UV-light aided photoelectrochemical synthesis of Au/TiO2 NTs for photoelectrocatalytic degradation of HPAM†
Di Gu,
Yang Wang,
Zhida Li,
Yue Liu,
Baohui Wang and
Hongjun Wu*
Provincial Key Laboratory of Oil & Gas Chemical Technology, College of Chemistry & Chemical Engineering, Northeast Petroleum University, Daqing 163318, China. E-mail: hjwu@nepu.edu.cn
First published on 30th June 2016
Abstract
TiO2 nanotube arrays (TiO2 NTs) loaded with Au nanoparticles were fabricated as an electrode for enhanced photoelectrocatalytic activity toward partially hydrolyzed polyacrylamide (HPAM) degradation. The honeycombed TiO2 NTs were prepared by a two-step anodization method and modified by Au nanoparticles via an UV-light aided photoelectrochemical process. The photoelectrocatalytic (PEC) activities of TiO2 NTs and Au/TiO2 NTs were characterized by decomposition of HPAM. The results showed that Au/TiO2 NTs exhibit much higher PEC activity than that of pristine TiO2 NTs. The size and amount of Au nanoparticles can be well controlled by adjusting the concentrations of metal ion precursor in the photoelectrochemical process. Structures, element components and morphologies of TiO2 NTs and Au/TiO2 NTs were measured by XRD, XPS, EDS and FESEM. Photoresponse of the as prepared samples were evaluated by UV-vis DRS. The UV-light aided photoelectrochemical synthesis of Au/TiO2 NTs contributes to the rational design of the plasmonic photocatalytic composite material based on wide band gap metal oxides for photoelectrochemical applications on degradation of polymers. As a consequence, an optimum loading amount of Au were obtained with weight percentage of 1.24 wt%.
Introduction
Vertically aligned TiO2 nanotube arrays (TiO2 NTs) as a nanostructure of TiO2 materials are expected to be a promising photocatalyst with unique properties such as large internal surface area, uniformly stable structure, strong adsorption capacity and convenient conduction channel.1,2 To date, numerous strategies have been adopted to enhance photoelectrocatalytic (PEC) activity of TiO2, such as surface modification with noble metal nanoparticles,3 transition metal cations doping,4 nonmetal anions doping,5 dye-sensitizing6 and coupling with narrow band gap semiconductor materials.7The method of noble metal nanoparticles deposition is much more attractive due to its excellent chemical stability and easy fabrication.8–10 Notably, deposited noble metal11,12 nanoparticles could form an extrinsic energy level in the band gap of TiO2, which extends the spectrum of TiO2 photo response. Then the lower energy of Fermi levels of a noble metal nanoparticle can induce the transfer of photoexcited electrons from TiO2 to the metal particles, thus significantly restrain the electron–hole recombination and lead to efficient charge separation followed by remarkably enhanced photocatalytic performance.13 Moreover, several noble metal9,14 nanoparticles may yield a surface plasmon resonance effect under visible light excitation. Au nanoparticles modified14–16 TiO2 NTs (Au/TiO2 NTs) were found to possess excellent activities for photocatalytic applications.
Thus far, a wealth of conventional approaches have been well documented that noble metals could be decorated on TiO2 surface effectively, including sol–gel,17 dipping-calcination,18 photo-assisted deposition,19 electrodeposition20 and deposition precipitation methods.21 Whereas, to date no satisfactory methods have been developed to uniformly decorate nanoparticles within high aspect ratio nanotubular structures. Photo-assisted deposition of metal nanoparticles cannot be applied here because of the short penetration depth of UV light in TiO2.19 Electrodeposition is not suitable for depositing metal particles inside high aspect ratio porous structures due to its preferred nucleation and growth of nanoparticles at the pore mouth.22 Colloid methods will never be of practical use for photocatalysis because of the long organic ligands inhibit the catalytic activity of the nanoparticle surfaces making them difficult to be completely removed.17 For another widely known technique, impregnation allows poor control over the particle size, dispersion and composition.23
In the study, an attempt was made to deposited Au nanoparticles on TiO2 NTs by a UV-light aided photoelectrochemical method. The distribution of metal nanoparticles could be well tuned by adjusting the doping concentrations. The PEC degradation of partially hydrolyzed polyacrylamide (HPAM) was also investigated to estimate the PEC activity of Au/TiO2 NTs. HPAM itself has no toxicity, however, it has been reported to be easily broken down by physical-chemical factors and its intermediate products are hazardous as their monomer is highly toxic.24 The degradation of acrylamide monomer has cumulative neurotoxicity, and it is identified as a genotoxic carcinogen.25 For the treatment of HPAM, many advanced technologies have been proposed by focusing on bio-degradation,26 thermal degradation,27 ultrasonic degradation,28 chemical oxidative degradation.25,29,30 However, most of them are either expensive or ineffective with a requirement of strict reaction conditions. Therefore, seeking out an effective way to deal with HPAM wastewater becomes urgent and important.
