon 6 fabric was measured via UV-vis spectrophotometric analysis. Fig. 8 shows the linear fitting curve of absorbance versus concentration for benzyl benzoate in ethanol, and the quantitative relationship between absorbance and concentration could be expressed by eqn (5), with a correlation coefficient of 0.999829. The following equation was used to quantify the content of benzyl benzoate on the treated nylon 6 fabric:where y is absorbance, x is concentration.
 |
| | Fig. 8 The linear fitting curve of absorbance vs. concentration for benzyl benzoate in ethanol. | |
The benzyl benzoate content of the nylon 6 fabric treated with microcapsules at different concentrations of blocked waterborne PU adhesive is shown in Table 2. The benzyl benzoate content of the treated nylon 6 fabric increased from 4.0 mg g−1 to 13.4 mg g−1 when the concentration of blocked waterborne PU adhesive was increased from 0 g L−1 to 70 g L−1, because the amount of the microcapsules fixed on the fabric increased with the increasing amount of blocked waterborne PU adhesive. However, increasing the blocked waterborne PU adhesive causes a decline in the “hand feel” of the treated PA6 fabric, due to the formation of blocked waterborne PU adhesive coating on the fabric surface. Thus, the concentration of the blocked PU waterborne adhesive in the finishing solution should be controlled within a proper range, in order to obtain the nylon 6 fabric with excellent anti-mite properties and good “hand feel”.
Table 2 The benzyl benzoate content of the treated nylon 6 fabric at different concentrations of blocked waterborne PU adhesive
| Concentration of blocked waterborne PU adhesive (g L−1) |
Benzyl benzoate content (mg g−1) |
| 0 |
4.0 |
| 10 |
5.5 |
| 30 |
8.5 |
| 50 |
11.7 |
| 70 |
13.4 |
In order to compare the laundering durability of nylon 6 fabric treated with non-microencapsulated and microencapsulated benzyl benzoate, the remaining benzyl benzoate content on the treated fabric after different washing times was measured via UV-vis spectrophotometry, and the results are shown in Fig. 9. The benzyl benzoate content of nylon 6 fabric treated with non-microencapsulated benzyl benzoate was decreased dramatically after being washed. After washing 20 times, the remaining benzyl benzoate content was only about 14% (wt) of the initial content of benzyl benzoate, for the non-microencapsulated benzyl benzoate treated fabric. However, for the microencapsulated benzyl benzoate treated fabric, the decrease rate of benzyl benzoate content was slower and the remaining benzyl benzoate content after washing 20 times was about 40% (wt) of the initial content of benzyl benzoate, which was significantly higher than the non-microencapsulated benzyl benzoate treated fabric. Therefore, it was concluded that the microencapsulated benzyl benzoate treated fabric had better laundering durability than the non-microencapsulated benzyl benzoate treated fabric. It was noticed that for the microencapsulated benzyl benzoate treated fabric, the decrease rate of benzyl benzoate content after the first washing was significantly higher than the decrease rates of benzyl benzoate content after washing 5, 10, 15, and 20 times. This was attributed to some easy washing microcapsules that were not tightly fixed onto the surface of the fabric. After the first washing, only firmly fixed microcapsules remained on the surface of the fabric, the decline in the benzyl benzoate content could be attributed to the slow-release of benzyl benzoate from the microcapsules, thus the decrease rate of benzyl benzoate content became slower.
 |
| | Fig. 9 Benzyl benzoate content of treated nylon 6 fabric after different washing times. | |
The morphologies of the treated nylon 6 fabric washed 1, 5, 10, 20 times are shown in Fig. 10. It could be seen from the SEM images that after different washing times, the treated microcapsules on the nylon 6 fabric still presented a spherical shape, which indicates that there was no morphological change in the microcapsules after being washed. However, the amount of microcapsules on the surface of the nylon 6 fabric declined since the microcapsules that were not firmly fixed were washed away during the washing test.
 |
| | Fig. 10 SEM images of nylon 6 fabric treated with benzyl benzoate microcapsules, with different washing times: (a) 1 time, (b) 5 times, (c) 10 times, and (d) 20 times. | |
3.8 Anti-mite properties
The anti-mite properties of the treated nylon 6 fabric were evaluated according to the method in the Chinese National Standard GB/T 24253-2009, and the results are presented in Table 3. All of the PA6 fabrics treated with microcapsules exhibited better anti-mite activity than the control fabric. The mite repellent rates of nylon 6 fabric treated with only microcapsules was 70.2% and was reduced to 43.3% after being washed 20 times. The nylon 6 fabric treated with microcapsules and blocked PU waterborne adhesive exhibited the best anti-mite activity and laundering durability; the mite repellent rate was 82.3% and reduced to 61.8% after being washed 20 times. The reasons for the above phenomenon are that the blocked waterborne PU adhesive could fix more microcapsules on the fabric during the finishing process, and immobilizing the microcapsules on the fabric with chemical bonds made the treated nylon 6 fabric durable under laundering.
