Hierarchical γ-BaB2O4 hollow microspheres: surfactant-assisted hydrothermal formation, phase conversion, optical properties and application as adsorbents†
Xiuping
Chen
,
Peiyan
Zhai
,
Jie
Li
,
Heng
Zhang
* and
Wancheng
Zhu
*
Department of Chemical Engineering, Qufu Normal University, Shandong 273165, China. E-mail: zhuwancheng@tsinghua.org.cn; qsdhxy@126.com; Tel: +86-537-4453130
First published on 27th June 2016
Abstract
Three-dimensional (3D) hierarchical metal borate nanoarchitectures have attracted extensive interest for their wide use owing to their versatile compositions and unique properties, and their rational design and facile synthesis with high uniformity and complex interiors have long remained a great challenge. In this contribution, hierarchical γ-BaB2O4 hollow microspheres assembled from one-dimensional (1D) nanorods were successfully synthesized by a facile ethylenediaminetetraacetic acid disodium salt (EDTA-2Na) assisted hydrothermal method (180 °C, 12 h), using Ba(OH)2·8H2O and H3BO3 as the raw materials. The effects of process parameters were investigated in detail, based on which a feasible formation mechanism was proposed. The hierarchical γ-BaB2O4 hollow microspheres exhibited a specific surface area of 46.25 m2 g−1. The subsequent mild phase conversion (700 °C, 3.0 h) led to β-BaB2O4 hollow microspheres with a well-preserved spherical morphology and high crystallinity, indicating hierarchical γ-BaB2O4 and β-BaB2O4 hollow microspheres of excellent thermostability. Both the hierarchical γ-BaB2O4 and β-BaB2O4 hollow microspheres exhibited a transparent characteristic from the ultraviolet to the visible region. Moreover, when employed as adsorbents, the hierarchical γ-BaB2O4 hollow microspheres presented satisfactory adsorption properties for the removal of Pb2+ from mimic waste water, suggesting the great potential applications of the 3D hierarchical porous or hollow nanoarchitectures of metal borates in the future.
1. Introduction
Over the past few decades, numerous three-dimensional (3D) hierarchical nanoarchitectures such as hollow,1 core–shelled,2 urchin-like,3 hollow urchin-like,4 and flower-like5 microspheres with unique structures and remarkable properties have attracted extensive attention owing to their great potential in versatile fields. Although much effort has been made, it is still a challenge to prepare 3D hierarchical nanostructures with desirable nano-building blocks and morphologies as well as expected properties. Among the varieties of 3D hierarchical nanoarchitectures, metal borates have been paid extensive attention due to their versatile compositions and unique properties, and they have been widely employed as adsorbents,6 anti-wear additives,7 photoluminescence host materials,8 micro-/nanoscale nonlinear photonic devices,9–11 catalyst supports,12 etc. So far however, in addition to some more frequently encountered one-dimensional (1D) borate nanostructures, there have been just a few 3D hierarchical alkaline earth metal borates reported, including hierarchical porous MgBO2(OH) superstructures,6 hierarchical Ba2(B5O9)Cl·(H2O)0.5 microspheres,8 flower-like Mg3B2O6,13 hierarchical porous Ca(BO2)2 microspheres,12 hierarchical laminar rhombic priceite (Ca4B10O19·7H2O) superstructures,14 and flower-like and urchin-like Mg3B2O6:Eu3+.15 Thus, much more work should be dedicated to the challenging controllable fabrication of 3D metal borate nanostructures with high uniformity and complex interiors through facile synthetic routes.As is known, inorganic nonlinear optical crystals have been serving as critical materials for fast response and optical fiber communications in modern telecommunications, among which barium borate (β-BaB2O4) has been recognized as one of the most important nonlinear optical (NLO) materials owing to its characteristic properties. For example, β-BaB2O4 exhibited wide optical transmission from 189 nm to 3500 nm, a large effective second-harmonic-generation (SHG) coefficient (d22 = ±2.3 pm V−1, d31 = ±0.16 pm V−1), and a high damage threshold of 10 GW cm−2 for 0.1 ns pulse-width at 1064 nm, as well as excellent mechanical performances.16 Also, β-BaB2O4 nanoparticles (NPs) could be employed as potential candidates for optical limiting applications in continuous wave (cw) and pulsed laser regimes due to their high linear transmittance and high laser damage threshold. To date, however, only a few attempts to prepare β-BaB2O4 nanostructures have been reported. Qu et al. reported an organic-free hydrothermal synthesis for optical quality single-crystal β-BaB2O4 microwires and nanowires.10 Liu et al. carried out controllable synthesis of photoluminescent europium-doped β-BaB2O4 nanorods, nanowires, and flower-like assemblies.17 Li et al. synthesized β-BaB2O4 nanospindles through heat treatment of Ba3B6O9(OH)6 nanorods at 810 °C for 3.0 h.18 Zhang et al. fabricated single-crystalline β-BaB2O4 nanorods by a hexadecyl trimethyl ammonium bromide (CTAB) assisted hydrothermal method, followed by a subsequent annealing.19 Taking the important applications and great potential of 3D nanoarchitectures into consideration, it is highly desirable to develop a rational, facile, and controllable route to 3D hollow BaB2O4 nanoarchitectures.