Experimental
Materials and methods
A 2 mm thick titanium sheet (99.6%, Strem Chemicals) was cut into pieces of 20 × 10 mm2. Ethylene glycol (EG, ≥99.5%), ammonia fluoride (NH4F, AR), chloroauric acid (HAuCl4·3H2O, 99.9%) and HPAM were purchased from Acros Organics and used as received. Deionized (DI) water with a resistivity of 18.2 MΩ cm prepared by the Millipore system was used throughout the study.Preparation of TiO2 NTs
The TiO2 NTs were prepared by a two-step electrochemical anodization process.31,32 The anodization was carried out using a conventional two-electrode system with the Ti foil as an anode and a Pt mesh (Aldrich, 100 mesh, 1 × 1 cm2) as a cathode in ethylene glycol solution (0.3 wt% NH4F and 2 vol% water, the concentration of NH4F used in this study was lower than in our previously work (0.50 wt%)19 because it was found that the concentration of NH4F was showed no significant difference in photocatalytic activity between 0.3 and 0.5 wt%, we choose 0.3 wt% of NH4F in economic terms) at room temperature. The Ti foil was anodized at 50 V for 30 min in the first-step to form 1-step TiO2 NTs, and then the as-grown nanotube layer was ultrasonically removed in DI water. The same Ti foil then underwent the second anodization at 20 V for 30 min. After the two-step anodization, the prepared TiO2 NTs were thoroughly cleaned with DI water and dried in N2 gas. The as-anodized TiO2 NTs samples were crystallized by annealing in air at 450 °C for 2 h with a heating rate of 5 °C min−1 to form 2-step TiO2 NTs (unless otherwise specified, the following TiO2 NTs are 2-step TiO2 NTs).Decoration of Au on TiO2 NTs
Au/TiO2 NTs was prepared by photoelectron-deposition method using HAuCl4·3H2O as gold resource. The photoelectron-deposition procedure was conducted using an electrochemical workstation in a home-made quartz reactor of a three-electrode system. It can be prepared as follows: the crystallized TiO2 NTs with active area of 1 cm2 served as the working electrode, a Pt sheet as the counter electrode and a saturated Ag/AgCl as a reference electrode; all the electrodes were soaked into HAuCl4·3H2O solution; a 300 W high pressure mercury lamp emitting at a wavelength of 365 nm served as UV-light source; distance between the light source and the photoanode was fixed at 10 cm; the decoration process run about 3 min with a doping voltage of −3 V at room temperature (ESI, Fig. S1 and 2†). Then, the as prepared Au/TiO2 NTs were carefully washed with DI water and dried in air.Characterization
The sample morphologies and structures were observed by field-emission scanning electron microscope (FESEM, Zeiss Sigma). The elemental composition of samples were evaluated with electronic differential system (EDS) attached to the FESEM. The crystalline structure of the samples was analyzed by grazing incidence X-ray diffraction (XRD) analysis on an X-ray diffractometer (Rigaku D/MAX 2200) using Cu Ka source (λ = 0.15406 nm) with 40 kV and 30 mA. The elemental composition was calculated from photoelectron spectroscopy (XPS) which were collected by an Axis Ultra instrument (Kratos Analytical) under ultrahigh vacuum (<10−8 Torr) and using a monochromatic Al Kα X-ray source operating at 150 W. The survey and high-resolution spectra were collected at fixed analyzer pass energies of 160 eV and 20 eV, respectively. Binding energies were referenced to the C 1s binding energy of adventitious carbon contamination which was set at 284.8 eV. Light absorption properties were obtained by UV-vis diffuse reflectance spectra (DRS) (UV-2550, Shimadzu, Japan) and BaSO4 was used as a reflectance standard.Photocurrent and PEC measurements
