Facile synthesis of mesoporous melamine-formaldehyde spheres for carbon dioxide capture†
Zhongfei Lva,
Dandan Zhaob and
Shiai Xu*a
aShanghai Key Laboratory of Advanced Polymeric Material, Key Laboratory for Ultrafine Materials of Ministry of Education, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: saxu@ecust.edu.cn; Fax: +86 021 6425 3775; Tel: +86 021 6425 3353
bChemical Experiment Center, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 2002237, China
First published on 15th June 2016
Abstract
Greenhouse effect and excessive carbon dioxide (CO2) emissions have caused widespread public concern in recent years. Tremendous efforts have been made towards CO2 capture promoting the development of numerous sorbents. In this study, mesoporous melamine-formaldehyde spheres (MMFSs) with high surface area (298 m2 g−1), uniform pore sizes (3.0 nm) and high nitrogen content (∼45%) have been successfully synthesized using Pluronic F127 and sodium dodecyl sulfonate (SDS) as the templates, glycol as the organic co-solvent, and melamine (M) and paraformaldehyde (PFA) as the precursors, respectively. These MMFSs are highly effective in capturing CO2, with the maximum adsorption capacity of 48.0 mg g−1 at 273 K for CO2. Moreover, the CO2/N2 selectivity at 298 K reaches 24.3, which is attributed to the high N content allowing for high binding affinity with CO2 and the well-defined mesopores (3.0 nm). This approach suggests a new direction in designing CO2 sorbents with excellent selectivity.
Introduction
Global warming and excessive carbon dioxide (CO2) emissions can pose a potential threat to the global economy and human health, and thus have caused widespread public concern over the past few years.1–3 To address this issue, tremendous efforts have been devoted to CO2 capture and sequestration, yielding numerous sorbents for CO2 uptake.4,5 Aqueous alkanolamines such as 30 wt% aqueous monoethanolamine (MEA) are conventionally used to capture CO2 via the chemisorptive formation of carbamates or carbonate species.6,7 However, these amines require higher heat treatment above 100 °C to release the chemically adsorbed CO2. As a result, the energy needed for the regeneration of amine solvents account for ∼70% of the total capture process, which making them commercially unattractive.8,9 A recent study reveals that solid amines supported on porous materials offer less energy intensive regeneration options as a result of the recovery of the sensible heat of the sorbent module, which thus can cut down the total energy consumption in the CO2 capture process.10–13 However, it would be more desirable if some important properties of these amine-functionalized porous materials, such as total CO2 uptake, CO2 selectivity over other gases especially nitrogen (N2), could be improved.The performance of solid adsorbents is highly dependent on their various material parameters including the specific surface area, functional groups, and the kinds of elements. It was revealed that high nitrogen (N) content and surface area can result in high uptake and selectivity simultaneously.14–17 Among those amines, melamine (2,4,6-triamino-1,3,5-triazine) is one of the most ideal moieties due to its low alkalinity (pKa = 5.5), versatile reactivity, low cost, highly abundant N content. More importantly, melamine resins can be produced in an industrial scale and widely used as a cross-linker in organic coatings and plastics.18,19 The modification of porous silica or carbon with melamine and the incorporation of melamine into the covalent organic frameworks allow for good CO2 adsorption capacity.20–23 If high surface areas and porosity can be achieved in such networks, their applications can be further extended due to rich amine groups on the pore walls. Indeed, porous materials with amine functionalities on the pore walls have recently attracted great interest, and some approaches to porous melamine resins have been developed using templating, emulsion strategies and solvothermal methods.24–32 However, owing to the weak self-assembly ability of precursor components and the serious cross-linking of neighboring nanospheres during the hydrothermal process, the synthesis of mesoporous melamine-formaldehyde spheres (MMFSs) through a simple, low-cost and environmentally friendly method is inherently nontrivial.