Herein, to the best of our knowledge, we reported for the first time the successful synthesis of uniform hierarchical γ-BaB2O4 hollow microspheres via a facile surfactant-assisted hydrothermal route, and also high crystallinity β-BaB2O4 hollow microspheres with well-preserved spherical morphology via subsequent mild phase conversion. The effects of process parameters, such as reactant concentration, surfactant, temperature and time, on the hydrothermal products as well as the EDTA-2Na assisted formation mechanism were investigated in detail. In addition, the phase conversion, optical properties and potential application of the as-synthesized hierarchical γ-BaB2O4 hollow microspheres as adsorbents for heavy metal Pb2+ ion removal were also presented. The results indicated that the surfactant and Ostwald ripening mechanism played the key role in the hydrothermal self-assembly for the hierarchical γ-BaB2O4 hollow microspheres, which exhibited a specific surface area of 46.25 m2 g−1. The mild phase conversion led to high crystallinity β-BaB2O4 hollow microspheres, and both the hierarchical γ-BaB2O4 and β-BaB2O4 hollow microspheres revealed unique optical properties. In addition, to explore the novel properties and potential applications of the hierarchical hollow barium borate nanoarchitectures in versatile fields, we have focused on and reported for the first time (as far as we know) the feasibility of using the as-obtained uniform γ-BaB2O4 hollow microspheres as an adsorbent. As confirmed, the hierarchical γ-BaB2O4 hollow microspheres exhibited a relatively satisfactory performance as the adsorbent for the removal of Pb2+ ions from mimic waste water.
2. Experimental
2.1 Synthesis of the hierarchical γ-BaB2O4 hollow microspheres
All reagents were of analytical grade and used directly without further purification. In a typical procedure, 3.150 g of Ba(OH)2·8H2O was dissolved in 20.0 mL of deionized (DI) water, and 1.237 g of H3BO3 (molar ratio: Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B = 1
B = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was dissolved in 30.0 mL of DI water. Then, 0.930 g of EDTA-2Na powder and the H3BO3 solution were sequentially added to the Ba(OH)2 solution under vigorous magnetic stirring at room temperature. Having been stirred for 15 min, the resultant slurry was transferred into a Teflon-lined stainless steel autoclave with a capacity of 63.0 mL. Then the autoclave was sealed, heated to 180 °C (heating rate: 5 °C min−1) and kept in an isothermal state for 12.0 h. After the hydrothermal synthesis, the autoclave was cooled down to room temperature naturally. The as-obtained precipitate was washed with DI water and ethanol three times, then filtered, and finally dried at 70 °C for 12.0 h. To evaluate the effects of the process parameters on the hydrothermal products, the reactant concentration, amount of EDTA-2Na, temperature, and time were tuned within the range of 0.05–0.40 mol L−1, 0–0.10 mol L−1, 120–210 °C and 1.0–18.0 h, respectively, with other conditions unchanged.
2) was dissolved in 30.0 mL of DI water. Then, 0.930 g of EDTA-2Na powder and the H3BO3 solution were sequentially added to the Ba(OH)2 solution under vigorous magnetic stirring at room temperature. Having been stirred for 15 min, the resultant slurry was transferred into a Teflon-lined stainless steel autoclave with a capacity of 63.0 mL. Then the autoclave was sealed, heated to 180 °C (heating rate: 5 °C min−1) and kept in an isothermal state for 12.0 h. After the hydrothermal synthesis, the autoclave was cooled down to room temperature naturally. The as-obtained precipitate was washed with DI water and ethanol three times, then filtered, and finally dried at 70 °C for 12.0 h. To evaluate the effects of the process parameters on the hydrothermal products, the reactant concentration, amount of EDTA-2Na, temperature, and time were tuned within the range of 0.05–0.40 mol L−1, 0–0.10 mol L−1, 120–210 °C and 1.0–18.0 h, respectively, with other conditions unchanged.