The photocurrent and PEC measurements were also carried out in the same system mentioned at part 2.3. Degradation rates of HPAM were used to compare the PEC activity of the prepared samples. In the PEC experiments, 0.025 M Na2SO4 solution was added as the supporting electrolyte to the 20 mL HPAM solution with an initial concentration of 50 mg L−1. Prior to the irradiation, 1 h premixing of catalysts and HPAM solution for absorption–desorption equilibrium was essential. The solution was continuously stirred during the whole PEC process. After UV-light irradiation with a 1.2 V bias potential for 3.5 h (ESI, Fig. S3†), concentrations of HPAM were monitored using a UV-visible spectrophotometer (UV-2550) at 585 nm through a starch–chromium iodinate method. HPLC analysis of degradation products was conducted using a Shimadzu LC-2010AHT Liquid Chromatograph that was equipped with a Spherisorb S5 ODS-2 column (5 μm, 150 mm × 4.6 mm). The mobile phase consisted of a water–methanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) mixture and the flow rate was 0.25 mL min−1. The wavelength of detection was 210 nm.
50) mixture and the flow rate was 0.25 mL min−1. The wavelength of detection was 210 nm.
Results and discussion
The morphology of the TiO2 NTs and Au/TiO2 NTs can be observed in Fig. 1. In case of the Au/TiO2 NTs, the photo assisted electrodeposition process used for the synthesis results in uniformly distributed and excellent monodispersed nanoparticles. Compared with the pristine TiO2 NTs in Fig. 1a, the tube mouth turns to be thick and is covered by a layer of Au nanoparticle clusters. It can be seen that Au nanoparticles aggregations with diameter about ∼26 nm randomly loaded on TiO2 NTs. The little aggregation states of Au nanoparticles can greatly enhance the active surface area of the substrate.33 Nevertheless, further nucleation and the amounts of noble metal nanoparticles are likely to narrow the pore of the top layer (Fig. 1f) and lead to a shield of active sites of the TiO2 NTs. | ||
| Fig. 1 SEM images of top views of (a) pristine TiO2 NTs; (b) 0.66 wt% Au/TiO2 NTs; (c) 0.92 wt% Au/TiO2 NTs; (d) 1.05 wt% Au/TiO2 NTs; (e) 1.24 wt% Au/TiO2 NTs; (f) 2.2 wt% Au/TiO2 NTs. | ||
Notably, both the loaded amount and the size of Au nanoparticles, which are extremely crucial to PEC activities, can be adjusted by varying the electrolyte concentration, deposition potential and deposition time. For a given deposition potential of −3 V and duration time of 180 s, the particle amount deposited increased with the prolonging electrolyte concentration for both Au nanoparticles. The XPS data in Table 1 further depicts the loading amounts of the elements from each sample respectively.
| Weight% | Concentration of HAuCl4 (g L−1) | ||||
|---|---|---|---|---|---|
| 0.005 | 0.01 | 0.015 | 0.02 | 0.025 | |
| Ti | 52.35 | 52.03 | 52.7 | 52.46 | 52.21 |
| O | 37.75 | 37.66 | 37.56 | 37.39 | 37.45 |
| Au | 0.66 | 0.92 | 1.05 | 1.24 | 2.2 |
| C | 9.24 | 9.39 | 9.12 | 8.93 | 9.14 |
XRD pattern and EDS spectra were used to characterize the Au/TiO2 NTs surfaces. Fig. 2 shows XRD patterns of three kinds of TiO2 NTs. The Au/TiO2 NTs samples used in results and discussion take 0.02 g L−1 HAuCl4 aqueous solution as the precursor for decorating TiO2 NTs. As shown in Fig. 2a, all diffraction peaks in the pattern could be indexed to the TiO2 anatase phase (JCPDS 21-1272) before modification. The peaks at 25.43°, 37.92°, 48.03°, 53.97° and 55.05°separately correspond to the (101), (004), (200), (105) and (211) crystal planes of anatase TiO2. The other diffraction peaks at about 38.34°, 40.2°, 53.1°, 70.8° and 75.8°, which are in good agreement with the Ti metal phase (JCPDS 44-1294), are assigned to the Ti substrate. No rutile phase was detected. Meanwhile, the major diffraction (101) planes of anatase TiO2 is broadened indicating high crystallinity.