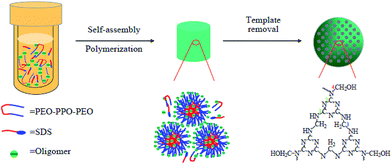
In this study, we report a facile soft-template approach for the synthesis of MMFSs using triblock copolymer Pluronic F127 and sodium dodecyl sulfonate (SDS) as templates, glycol as the organic co-solvent and melamine (M) and paraformaldehyde (PFA) as precursors, respectively. The synthesis scheme is shown in Fig. 1. The resultant MMFSs samples have a high N content of above 42% and mesopores of 3.0–5.6 nm in diameter. The high N content originating from the triazine units of melamine facilitates CO2 binding affinity, enabling “CO2-philicity”, and well-defined pores in a mesoscopic dimension (∼3.0 nm) disrupt N2 adsorption due to the weakened interaction with pore walls. This orthogonal approach to the two different gases results in excellent CO2-to-N2 selectivities, implying that N-containing mesopores are useful in enhancing the selectivity of the sorbents. This study may also provide an insight into the inconsistent selectivities of porous polymers with the same N-containing functional groups.
Experiment
Materials and characterizations
Triblock poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) copolymer Pluronic F127 (PEO106-PPO70-PEO106, molecular weight = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) was purchased from Sigma-Aldrich Co. LLC (USA). Melamine (M), sodium dodecyl sulfate (SDS), paraformaldehyde (PFA), sodium hydroxide, hydrochloric acid, ethylene glycol and ethanol were purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd (Shanghai, China). All chemicals were analytical grade and used without any purification. Scanning electron microscopic (SEM) images were obtained using a S4800 field emission scanning electron microscopic (FE-SEM) and a thin gold film was sprayed on the samples before the characterization. Transmission electron microscopy (TEM) images were obtained using a JEM-2100 microscope operated at 200 kV. Fourier transform infrared (FT-IR) spectra were obtained using a Nicolet 6700 FTIR spectrometer in the range of 400–4000 cm−1. Solid-state 13C NMR spectra were obtained using a Bruker Avance-400 spectrometer at 11 kHz using densely packed MMFSs powders. Elemental analyses were performed on a VARIO EL III elemental analyzer (Thermo Scientific, MA, USA). Thermogravimetric (TGA) analysis was performed on a STA449C thermal analyzer from room temperature to 800 °C at a heating rate of 5° min−1 under a nitrogen atmosphere. Gas sorption isotherms were measured on a Micromeritics Tristar 2460 system at liquid nitrogen temperature. Samples were outgassed for 10 h at 150 °C before the measurements. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method, and the pore volume and pore size distributions were derived from the adsorption branches for isotherms using the Barrett–Joyner–Halenda (BJH) model. To evaluate the gas uptakes and selectivities of the samples, the CO2 and N2 adsorption/desorption isotherms were collected at 273 K and 298 K in the pressure range of 0–1 bar by using the same porosity analyzer.
600) was purchased from Sigma-Aldrich Co. LLC (USA). Melamine (M), sodium dodecyl sulfate (SDS), paraformaldehyde (PFA), sodium hydroxide, hydrochloric acid, ethylene glycol and ethanol were purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd (Shanghai, China). All chemicals were analytical grade and used without any purification. Scanning electron microscopic (SEM) images were obtained using a S4800 field emission scanning electron microscopic (FE-SEM) and a thin gold film was sprayed on the samples before the characterization. Transmission electron microscopy (TEM) images were obtained using a JEM-2100 microscope operated at 200 kV. Fourier transform infrared (FT-IR) spectra were obtained using a Nicolet 6700 FTIR spectrometer in the range of 400–4000 cm−1. Solid-state 13C NMR spectra were obtained using a Bruker Avance-400 spectrometer at 11 kHz using densely packed MMFSs powders. Elemental analyses were performed on a VARIO EL III elemental analyzer (Thermo Scientific, MA, USA). Thermogravimetric (TGA) analysis was performed on a STA449C thermal analyzer from room temperature to 800 °C at a heating rate of 5° min−1 under a nitrogen atmosphere. Gas sorption isotherms were measured on a Micromeritics Tristar 2460 system at liquid nitrogen temperature. Samples were outgassed for 10 h at 150 °C before the measurements. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method, and the pore volume and pore size distributions were derived from the adsorption branches for isotherms using the Barrett–Joyner–Halenda (BJH) model. To evaluate the gas uptakes and selectivities of the samples, the CO2 and N2 adsorption/desorption isotherms were collected at 273 K and 298 K in the pressure range of 0–1 bar by using the same porosity analyzer.