2.2 Phase conversion of γ-BaB2O4 hollow microspheres
The as-synthesized γ-BaB2O4 powder was transferred to a porcelain boat located in a horizontal quartz tube furnace, which was then heated to 500–700 °C (heating rate: 2.5 °C min−1) and kept in an isothermal state for 3.0 h. After calcination, the product was cooled down to room temperature naturally within the tube furnace. Then, the as-obtained product was washed with DI water and ethanol three times, then filtered, and ultimately dried at 70 °C for 12.0 h.2.3 Hierarchical γ-BaB2O4 hollow microspheres as an adsorbent for Pb2+ removal
The as-obtained hierarchical γ-BaB2O4 hollow microspheres were evaluated for the removal of Pb2+ ions from mimic waste water. In a typical procedure, 20 mg of the γ-BaB2O4 hollow microspheres was mixed with 50 mL of Pb2+-containing solution (concentration: 50.0 mg L−1) under magnetic stirring at room temperature, with the stirring time ranging from 5.0 to 300.0 min. After the adsorption systems had been stirred for their designated times, the resultant slurries were filtered individually, leading to a series of transparent filtrates. The amount of Pb2+ absorbed onto the adsorbent was calculated using eqn (1):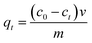 | (1) |
2.4 Characterization
The crystal structures of the samples were identified using an X-ray powder diffractometer (XRD, MiniFlex600, Rigaku, Japan) with Cu-Kα radiation (λ = 1.5406 Å) and a fixed power source (30 kV, 10.0 mA). The morphology and microstructure of the samples were examined using field emission scanning electron microscopy (SEM, JSM 6700F, JEOL, Japan, at 5.0 kV), and high resolution transmission electron microscopy (TEM, JEM-2010, JEOL, Japan, at 100.0 kV). The size distribution of the microspheres was estimated by directly measuring ca. 100 particles from the typical SEM images. N2 adsorption–desorption isotherms were measured at 77 K using a chemisorption–physisorption analyzer (Autosorb-IQ2-MP, Quantachrome, USA) after the samples had been degassed at 300 °C for 1.0 h. The specific surface area was calculated from the adsorption branches in the relative pressure range of 0.05–0.30 using the multipoint Brunauer–Emmett–Teller (BET) method, and the pore size distribution was evaluated from the N2 desorption isotherm using the Barrett–Joyner–Halenda (BJH) model. The chemical bonds in the molecules of the hydrothermal product were determined by Fourier transform infrared spectroscopy (FT-IR, Nexus 470, Nicolet, USA). The optical properties were determined using a UV-vis spectrophotometer (UV-756 CRT, Shanghai Yoke Instrument and Meter Co., LTD, China). The actual residual amount of Pb2+ ions within the filtrate was determined by inductively coupled plasma analysis (ICP, Optima 4300DV Spectrometer, PerkinElmer instruments, USA).3. Results and discussion
3.1 Structure and morphology characterization of the hierarchical γ-BaB2O4 hollow microspheres
The structure and morphology related information on the hydrothermal product obtained by heating at 180 °C for 12.0 h, with a molar ratio of Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B of 1
B of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and reactant concentration of Ba(OH)2 of 0.20 mol L−1, is shown in Fig. 1. Most of the diffraction peaks of the XRD pattern coincide well with those of γ-BaB2O4 (JCPDS no. 35-0181) (Fig. 1(a)), with sporadic peaks of low intensity located at 2θ = 13.90° and 41.32° which failed to be indexed. Those few detected diffraction peaks for the present samples were also found to be distinctly present for γ-BaB2O4 micro-/nanowires.22 The SEM characterization showed that the as-obtained product particles exhibited a uniform microsphere morphology (Fig. 1(b)). Interestingly, the high magnification SEM image indicated typical hydrothermally synthesized γ-BaB2O4 microspheres of hollow structure. The hollow microspheres consisted of 1D nanorods, with a shell thickness of ca. 2.15 µm (Fig. 1(b1)). Meanwhile, the statistical data revealed that ca. 73% of the as-synthesized uniform microspheres had a size ranging from 12.0–18.0 µm, and the average size of the microspheres was 14.7 µm (Fig. 1(b2)). The TEM image showed that the constituent nanorods derived from the microspheres, owing to the ultrasonic dispersion during the TEM sample preparation, exhibited a distinct, straight rod-like shape with a smooth surface (Fig. 1(c)), a length of 56–651 nm and a diameter of 14–40 nm. The HRTEM image (Fig. 1(e)) recorded from the red dashed rectangular region in Fig. 1(d) demonstrated explicit lattice fringes with an interplanar spacing of 0.485 nm (Fig. 1(e)), which was somewhat similar to the standard value of the crystal planes (0.452 nm) corresponding to the diffraction peak located at 2θ = 19.62°. This confirmed the presence of as-obtained γ-BaB2O4 hollow microspheres of relatively high crystallinity.
