Identifying the preferred interaction mode of naringin with gold nanoparticles through experimental, DFT and TDDFT techniques: insights into their sensing and biological applications†
Abstract
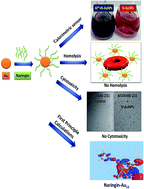
In this work, the binding behaviour of naringin – a flavonoid with AuNPs is explained by combining experimental and theoretical approaches. We have systematically analysed the effect of temperature and concentration of naringin and gold (Au) in the formation of naringin stabilized Au nanoparticles (N-AuNPs). The interaction of naringin with gold nanoparticles (AuNPs) is investigated by various techniques such as UV-visible spectroscopy, TEM, FT-IR, XRD and gel electrophoresis. These studies indicate that naringin acts as a reducing and stabilizing agent. Further, we have modelled the two side chains of naringin with the functional groups [C10H7O2] and [C6H5O]−, and identified the lowest energy configurations of these groups with AuNPs with the help of density functional theory (DFT). The [C10H7O2]–Au13 has higher binding energy than [C6H5O]−–Au13 and it is attributed to delocalized molecular orbitals in [C10H7O2], hence higher charge transfer to the Au13 cluster. On the basis of the resulting structures, we examine the optical properties using time-dependent density functional theory (TDDFT). We observe significant changes in the optical spectra of the representative structures of side chains with the AuNPs. The peak in the spectra of the Vis region of [C10H7O2]–Au13 undergoes a shift towards lower wavelength in comparison to [C6H5O]−–Au13. Natural transition orbitals (NTOs) of hole and particle states of the [C10H7O2]–Au13 conjugate system are localized on [C10H7O2] and Au13, respectively, whereas for the [C6H5O]−–Au13 both hole and particle states are localized on the Au13 cluster. These N-AuNPs show their applicability as a sensor for detecting aluminium ions (Al3+) in aqueous solution. These NPs are also found to be biocompatible with normal red blood cells and MDAMB-231 breast carcinoma cell lines, as evaluated from hemolysis and cytotoxicity assays. Thus, naringin offers non-toxic and bio friendly N-AuNPs, which are considered to be the best vehicle for drug release and other possible biomedical and sensing applications.

 Please wait while we load your content...
Please wait while we load your content...