Synthesis of MMFSs
MMFSs were synthesized from a mixture of melamine, (HCHO)n, F127, SDS, NaOH, ethylene glycol and H2O at a molar ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0–4.5
2.0–4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.002
0.002![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02
0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.04
0.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2
3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28.3 at 150 °C for 24 h. In a typical run, 6.3 g of melamine, 4.5 g of PFA and 0.10 g of NaOH were dissolved in 16 mL of deionized water and stirred at 80 °C for 15 min. Pluronic F127 was dissolved in 10 mL of deionized water and 10 mL of ethylene glycol containing 3.5 mL of HCl aqueous solution (wt 36%) was stirred at room temperature for 2 h. Then, this solution was poured into the prepolymer solution and then stirred at room temperature for another 2 h, after which the mixture was transferred to an autoclave and heated at 150 °C for 24 h. After filtrating, washing and drying, a faint yellow solid was obtained and the template of F127 and SDS were removed by extraction with ethanol and hydrochloric aid at 60 °C for 12 h. The synthesis route was proposed in Fig. 1.
28.3 at 150 °C for 24 h. In a typical run, 6.3 g of melamine, 4.5 g of PFA and 0.10 g of NaOH were dissolved in 16 mL of deionized water and stirred at 80 °C for 15 min. Pluronic F127 was dissolved in 10 mL of deionized water and 10 mL of ethylene glycol containing 3.5 mL of HCl aqueous solution (wt 36%) was stirred at room temperature for 2 h. Then, this solution was poured into the prepolymer solution and then stirred at room temperature for another 2 h, after which the mixture was transferred to an autoclave and heated at 150 °C for 24 h. After filtrating, washing and drying, a faint yellow solid was obtained and the template of F127 and SDS were removed by extraction with ethanol and hydrochloric aid at 60 °C for 12 h. The synthesis route was proposed in Fig. 1.
Results and discussion
Adsorbent characterizations
MMFSs were prepared through a facile route as described in the Experimental section and Fig. 1. The N2 adsorption/desorption isotherms, SEM and TEM images were used to characterize the mesoporous structure of these MMFSs. The N2 adsorption/desorption isotherms offer a more quantitative set of information on the porosity of MMFS-3.0 as shown in Fig. 2A. MMFS-3.0 shows a type IV isotherm with a sharp capillary condensation step at P/P0 = ∼0.45 indicating the dominant presence of mesopores.33 The Brunauer–Emmet–Teller (BET) specific surface area and pore volume are 298 m2 g−1 and 0.46 cm3 g−1, respectively (Table S1†). The main pore size of ∼3.0 nm calculated by the Barrett–Joyner–Halenda (BJH) method from the adsorption branch (Fig. 2a) is in well agreement with the core dimension of each pore in the SEM (Fig. 2B and b) and TEM (Fig. 2C and c) images. Moreover, to optimize the porosity and CO2 adsorption characteristics, a series of MMFSs with different PFA/M molar ratios of 2.0 to 4.5 were synthesized. The N2 adsorption/desorption isotherms and textural parameters were given in ESI Fig. S1 and Table S1,† respectively. From the above results, theses MMFSs could achieve surface areas changing from 63 to 298 m2 g−1, average pore sizes varying from 3.0 to 5.6 nm, large total pore volumes ranging from 0.10 to 0.46 cm3 g−1, indicating that a proper PFA/M molar ratio is needed for MMFSs to obtain high surface area and uniform pore size