2 and reactant concentration of Ba(OH)2 of 0.20 mol L−1, is shown in Fig. 1. Most of the diffraction peaks of the XRD pattern coincide well with those of γ-BaB2O4 (JCPDS no. 35-0181) (Fig. 1(a)), with sporadic peaks of low intensity located at 2θ = 13.90° and 41.32° which failed to be indexed. Those few detected diffraction peaks for the present samples were also found to be distinctly present for γ-BaB2O4 micro-/nanowires.22 The SEM characterization showed that the as-obtained product particles exhibited a uniform microsphere morphology (Fig. 1(b)). Interestingly, the high magnification SEM image indicated typical hydrothermally synthesized γ-BaB2O4 microspheres of hollow structure. The hollow microspheres consisted of 1D nanorods, with a shell thickness of ca. 2.15 µm (Fig. 1(b1)). Meanwhile, the statistical data revealed that ca. 73% of the as-synthesized uniform microspheres had a size ranging from 12.0–18.0 µm, and the average size of the microspheres was 14.7 µm (Fig. 1(b2)). The TEM image showed that the constituent nanorods derived from the microspheres, owing to the ultrasonic dispersion during the TEM sample preparation, exhibited a distinct, straight rod-like shape with a smooth surface (Fig. 1(c)), a length of 56–651 nm and a diameter of 14–40 nm. The HRTEM image (Fig. 1(e)) recorded from the red dashed rectangular region in Fig. 1(d) demonstrated explicit lattice fringes with an interplanar spacing of 0.485 nm (Fig. 1(e)), which was somewhat similar to the standard value of the crystal planes (0.452 nm) corresponding to the diffraction peak located at 2θ = 19.62°. This confirmed the presence of as-obtained γ-BaB2O4 hollow microspheres of relatively high crystallinity.
3.2 Effects of temperature and time on the hydrothermal products
To understand the hydrothermal growth of the hierarchical γ-BaB2O4 hollow microspheres, temperature- and time-dependent experiments were carried out. As shown in Fig. S1,† the reaction temperature had great influence on the compositions and the morphologies of the products. The crystallinity of the hydrothermal products got higher with the increase in the temperature from 120 °C to 210 °C (Fig. S1(a1)–(a3)† and 1(a1)). When hydrothermally treated at 120 °C, only low crystallinity or almost amorphous (Fig. S1(a1)†) irregular NPs were obtained (Fig. S1(b)†). When treated at 150 °C to 210 °C, the products were a pure phase of γ-BaB2O4 (JCPDS no. 35-0181) (Fig. S1(a2) and (a3)† and 1(a1)). Apparently, with the temperature going up to 150 °C, γ-BaB2O4 hollow microspheres emerged (Fig. S1(c)†). The hollow microspheres consisted of short 1D nanostructured subunits (Fig. S1(c1)†), but with a wide diameter distribution within the range of 12.0–30.0 µm (average diameter: 21.0 µm). As mentioned above, when treated at 180 °C, the as-obtained γ-BaB2O4 hollow microspheres exhibited relatively uniform morphology and ca. 73% of them had a diameter within the range of 12–18 µm (Fig. 1(b) and (b2)). With the temperature going up further to 210 °C, the products were composed of γ-BaB2O4 hollow microspheres (diameter: 10.0–20.0 µm) and assembled microrods (Fig. S1(d) and (d1)†).With the hydrothermal treatment time increased from 1.0 h to 3.0 h, 12.0 h, and 18.0 h, the majority of the hydrothermal products synthesised at 180 °C were all confirmed as γ-BaB2O4, with the crystallinity getting higher and higher (Fig. S2(a)†). Meanwhile, the product particles almost all emerged as microspheres and the average diameter of the microspheres tended to be larger with the elongation of the time (Fig. S2(b)–(d)†). Also, as shown, even when treated at 180 °C for 1.0 h, γ-BaB2O4 microspheres were formed (Fig. S2(b)†). When treated for a longer time, more distinct microspheres with hollow structures appeared (Fig. S2(c)† and 1(b)). When treated for an excessive longer time such as 18.0 h, however, the occurrence of some discrete NPs was observed (Fig. S2(d)†). With the aim of uniform hollow γ-BaB2O4 microspheres, the optimum hydrothermal time was fixed at 12.0 h.