Table 3 Mite repellent rates of the treated nylon 6 fabrica
| |
Mite repellent rate (%) |
| Before washing |
After washing 20 times |
| 1# treated with only CaCO3 encapsulating benzyl benzoate microcapsules, no blocked waterborne PU adhesives, 2# treated with CaCO3 encapsulating benzyl benzoate microcapsules and blocked waterborne PU adhesives. |
| Control |
10.0 |
8.6 |
| 1# |
70.2 |
43.3 |
| 2# |
82.3 |
61.8 |
4 Conclusion
Microcapsules based on a benzyl benzoate core and a CaCO3 shell, were synthesized using an interfacial co-precipitation method. The results of FTIR, SEM and upright metallurgical microscope indicated the successful encapsulation of the benzyl benzoate with the CaCO3 shell. Increasing the concentration of SDBS and the emulsifying speed decreased the particle size and narrowed the size distribution. The encapsulation ratio and encapsulation efficiency of the microcapsules were affected by the concentration of SDBS and core–shell mass ratio, but the influence of temperature was not significant. The synthesized microcapsules were used to treat the nylon 6 fabric by using a blocked waterborne PU adhesive to immobilize the microcapsules on the fabric with chemical bonds. The SEM images of the treated nylon 6 fabric confirmed that the microcapsules were successfully fixed onto the surface of the fabric, and increasing the concentration of the blocked waterborne PU adhesive would increase the microcapsules and benzyl benzoate content on the treated fabric. The mite repellent tests showed that the mite repellent rate of the anti-mite nylon 6 fabric was up to 82.3% and remained at 61.8% after being washed 20 times.
Acknowledgements
Financial support from the Key Project of Department of Education of Guangdong Province (No. 2012CXZD0007) is highly appreciated.
References
- R. Voorhorst, F. T. M. Spieksma, H. Varekamp, M. J. Leupen and A. W. Lyklema, J. Allergy, 1967, 39, 325–339 CrossRef.
- W. R. Thomas, W. A. Smith, B. J. Hales, K. L. Mills and R. M. O'brien, Int. Arch. Allergy Immunol., 2002, 129, 1–18 CrossRef CAS PubMed.
- R. Ji-Hwan, Y. Jung-Yeon, K. Min-Ji, H. Sang-Gyu, A. K. Chul, R. Jae-Chan, C. Mi-Kyung, C. Jung Hee, K. Chang-Hoon and L. Sang-Nam, J. Allergy Clin. Immunol., 2013, 131, 549–561 CrossRef PubMed.
- A. Niekraszewicz, J. Lebioda, J. Hoffman, K. Ruszkowski and H. Struszczyk, Fibres Text. East. Eur., 2005, 13, 24–27 CAS.
- A. Woodcock, L. Forster and E. Matthews, N. Engl. J. Med., 2003, 349, 225–236 CrossRef PubMed.
- A. Dietemann, J. C. Bessot, C. Hoyet, M. Ott, A. Verot and G. Pauli, J. Allergy Clin. Immunol., 1994, 34, 354–362 Search PubMed.
- M. L. Hayden, G. Rose, K. B. Diduch, P. Domson, M. D. Chapman, P. W. Heymann and T. A. Platts-Mills, J. Allergy Clin. Immunol., 1992, 89, 536–545 CrossRef CAS PubMed.
- S. Lau-Schadendorf, A. F. Rusche, A. K. Weber, P. Buettner-Goetz and U. Wahn, J. Allergy Clin. Immunol., 1991, 87, 41–47 CrossRef CAS PubMed.
- W. F. Green, N. R. Nicholas, C. M. Salome and A. J. Woolcock, Clin. Exp. Allergy, 1989, 19, 203–207 CrossRef CAS PubMed.
- E. Sertkaya, K. Kaya and S. Soylu, Ind. Crops Prod., 2010, 31, 107–112 CrossRef CAS.
- V. Mahakittikun, N. Soonthornchareonnon, S. Foongladda, J. J. Boitano, T. Wangapai and P. Ninsanit, Asian Pac. J. Allergy Immunol., 2014, 32, 46 Search PubMed.
- S. Wongkamchai, K. Rongsriyam, H. Nochot, V. Mahakittikun, B. Sermsart, W. Choochote and K. Kanjanopart, Exp. Appl. Acarol., 2005, 35, 293–300 CrossRef CAS PubMed.