distributions. In our experiments, the PFA/M molar ratio centered at 3.0 could be chosen as the optimal experimental condition. In fact, most mesoporous polymers with highly developed surface area and most pores arise from the presence of templates and some pores formed during the cross-linking process.34 However, as MMFSs were synthesized using the same amount of template F127 and SDS, the differences in the mesopores and specific surface areas among different MMFSs can be mainly attributed to the cross-linking process. There are three steps in the synthesis of melamine-formaldehyde resins: addition of formaldehyde to melamine, condensation reaction between methylolmelamines and crosslinking.35 At the start of the resin synthesis, melamine firstly reacts with formaldehyde, leading to the formation of nine different methylolmelamines. Next, two different types of condensation reactions can occur, leading to oligomerization by the formation of bridges between the triazine rings. At low pH and PFA/M molar ratio there is a predominance of methylene bridge formation, whereas at high pH and PFA/M ratio ether bridges are formed as well.36 This formation is of great importance in characterizing the network structure of melamine-formaldehyde resins, thus could influence the production of mesopores in the sample. More importantly, the similar results were also reported by Tan et al.30,31 In addition, the SEM images (Fig. 2B and b and S2†) show that MMFSs have a foam-like interconnected mesoporous network structure consisting of numerous spherical aggregates of submicron-sized particles. Ideally, the reaction between melamine and PFA can result in the formation of a perfect network structure as illustrated in Fig. 1. Moreover, the surfactant of SDS and F127 may have a synergistic regulation on the formation of sample morphology. However, under the reaction conditions, the difficulty in the polymerization and the low solubility of the prepolymer make it difficult to adjust the compatibility between the template and the polymer precursor.37 Then, the MMFSs with particle size from 100 nm to 200 nm tend to aggregate, which can also be seen from the TEM images (Fig. 2C and c).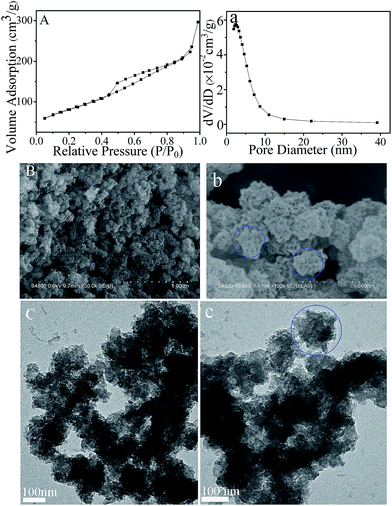 | ||
| Fig. 2 N2 sorption isotherms (A), pore size distributions (a), SEM images (B and b) and TEM images (C and c) of MMFS-3.0. | ||
The chemical structure of MMFSs after extraction the template of F127 and SDS was analyzed by elemental analysis, Fourier transform infrared (FT-IR) spectroscopy and solid-state 13C nuclear magnetic resonance (NMR) measurements. Fig. 3A–C show the solid-state 13C NMR spectra of MMFS-2.5, MMFS-3.0 and MMFS-3.5, synthesized using a PFA/M molar ratio of 2.5, 3.0, and 3.5, respectively. All the NMR spectra clearly show four peaks at 46, 53, 153 and 163 ppm. The signals at 153 ppm and 163 ppm can be assigned to the triazine rings, whereas the peak at 53 and 46 ppm is associated with the –NH–CH2–NH– and –NHCH2OH species, respectively.38 Moreover, the content of different C (C1,2, C3, C4) species (Fig. 1) in MMFSs was calculated and showed in Table S2.