3.3 Effect of reactant concentration on the hydrothermal products
To investigate the effect of reactant concentration on the composition and morphology of the hydrothermal products, the concentration of Ba(OH)2 was tuned within the range of 0.05–0.40 mol L−1, with other conditions kept the same. As shown in Fig. 2(a1)–(a3), all the products coincided well with γ-BaB2O4. Apparently, with the increase in the concentration from 0.05–0.40 mol L−1, the crystallinity of the product became lower first and then somehow higher. Significantly, when the reactant concentration was 0.05 mol L−1, the product was confirmed as γ-BaB2O4 urchin-like microspheres, which consisted of radially-grown 1D nanorods (Fig. 2(b)) with a length of ca. 20 µm and a smooth surface (Fig. 2(b1)). With the concentration increased to 0.10 mol L−1, the product turned to be γ-BaB2O4 microspheres whereas with non-uniform diameters (Fig. 2(c)), which were constituted of short nanorods-like subunits (Fig. 2(c1)). With the concentration increased to 0.20 mol L−1, uniform hollow microspheres were obtained, as previously shown in Fig. 1(b). Nevertheless, when the concentration was further doubled to 0.40 mol L−1, the product became almost discrete irregular nanorods (Fig. 2(d) and (d1)). As reported, a relatively high degree of supersaturation originating from the high reactant concentration could favor the 3D growth or self-assembly of the microspheres.20 In the present case, the optimum concentration of Ba(OH)2 for the formation of γ-BaB2O4 hollow microspheres was confirmed as 0.20 mol L−1, as demonstrated above in Fig. 1.3.4 Effect of EDTA-2Na on the hydrothermal products
To explore the effect of EDTA-2Na on the composition and morphology of the hydrothermal products, the concentration of EDTA-2Na was tuned within the range of 0.00–0.10 mol L−1, with other conditions remaining unchanged. As shown in Fig. 3(a1), the hydrothermal product synthesized in the absence of EDTA-2Na was the pure phase of monoclinic Ba3B6O9(OH)6 (JCPDS no. 01-071-2501)18 with nano-/microrods or plate-like morphology (Fig. 3(b)). With the concentration of EDTA-2Na changed from 0.025 mol L−1 to 0.038 mol L−1 and 0.050 mol L−1, all the hydrothermal products consisted of the pure phase of γ-BaB2O4 and the crystallinity of the products did not change much (Fig. 3(a2) and (a3) and 1(a)).However, a distinct change in the product morphology emerged with a slight variation of the concentration of EDTA-2Na. When the concentration was 0.025 mol L−1, the product was detected as microrods and irregular NPs (Fig. 3(c)). With the concentration increased to 0.038 mol L−1, distinct self-assembled γ-BaB2O4 hollow microspheres and nonuniform assemblies appeared (Fig. 3(d) and (d1)). When the concentration was increased to 0.050 mol L−1, uniform γ-BaB2O4 microspheres were obtained as shown previously (Fig. 1). Nevertheless, with the concentration of EDTA-2Na further increased to 0.10 mol L−1, the composition of the product failed to be indexed at present (Fig. 3(a4)). The product contained bulky blocks and also distinct, bigger, compact microspheres constructed from NPs (Fig. 3(e)). Thus, the concentration of the surfactant EDTA-2Na played a key role in the hydrothermal formation of the uniform γ-BaB2O4 microspheres, and the optimal concentration was as 0.050 mol L−1.