For the Au/TiO2 NTs samples, three more peaks at 2 theta values of 38.21°, 44.32° and 63.8° represent the (111), (200) and (220) planes of the metallic Au phase (JCPDS 65-2870) in their XRD patterns (Fig. 3b), indicating the existence of metallic Au. Compared with the XRD spectra of the pristine TiO2 NTs (Fig. 2a), there was no obvious difference of anatase TiO2 phase in Au/TiO2 samples (Fig. 2b), indicating that deposition of noble metal nanoparticles had no impacts on the crystalline structure of TiO2 nanotubes. The XRD data further confirms the successful preparation of the Au/TiO2 NTs photocatalysts.
Fig. 3 shows a set of EDS spectra acquired for the Au-loaded and a reference anatase TiO2 NTs. The EDS spectrum clearly shows the Au peak as well as those of Ti and O on the Au/TiO2 NTs samples. The results confirm the presence of the elements of Au on the surface of TiO2 nanotubes, which is consistent with the observations by XRD (Fig. 2).
To further demonstrate the metallic state of Au nanoparticles, the high resolution X-ray photoelectron spectroscopy (XPS) technique was employed to analyze the surface elemental compositions of the Au/TiO2 NTs and acquire in-depth fundamental information on the interaction of metals with TiO2 NTs. Fig. 4a and S4 (ESI†) show the XPS pattern of Au/TiO2 NTs. In Fig. 4b and c, the sharp peaks assigned to Ti 2p3/2, Ti 2p1/2, O 1s at 458.2 eV, 464.0 eV, 530.2 eV are detected to confirm the main major elements of the sample. The peak of C 1s at 284.7 eV existing in the sample was also detected. The introduction of carbon may derive from three possible paths: the testing process of XPS, the atmosphere during calcinations or EG of electrolyte.
 | ||
| Fig. 4 (a) XPS spectra of Au/TiO2 NTs; (b–d) high-resolution Ti 2p, O 1s, Au 4f spectra of Au/TiO2 NTs. | ||
In addition to the peaks observed in TiO2 NTs, doublet peaks of Au 4f7/2 located at 83.47 eV and Au 4f5/2 at 87.07 eV (in Fig. 4d) indicate that the as-formed Au species exist in mainly zero oxidative state on the surface of TiO2 NTs, which is well supported by the XRD analysis. A significant negative shift (ca.0.6 eV) of the binding energy for Au 4f7/2 relative to 84.0 eV of the bulk Au is apparent, which can be attributed to the electron transfer from oxygen vacancies of the TiO2 to Au, leading to a lower Au 4f7/2 core level binding energy in the Au/TiO2 NTs.14
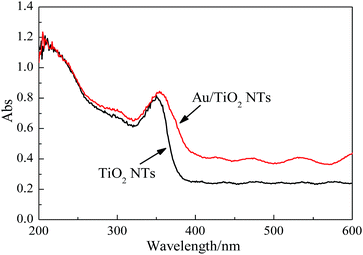
The optical absorption is a key factor for PEC performance of the materials. Fig. 5 depicted the UV-vis DRS spectra of the pristine TiO2 NTs and as-synthesized samples with 1.24 wt% gold loading. With regard to the two samples, excellent adsorptions in the UV region were observed corresponding to electrons transition from the valence band to the conduction band of TiO2. However the absorbance of the gold doped sample was significantly enhanced in comparison with the pristine TiO2 NTs and exhibited a red-shifted adsorption edge. Besides, the visible light absorption of Au/TiO2 NTs were also higher than those of the pristine TiO2 NTs, which show that the lower energy transitions are possible. The result shows compliance with what reported in the published literatures.34,35 All the enhanced absorptions and red-shifts mentioned above can be principally assigned to the consequence of the localized surface plasmon resonance (LSPR) of metallic nanoparticles when free conduction band electrons induced by the incident electromagnetic radiation oscillate collectively, basing on the relevant literatures.14,36 Thus, this further indicates the their intense photosensitivity to UV light for photoelectrocatalytic applications.