† It is clear that the content of C1,2 species was increased as decreasing the mass of PFA, and thus MMFS-2.5 contains a relatively higher content of C1,2 species (∼60.13%), which may play an important role in improving the adsorption property of MMFSs for CO2. Meanwhile, the element analysis of MMFSs (Tables S3 and S4†) after template removal gives that the N/C molar ratio is between 1.07 and 1.18, and MMFSs contain a high N content of above 42%. The solid-state 13C NMR spectra and the element analysis show that the content of triazine species or N element increases with decreasing the PFA mass, which plays an important role in increasing the adsorption capacity and selectivity of CO2 as will be discussed later. The IR spectra (Fig. 3D) of MMFS-3.0 show three vibration bands at 1557, 812 and 790 cm−1, indicating the existence of triazine rings on the pore walls. A broad peak at ∼3400 cm−1 points to the free hydroxyl and amino groups in the network and the physisorbed water on the sample surface. Moreover, the peak at 1341 cm−1 indicates that the presence of methylene bridges in the MMFS network, which is in line with the solid-state 13C NMR spectra. Notably, methylene rather than methylene ether linkage is observed in the sample, which is more stable than the ether linkage.39 The thermo gravimetric (TGA) profile shows that MMFS is stable up to ∼300 °C (Fig. S3†), which is important for expanding the application of MMFSs.40
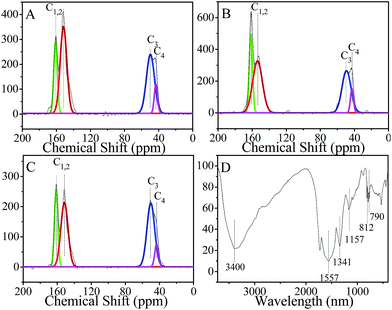 | ||
| Fig. 3 13C solid-state NMR spectra of MMFS-2.5 (A), MMFS-3.0 (B), MMFS-3.5 (C) and FT-IR spectra of MMFS-3.0 (D). | ||
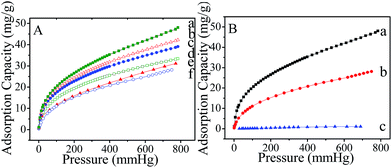
To investigate the effects of the intrinsic pore structure characteristics and N-containing or triazine groups inside MMFSs on the CO2 capture, the CO2 uptake of MMFSs synthesized with different PFA/M molar ratios (2.0–4.5) was measured up to 1 bar at 273 K (Fig. 4A). It is noticeable that the maximum adsorption capacity for CO2 increases up to 48.0 mg g−1 (Fig. 4Aa, Table S1†) in MMFS-3.0, which could be attributed to its higher BET area and total pore volume to a certain extent. For comparison, MMFS-2.0, MMFS-2.5, MMFS-3.5, MMFS-4.0 and MMFS-4.5 synthesized using the same template and a similar approach exhibit similar pore structure characteristics with an adsorption capacity for CO2 gas of 42.2, 39.1, 33.4, 32.5 and 30.2 mg g−1, respectively (Table S1†). Thus, it could be concluded that the adsorption capacity for CO2 increases as the initial PFA/M molar ratio decreases. Usually, adsorption can be divided into two types, namely physisorption and chemisorption, which is determined by the pore structure characteristics and the chemical structure of the sorbents, respectively.41–44 In our experiments, the triazine rings of melamine are Lewis base, which could react with CO2 molecules through chemisorption. In addition, the higher surface area and total volume are in indeed needed to improve the adsorption of MMFSs for CO2 capture as discussed before. The maximum adsorption capacity for CO2 is observed in MMFS-3.0, which can be attributed to its similar chemical structure to the other MMFSs, but excellent pore structure characteristics greatly improve its physisorption between sample and CO2 molecule. For the other samples, MMFS-2.0, MMFS-2.5, MMFS-3.5, MMFS-4.0 and MMFS-4.5 exhibit similar pore structure indicating that there is no major difference in the physisorption process between them. However, there is a significant difference in the N content between the above samples, which may play a role in the chemisorption between the samples and CO2 molecules and finally influence the performance of CO2 capture. Then, during the CO2 uptake experiments, MMFS-2.0 with a higher N/C molar ratio of 1.18 achieved higher adsorption capacity for CO2. Moreover, as the temperature is increased from 273 K to 298 K at 1 bar, MMFS-3.0 retains 48.5% of its initial CO2 uptake