3.5 Shape evolution of the hydrothermal product
Since the transformation of hierarchical γ-BaB2O4 hollow microspheres at 180 °C was too fast, a series of time dependent experiments were carried out at 150 °C so as to better understand the hydrothermal growth of the unique γ-BaB2O4 hollow microspheres. As shown, the room-temperature coprecipitate was almost amorphous NPs (Fig. 4(a1) and (b)), and the hydrothermal product grown at 150 °C for 120 min was irregular NPs with very low crystallinity (Fig. 4(a2) and (c)). Taking the XRD patterns of the room-temperature coprecipitated NPs (Fig. 4(a1)), reactants (Fig. S3(a)–(c)†) and EDTA–Ba (complex) (Fig. S3(d)†) into consideration, it was obvious that the phase transformation of γ-BaB2O4 had gradually taken place, and the hydrothermal treatment led to somehow bigger NPs. With the time extended to 130 min, some relatively loose microspheres with a diameter of ca. 25.0 µm emerged, which were obviously self-assembled from multitudes of tiny NPs (Fig. 4(a3), (d) and (d1)). As the time was prolonged to 150 min, relatively high crystallinity and distinct compact γ-BaB2O4 microspheres and hemispheres were formed, with an average size of ca. 24.5 µm (Fig. 4(a4), (e) and (e3)). Simultaneously, some slender nanorods appeared on the surfaces of the γ-BaB2O4 spheres (Fig. 4(e1) and (e2)). When the hydrothermal time was further prolonged to 180 min, distinct γ-BaB2O4 hollow microspheres were obtained (Fig. 4(a5) and (f)). Apparently, the γ-BaB2O4 hollow structures were formed and the rod-like subunits became thicker and shorter (Fig. 4(f1) and (f2)), and the microspheres exhibited an average diameter of ca. 21.6 µm (Fig. 4(f3)), somehow larger than those uniform γ-BaB2O4 hollow microspheres acquired at 150 °C for 720 min (Fig. S1(c) and (c2)†) with a wide size distribution within the range of 12.0–30.0 µm (average diameter: 21.2 µm). Thus, with the extension of the hydrothermal treatment time, the self-assembled microspheres became more compact, and the average diameter decreased on the whole with the increase in the hydrothermal treatment time from 150 to 180 and 720 min.3.6 EDTA-2Na assisted formation mechanism of the hierarchical γ-BaB2O4 hollow microspheres
Based on the above experimental results, a possible formation mechanism of the hierarchical γ-BaB2O4 hollow microspheres is proposed. From a chemical point of view, the dissolution of Ba(OH)2·8H2O, H3BO3 and EDTA-2Na led to the corresponding aqueous ions first, as shown in eqn (2)–(4).| Ba(OH)2·8H2O(s) → Ba2+(aq.) + 2OH−(aq.) + 8H2O | (2) |
| H3BO3(s) + H2O → B(OH)4−(aq.) + H+(aq.) | (3) |
| EDTA-2Na(s) → EDTA2−(aq.) + 2Na+(aq.) | (4) |
| EDTA2−(aq.) + Ba2+(aq.) → EDTA–Ba (complex) | (5) |
| Ba2+(aq.) + 2B(OH)4−(aq.) + 2H+(aq.) + 2OH−(aq.) → BaB2O4(s) + 6H2O | (6) |
| EDTA–Ba (complex) + 2B(OH)4−(aq.) + 2H+(aq.) → BaB2O4(s) + 6H2O + EDTA2−(aq.) | (7) |
| Ba(OH)2·8H2O(s) + 2H3BO3(s) → BaB2O4(s) + 12H2O | (8) |
Since Ba(OH)2·8H2O has a very low solubility in DI water, even under vigorous magnetic stirring at room temperature, the powder added could not be readily dissolved. With the introduction of EDTA-2Na (Fig. S4(a)†), the EDTA2− ions could immediately chelate the newly dissociated Ba2+ ions, resulting in the chelate EDTA–Ba (complex) (Fig. S4(b)†) due to the strong chelation between Ba2+ and EDTA2− ions (eqn (5)). In the chelate complex, EDTA2− provided two nitrogen atoms and four oxygen atoms, bringing about tightly wrapped five-membered chelate rings with high stability. Accordingly, the concentration of the free Ba2+ ions in the solution was reduced, which promoted the dissolution of Ba(OH)2·8H2O. Then, with the dropwise addition of H3BO3 solution into the above system, the free Ba2+ ions were combined with OH−, H+ and B(OH)4− ions within the solution, giving rise to the more stable γ-BaB2O4 phase (eqn (6)). When the slurry derived from the room temperature coprecipitation was transferred into the stainless steel autoclave for hydrothermal treatment at a specific temperature (higher than 150 °C) for a specific time (longer than 130 min), the chelate complex EDTA–Ba gradually decomposed21,22 and the quantity of the γ-BaB2O4 phase gradually increased, as shown in eqn (7). Finally, the overall reaction could be written out, as described in eqn (8).