The photocurrent response of TiO2 NTs and Au/TiO2 NTs were investigated for further evaluation of the enhancing of PEC performance. It can been seen from Fig. 6 that the current measured on the two samples are all extremely low without UV illumination. The sharp increase and decrease of the currents caused by the periodical UV light irradiation are observed with good repeatability in all the cycles, which indicated good quality of optical response. The photocurrent density of Au/TiO2 NTs is ∼16 μA cm−2, which is about 3 times larger than that of the pristine TiO2 NTs and 1-step TiO2 NTs (ESI, Fig. S5†). The results demonstrate that Au can capture the photoinduced electrons of TiO2 NTs to facilitate the separation of the hole–electron pairs, thus significantly reinforce the photocurrent response of NTs. The reduced recombination of photogenerated charges in Au/TiO2 NTs would make them good photocatalysts for organic pollutant degradation.
The HPAM was selected as the target pollutant to evaluate the PEC characteristics of TiO2 NTs under UV light irradiation at room temperature. The degradation rates of HPAM aqueous solution during the PEC degradation reaction with different photocatalysts under different reaction systems were presented in Fig. 7a. Prior to the PEC measurements, photocatalytic (PC) and electrocatalytic (EC) processes for degrading HPAM in aqueous solution were performed on the pristine TiO2 NTs electrode under the same conditions. As shown in Fig. 7a, no obvious degradation of HPAM could be observed in absence of the TiO2 NTs (UV) or UV light irradiation (dark), confirming that the degradation reaction is really driven by a photocatalytic process. We also noticed that only 6.8% and 10.03% removal rates of HPAM were obtained during the 1-step TiO2 NTs and 2-step TiO2 NTs PC processes. Such low PC-induced activity could be attributed to the low quantum efficiency of the photoanodes. When the degradation of HPAM was carried out with extra field (a potential of 1.2 V) under dark condition (EC), the concentration of HPAM decreased a little more to 15%. Whereas as expected, the degradation rate of PEC process was twice higher up to 29.36%, which indicated that the presence of both extra electric field and illumination are necessary for the outstanding PC performance of TiO2 NTs. The excellent performance of PEC process could be attributed to the boosts in the separation of photogenerated electron–hole pairs and promotions in the quantum efficiency of TiO2 NTs from the assistance of the extra electric field.
 | ||
| Fig. 7 (a) Degradation rate of HPAM under different reaction systems; (b) PEC degradation of HPAM using Au/TiO2 NTs with different concentrations of HAuCl4. | ||
To further increase the photoelectroactivity of TiO2 NTs, different catalysts obtained by distinguishing concentrations of HAuCl4 are also tested on PEC degradation of HPAM solution. As shown in Fig. 7b, the PEC activity of Au/TiO2 NTs composites correlates with metal load, although all catalysts have similar degradation efficiencies after 3.5 h irradiation (ESI, Fig. S3†). It is demonstrated that the loading amount of noble metals is an important parameter affecting the PEC activities of Au/TiO2 NTs nanocomposites. With the increase of the loading amount of Au nanoparticles, the PEC activities of Au/TiO2 NTs increased firstly and then decreased due to the weaker ultraviolet light absorption and the less exposure of active sites of TiO2 NTs as well as the possible additional e−–h+ recombination centers derived from the excessive loading of the large Au nanoparticles. As a result, TiO2 NTs infiltrating in 0.02 g L−1 HAuCl4 solution, which corresponds to the loading weight percentage of 1.24 wt%, achieved the optimum catalytic performance with degradation rates of 43.84%. It is noticeable that the introduction of Au did increase the degradation efficiency of TiO2 NTs.
When noble metal nanoparticles are loaded on TiO2 nanotubes, the formation of locally Schottky junctions established by the Schottky barrier between metals and TiO2 would build a higher potential gradient than at the TiO2/electrolyte interface. Thus metal nanoparticles may play imperative roles as “electron reservoirs”16 where the photogenerated electrons be captured by them easily, and then the electron–hole pair separation lifetime is prolonged.