capacity, verifying the effective binding of the N-containing units with CO2. By contrast, at the same temperature and pressure, the N2 and O2 uptake capacity of MMFS-3.0 is 1.1 and 0.4 mg g−1 (Fig. S4†) respectively. The estimated CO2/N2 and CO2/O2 adsorption selectivity is 24.3 and 62.9, respectively, which can be ascribed to N sited in the triazine rings facilitating the interaction with the polarizable CO2 molecules and the ease of release N2 and O2 from mesopores.45,46 Moreover, compared with melamine modified SBA-15 (ref. 20) exhibited a similar surface area and pore structure with MMFS-3.0 and the maximum adsorption capacity of 20.4 mg g−1, our samples MMFS-3.0 shows more higher adsorption capacity, which is attributed to the higher N sited in the triazine rings facilitating the interaction with the polarizable CO2 molecules. However, mesoporous carbon adsorbents synthesized via nanocasting technique with melamine-formaldehyde resin as precursor and mesoporous silica as template40 showed more higher CO2 adsorption capacity of 0.782 m mol g−1 (34.4 mg g−1), which is attributed to its higher micropores for capturing the small CO2 molecules.47 Thus, this comparison results prove that excellent pore structure characteristics facilitated with trazine basic rings could greatly improve the physisorption and chemisorption between the adsorbents and CO2 molecules, which is in agreement with our previous explanation.
A practical CO2 capture application not only requires high adsorption capacity and selectivity but also a stable cyclic adsorption–desorption performance by the adsorbents. In order to evaluate the long term stability and CO2 adsorption regenerability for the adsorbents, the reproducibility of MMFS-4.5 was tested by four consecutives CO2 adsorption at 273 K and desorption by simple deflating run and the results were given in Fig. S5.† It is obvious that there is no notable change in CO2 adsorption capacity of MMFS-4.5 with the increase of cycle time (29.7, 29.3, 29.0 and 28.4 mg g−1 respectively), indicating complete regeneration of MMFS-4.5. This is due to the adsorption of CO2 on MMFS surface by weak electrostatic interaction between the active sites of adsorbents and CO2 molecules, which demonstrated MMFSs provide a good regeneration ability and reusability.
Conclusions
In summary, we have developed a facile route for the synthesis of MMFSs with high N-content and mesopores for CO2 capture by using SDS and F127 as the template. These obtained MMFSs were investigated to be intrinsically mesoporous with a high BET surface area of 298 m2 g−1 and a pore volume of 0.46 m3 g−1. At 273 K and 1 bar, MMFS-3.0 exhibited the maximum CO2 adsorption capacity of 48 mg g−1. The functionalized triazine rings enable these MMFSs to have a high selectivity at the gas adsorption of CO2 over N2. The co-presence of high N content and mesoporosity handles CO2 and N2 in an orthogonal manner, giving rise to unusual high slectivities. Hence, this study offers a useful design principle for superior selectivities of porous solid sorbents by combining mesopores and functional groups with high binding affinity with CO2.Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 51463020) and Kunlun Scholar Award Program of Qinghai Province.Notes and references
- T. J. Crowley, Science, 2000, 289, 270–277 CrossRef CAS PubMed.
- S. J. Datta, C. Khumnoon, Z. H. Lee, W. K. Moon, S. Docao, T. H. Nguyen, I. C. Hwang, D. Moon, P. Oleynikov, O. Terasaki and K. B. Yoon, Science, 2015, 350, 302–306 CrossRef CAS PubMed.