- S. Rodrigues, I. Martins, I. Fernandes, P. Gomes, V. Mata, M. Barreiro and A. Rodrigues, Chem. Eng. J., 2009, 149, 463–472 CrossRef CAS.
- G. Nelson, Int. J. Pharm., 2002, 242, 55–62 CrossRef CAS PubMed.
- P. Sánchez, M. V. Sánchez-Fernandez, A. Romero, J. F. Rodríguez and L. Sánchez-Silva, Thermochim. Acta, 2010, 498, 16–21 CrossRef.
- M. M. Specos, J. Garcia, J. Tornesello, P. Marino, M. Della Vecchia, M. D. Tesoriero and L. Hermida, Trans. R. Soc. Trop. Med. Hyg., 2010, 104, 653–658 CrossRef CAS PubMed.
- S. Giraud, S. Bourbigot, M. Rochery, I. Vroman, L. Tighzert and R. Delobel, Polym. Degrad. Stab., 2002, 77, 285–297 CrossRef CAS.
- J. Liu, C. Liu, Y. Liu, M. Chen, Y. Hu and Z. Yang, Colloids Surf., B, 2013, 109, 103–108 CrossRef CAS PubMed.
- M. Fujiwara, K. Shiokawa, K. Morigaki, Y. Zhu and Y. Nakahara, Chem. Eng. J., 2008, 137, 14–22 CrossRef CAS.
- M. Fujiwara, K. Shiokawa, M. Araki, N. Ashitaka, K. Morigaki, T. Kubota and Y. Nakahara, Cryst. Growth Des., 2010, 10, 4030–4037 Search PubMed.
- A. I. Petrov, D. V. Volodkin and G. B. Sukhorukov, Biotechnol. Prog., 2005, 21, 918–925 CrossRef CAS PubMed.
- S. Yu, X. Wang and D. Wu, Energy Fuel, 2014, 28, 3519–3529 CrossRef CAS.
- S. Yu, X. Wang and D. Wu, Appl. Energy, 2014, 114, 632–643 CrossRef CAS.
- G. Yan, L. Wang and J. Huang, Powder Technol., 2009, 192, 58–64 CrossRef CAS.
- T. Kato, T. Suzuki, T. Amamiya, T. Irie, M. Komiyama and H. Yui, Supramol. Sci., 1998, 5, 411–415 CrossRef CAS.
- C. Wang, C. He, Z. Tong, X. Liu, B. Ren and F. Zeng, Int. J. Pharm., 2006, 308, 160–167 CrossRef CAS PubMed.
- G. B. Sukhorukov, D. V. Volodkin, A. M. Günther, A. I. Petrov, D. B. Shenoy and H. Möhwald, J. Mater. Chem., 2004, 14, 2073–2081 RSC.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 
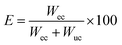
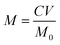
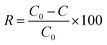

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in benzyl benzoate. The absorption peak at 1264 cm−1 in Fig. 2(b) corresponds to the asymmetric stretching of C–O in benzyl benzoate. Fig. 2(c) shows the spectrum of the synthesized benzyl benzoate microcapsule. The characteristic absorption peaks of CaCO3 at 1481 cm−1, 834 cm−1 and 731 cm−1 were also observed in the spectrum of the microcapsule, which indicates the formation of the CaCO3 shell. The characteristic absorption peaks of benzyl benzoate at 3064 cm−1, 3033 cm−1, 1722 cm−1 and 1264 cm−1 could also be observed in microcapsules' spectrum. This result indicates that there was no chemical interaction between benzyl benzoate and CaCO3; the benzyl benzoate is easily encapsulated by the CaCO3 shell through an interfacial co-precipitation method.
O in benzyl benzoate. The absorption peak at 1264 cm−1 in Fig. 2(b) corresponds to the asymmetric stretching of C–O in benzyl benzoate. Fig. 2(c) shows the spectrum of the synthesized benzyl benzoate microcapsule. The characteristic absorption peaks of CaCO3 at 1481 cm−1, 834 cm−1 and 731 cm−1 were also observed in the spectrum of the microcapsule, which indicates the formation of the CaCO3 shell. The characteristic absorption peaks of benzyl benzoate at 3064 cm−1, 3033 cm−1, 1722 cm−1 and 1264 cm−1 could also be observed in microcapsules' spectrum. This result indicates that there was no chemical interaction between benzyl benzoate and CaCO3; the benzyl benzoate is easily encapsulated by the CaCO3 shell through an interfacial co-precipitation method.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (c) broken microcapsules.
000 (c) broken microcapsules.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mCaCl2 (g g−1)
mCaCl2 (g g−1)