With the help of a small bias voltage between working electrode and counter electrode, the electrons would transfer along the TiO2 nanotube arrays to the Ti substrate and eventually reach the counter electrode through outer circuit. Subsequently, the transferred electrons would further reduce the oxygen absorbed on the surface of counter electrode to form superoxide anions (˙O2−). Following a series of reactions with H+, the activated ˙O2− further produces hydroxyl radicals (˙OH), which are responsible for the degradation of organic pollutants. As a result, a enhanced PEC activity is obtained. The probable pathway of HPAM degradation, according to HPLC analysis (ESI, Fig. S6–8†), is shown in eqn (1)–(8).
| TiO2 + hν → e− + h+ | (1) |
| h+ + OH− → ˙OH | (2) |
| h+ + H2O → ˙OH + H+ | (3) |
| O2 + e− → ˙O2− | (4) |
| Au + e− → Au− | (5) |
| Au− + O2 → ˙O2− | (6) |
| ˙O2− + H+ → ˙OOH → ˙OH | (7) |
 | (8) |
Conclusions
In summary, nanosized Au (∼26 nm) nanoparticles uniformly dispersed on the surface of TiO2 NTs by photoelectrochemical deposition using HAuCl4 solution as metal source. Compared with the pristine TiO2 NTs, various loading amounts of Au nanoparticles modified TiO2 NTs all exhibited excellent PEC activity for HPAM degradation under UV light irradiation, which can be attributed to the increased photoresponse both in UV and visible region and high electron–hole separation efficiency, resulting from the small bias potential as well as the synergy effects of TiO2 and noble metal nanoparticles. Corresponding to the most effective PEC system, the proper dosage of Au deposited on TiO2 NTs was found, which resulted in a loading weight percentage of 1.24 wt%.It is expected that our work provides an insight into designing highly efficient and environmentally friendly photocatalyst for practical application in the purification of organic pollutants. The proposed route is looking forward to be extended to the design of other bimetallic nanoparticles hybrid semiconductor-based nanotube electrodes, such as Ag–Pt/TiO2 NTs, Pt–Au/TiO2-NTs and Ag–Au/TiO2-NTs, for further PEC applications.
Acknowledgements
The authors are grateful to the partially supported by National Natural Science Foundation of China (No. 21306022 and No. 21476046), China Postdoctoral Science Foundation (No. 2013M540269), Postdoctoral Science Foundation of Heilongjiang Province of China (No. LBH-TZ0417) and Northeast Petroleum University Foundation (NEPUQN2015-1-06).Notes and references
- G. K. Mor, K. Shankar, M. Paulose, O. K. Varghese and C. A. Grimes, Nano Lett., 2006, 6, 215 CrossRef CAS PubMed.
- N. Liu, X. Chen, J. Zhang and J. W. Schwank, Catal. Today, 2014, 225, 34 CrossRef CAS.
- K. Lee, A. Mazare and P. Schmuki, Chem. Rev., 2014, 114, 9385 CrossRef CAS PubMed.
- S. Hashemian and A. Foroghimoqhadam, Chem. Eng. J., 2014, 235, 299 CrossRef CAS.
- J. H. Park, S. Kim and A. J. Bard, Nano Lett., 2006, 6, 24 CrossRef CAS PubMed.
- J. R. Jennings, A. Ghicov, L. M. Peter, P. Schmuki and A. B. Walker, J. Am. Chem. Soc., 2008, 130, 13364 CrossRef CAS PubMed.
- A. Kongkanand, K. Tvrdy, K. Takechi, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2008, 130, 4007 CrossRef CAS PubMed.
- C. Zhang, H. Yu, Y. Li, L. Fu, Y. Gao, W. Song, Z. Shao and B. Yi, Nanoscale, 2013, 5, 6834 RSC.
- F. Xiao, J. Mater. Chem., 2012, 22, 7819 RSC.
- T. Akita, M. Okumura, K. Tanaka, K. Ohkuma, M. Kohyama, T. Koyanagi, M. Date, S. Tsubota and M. Haruta, Surf. Interface Anal., 2005, 37, 265 CrossRef CAS.
- X. Li, G. Chen, L. Yang, Z. Jin and J. Liu, Adv. Funct. Mater., 2010, 20, 2815 CrossRef CAS.
- W. Fan, S. Jewell, Y. She and M. K. H. Leung, Phys. Chem. Chem. Phys., 2014, 16, 676 RSC.