- M. Burke, S. M. Hsiang and E. Miguel, Nature, 2015, 527, 235–240 CrossRef CAS PubMed.
- N. MacDowell, N. Florin, A. Buchard, J. Hallett, A. Galindo, G. Jackson, C. S. Adjiman, C. K. Williams, N. Shah and P. Fennell, Energy Environ. Sci., 2010, 3, 1645–1669 CAS.
- D. Y. C. Leung, G. Caramanna and M. M. Maroto-Valder, Renewable Sustainable Energy Rev., 2014, 39, 426–443 CrossRef CAS.
- G. T. Rochelle, Science, 2009, 325, 1652–1654 CrossRef CAS PubMed.
- M. Wang, A. Lawal, P. Stephenson, J. Sidders and C. Ramshaw, Chem. Eng. Res. Des., 2011, 89, 1609–1624 CrossRef CAS.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- J. Liu, P. K. Thallapally, B. P. McGrail, D. R. Brown and J. Liu, Chem. Soc. Rev., 2012, 41, 2308–2322 RSC.
- X. Xu, C. Song, J. M. Andresen, B. G. Miller and A. W. Scaroni, Energy Fuels, 2002, 16, 1463–1469 CrossRef CAS.
- Q. Wang, J. Luo, Z. Zhong and A. Borgna, Energy Environ. Sci., 2011, 4, 42–55 CAS.
- J.-R. Li, Y. Ma, M. C. McCarthy, J. Sculley, J. Yu, H.-K. Jeong, P. B. Balbuena and H.-C. Zhou, Coord. Chem. Rev., 2011, 255, 1791–1823 CrossRef CAS.
- S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796–854 CrossRef CAS PubMed.
- Q. Li, J. Yang, D. Feng, Z. Wu, Q. Wu, S. S. Park, C.-S. Ha and D. Zhao, Nano Res., 2010, 3, 632–642 CrossRef CAS.
- C. Goel, H. Bhunia and P. K. Baipai, RSC Adv., 2015, 5, 46568–46582 RSC.
- K. Huang, S.-H. Chai, R. T. Mayes, G. M. Veith, K. L. Browning, M. A. Sakwa-Novak, M. E. Potter, C. W. Jones, Y.-T. Wu and S. Dai, Chem. Commun., 2015, 51, 17261–17264 RSC.
- P. P. Sharma, J. Wu, R. M. Yadav, M. Liu, C. J. Wright, C. S. Tiwary, B. I. Yakobson, J. Lou, P. M. Ajayan and X.-D. Zhou, Angew. Chem., Int. Ed., 2015, 54, 13701–13705 CrossRef CAS PubMed.
- K. H. Lund and J. H. Petersen, Food Addit. Contam., 2006, 23, 948–955 CrossRef CAS PubMed.
- K. Hong and S. Park, Mater. Chem. Phys., 1999, 58, 128–131 CrossRef CAS.
- Y. Jing, L. Wei, Y. Wang and Y. Yu, Microporous Mesoporous Mater., 2014, 183, 124–133 CrossRef CAS.
- E. A. Prasetyanto, M. B. Ansari, B.-H. Min and S.-E. Park, Catal. Today, 2010, 158, 252–257 CrossRef CAS.
- K. Ahmad, O. Mowla, E. M. Kennedy, B. Z. Dlugogorski, J. C. Mackie and M. Stockenhuber, Energy Technol., 2013, 1, 345–349 CrossRef CAS.
- S. He, Y. Bi, Y. Zhang, H. Cao, X. Shi, X. Luo and L. Zhang, J. Sol-Gel Sci. Technol., 2015, 74, 175–180 CrossRef CAS.
- Y.-R. Dong, N. Nishiyama, M. Kodama, Y. Eqashira and K. Ueyama, Carbon, 2009, 47, 2112–2142 CrossRef.