- M. D. Ye, J. J. Gong, Y. K. Lai, C. J. Lin and Z. Q. Lin, J. Am. Chem. Soc., 2012, 134, 15720 CrossRef CAS PubMed.
- Z. Zhang, L. Zhang, M. N. Hedhili, H. Zhang and P. Wang, Nano Lett., 2013, 13, 14 CrossRef CAS PubMed.
- Z. W. Seh, S. Liu, S. Y. Zhang, M. S. Bharathi, H. Ramanarayan, M. Low, K. W. Shah, Y. W. Zhang and M. Y. Han, Angew. Chem., Int. Ed., 2011, 50, 10140 CrossRef CAS PubMed.
- W. Seh, S. Liu, M. Low, S. Y. Zhang, Z. Liu, A. Mlayah and M. Y. Han, Adv. Mater., 2012, 24, 2310 CrossRef PubMed.
- F. Xiao, J. Phys. Chem. C, 2012, 116, 16487 CAS.
- M. Wang, L. Sun, J. Cai, P. Huang, Y. Su and C. Lin, J. Mater. Chem. A, 2013, 1, 12082 CAS.
- D. Gu, H. Wu, Y. Zhu and B. Wang, RSC Adv., 2015, 5, 57937 RSC.
- L. Sun, J. Li, C. L. Wang, S. F. Li, H. B. Chen and C. J. Lin, Sol. Energy Mater. Sol. Cells, 2009, 93, 1875 CrossRef CAS.
- S. Tsubota, M. Haruta, T. Kobayashi, A. Ueda and Y. Nakahara, Stud. Surf. Sci. Catal., 1991, 63, 695 CrossRef CAS.
- B. Lei, J. Xue, D. Jin, S. Ni and H. Sun, Rare Met., 2008, 27, 445 CrossRef CAS.
- A. Furube, T. Asahi, H. Masnhara, H. Yamashita and M. Anpo, Chem. Phys. Lett., 2001, 336, 424 CrossRef CAS.
- Y. L. Luo, Z. H. Yang, Z. Y. Xu, L. J. Zhou, G. M. Zeng, J. Huang, Y. Xiao and L. K. Wang, J. Hazard. Mater., 2011, 189, 69 CrossRef CAS PubMed.
- Y. Pi, Z. Zheng, M. Bao, Y. Li, Y. Zhou and G. Sang, Chem. Eng. J., 2015, 273, 1 CrossRef CAS.
- M. Bao, Q. Chen, Y. Li and G. Jiang, J. Hazard. Mater., 2010, 184, 105 CrossRef CAS PubMed.
- M. Yang, Polym. Test., 1998, 17, 191 CrossRef CAS.
- S. P. Vijayalakshmi and G. Madras, Polym. Degrad. Stab., 2004, 84, 341 CrossRef CAS.
- C. Cao, Y. Zhao and Y. Zhou, Int. J. Green Energy, 2016, 13, 80 CrossRef CAS.
- A. L. Prajapat and P. R. Gogate, Ultrason. Sonochem., 2016, 31, 371 CrossRef CAS PubMed.
- M. Paulose, K. Shankar, S. Yoriya, H. E. Prakasam, O. K. Varghese, G. K. Mor, T. A. Latempa, A. Fitzgerald and C. A. Grimes, J. Phys. Chem. B, 2006, 110, 16179 CrossRef CAS PubMed.
- G. K. Mor, O. K. Varghese, M. Paulose, K. Shankar and C. A. Grimes, Sol. Energy Mater. Sol. Cells, 2006, 90, 2011 CrossRef CAS.
- R. Zanella, S. Giorgio, C. H. Shin, C. R. Henry and C. Louis, J. Catal., 2004, 222, 357 CrossRef CAS.
- W. Sun, S. Q. Zhang, Z. X. Liu, C. Wang and Z. Q. Mao, Int. J. Hydrogen Energy, 2008, 33, 1112 CrossRef CAS.
- K. W. Park, S. B. Han and J. M. Lee, Electrochem. Commun., 2007, 9, 1578 CrossRef CAS.
- C. Y. Wang, C. Y. Liu, X. Zheng, J. Chen and T. Shen, Colloids Surf., A, 1998, 131, 271 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12031h |
| This journal is © The Royal Society of Chemistry 2016 |