- D. F. Schmidt, C. F. Hohenesche, A. Weiss and V. Schädler, Chem. Mater., 2008, 20, 2851–2853 CrossRef CAS.
- C. F. Hohenesche, D. F. Schmidt and V. Schädler, Chem. Mater., 2008, 20, 6124–6129 CrossRef.
- C. C. Egger, C. Fresne, D. Schmidt, J. Yang and V. Schädler, J. Sol-Gel Sci. Technol., 2008, 48, 86–94 CrossRef CAS.
- K. Kailasam, Y.-S. Jun, P. Katekomol, J. D. Epping, W. H. Hong and A. Thomas, Chem. Mater., 2010, 22, 428–434 CrossRef CAS.
- Y. Huang, F. Yang, Z. Xu and J. Shen, J. Colloid Interface Sci., 2011, 363, 193–198 CrossRef CAS PubMed.
- M. X. Tan, Y. Zhang and J. Y. Ying, ChemSusChem, 2013, 6, 1186–1190 CrossRef CAS PubMed.
- M. X. Tan, L. Gu, N. Li, J. Y. Ying and Y. Zhang, Green Chem., 2013, 15, 1127–1132 RSC.
- D. Yang, P. Liu, N. Zhang, W. Wei, M. Yue, J. You and H. Wang, ChemCatChem, 2014, 6, 3434–3439 CrossRef CAS.
- M. Kruk and M. Jaroniec, Chem. Mater., 2003, 15, 2942–2949 CrossRef CAS.
- A. Derylo-Marczewska, J. Goworek, R. Kusak and W. Zgrajka, Appl. Surf. Sci., 2002, 195, 117–125 CrossRef CAS.
- G. Coullerez, D. Léonard, S. Lundmark and H. J. Mathieu, Surf. Interface Anal., 2000, 29, 431–443 CrossRef CAS.
- R. J. Meier, J. Phys. Chem., 1995, 99, 5457–5464 CrossRef CAS.
- Y.-X. Wang, J. Yang, S.-L. Chou, H. K. Liu, W.-X. Zhang, D. Zhao and S. X. Dou, Nat. Commun., 2015, 6, 8689–8690 CrossRef CAS PubMed.
- Z. Lv, Q. Sun, X. Meng and F.-S. Xiao, J. Mater. Chem. A, 2013, 1, 8630–8635 CAS.
- D. Schwarz and J. Weber, Macromol. Mater. Eng., 2015, 300, 531–541 CrossRef CAS.
- C. Goel, H. Bhunia and P. K. Bajpai, J. Environ. Sci., 2015, 238–248 CrossRef PubMed.
- J. A. Dunne, M. Rao, S. Sircar, R. J. Gorte and A. L. Myers, Langmuir, 1996, 12, 5896–5904 CrossRef CAS.
- D. W. Breck, W. G. Eversole, R. M. Milton, T. B. Reed and T. L. Thomas, J. Am. Chem. Soc., 1956, 78, 5963–5971 CrossRef CAS.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS PubMed.
- S. Sircar, T. C. Golden and M. B. Rao, Carbon, 1996, 34, 1–12 CrossRef CAS.
- X. Zhu, C. Tian, S. M. Mahurin, S.-H. Chai, C. Wang, S. Brown, G. M. Veith, H. Luo and S. Dai, J. Am. Chem. Soc., 2012, 134, 10478–10484 CrossRef CAS PubMed.
- J. H. Lee, H. J. Lee, S. Y. Lim, B. G. Kim and J. W. Choi, J. Am. Chem. Soc., 2015, 137, 7210–7216 CrossRef CAS PubMed.
- Z. S. Zhang, J. Zhou, W. Xing, Q. Z. Xue, Z. F. Yan and S. P. Zhuo, Phys. Chem. Chem. Phys., 2013, 17, 2523–2529 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra09508a |
| This journal is © The Royal Society of Chemistry 2016 |