According to all the above results and analysis, a feasible mechanism was proposed addressing the EDTA-2Na assisted formation of hierarchical γ-BaB2O4 hollow microspheres (Fig. 5). Firstly, dissolution of the solid powder Ba(OH)2·8H2O in DI water led to aqueous Ba2+ and OH− ions, some of which remained in the solid state owing to their corresponding low solubility at room temperature (Fig. 5(a)). Secondly, addition of EDTA-2Na into the solution resulted in the coordinate compounds of the EDTA–Ba complex due to the strong chelation between Ba2+ ions and EDTA2− (Fig. 5(b)). Thirdly, addition of B(OH)4− into the solution facilitated the room temperature coprecipitation of the aqueous ions and EDTA–Ba, bringing about the original nucleation and primary growth of the amorphous NPs (Fig. 5(c)). Subsequently, hydrothermal treatment gradually promoted the phase conversion of the above amorphous NPs (Fig. 5(d)) and also the occurrence of self-assembled loose microspheres with EDTA2− preferentially adsorbed on the surfaces (Fig. 5(e)). With the hydrothermal time prolonged, the constituent NPs began to crystallize into nanorod-like subunits due to the preferential growth, leading to relatively compact hierarchical γ-BaB2O4 microspheres (Fig. 5(f)). Finally, when the reaction time was further extended, the nanorod-like subunits further grew into thicker nanorods, attributed to the Ostwald ripening, simultaneously giving rise to the uniform hollow microspheres (Fig. 5(g)). The subsequent cooling, washing and drying enabled the ultimate formation of the hierarchical γ-BaB2O4 hollow microspheres.
As confirmed above, the surfactant EDTA-2Na played a key role as a chelating and capping agent for the self-assembly of the NPs at the early stage of the hydrothermal treatment, leading to the relatively compact microspheres, very similar to the hydrothermal formation of hierarchical Ba2(B5O9)Cl·(H2O)0.5 (ref. 8) and mesoporous SrCO3 (ref. 23) microspheres. As is known, EDTA2− has four carboxylic groups and two lone pairs of electrons on two nitrogen atoms as binding sites.24 The carboxyl groups afford a firm spatial symmetric configuration, constraining the Ba2+ within the symmetric regions. Based on this, by using EDTA-2Na as the surfactant, self-assembled loose microspheres were facilely acquired via the selective adsorption of EDTA2− on the surfaces of the originally formed amorphous NPs. The weak interactions between the amorphous NPs with EDTA2− adsorbed on the surfaces provided the driving force, and the hydrothermal treatment obviously promoted the self-assembly of the loose hierarchical microspheres. Subsequent hydrothermal treatment enabled the preferential growth of the constituent NPs and the evolution from the loose microspheres to the relatively compact γ-BaB2O4 microspheres, owing to the traditional Ostwald ripening mechanism. In addition, according to the previous experimental results and analysis, it was believed that Ostwald ripening was also responsible for the transformation from relatively compact microspheres to hollow ones.25,26 The outer crystalline shells grew on the solid particles accompanied by continuous dissolution and recrystallization of the interior structures.27 During this process, the inner crystallites, which had a higher surface energy, would dissolve and transfer outwards, producing channels connecting the inner space and outer space in the shells.28 This hollowing process was quite similar to that of the SnO2 hollow nanostructures29 and VO2 hollow microspheres.30 Thus, on the whole, the synthesis (including room temperature coprecipitation and hydrothermal conversion) and hydrothermal self-assembly, as well as the subsequent Ostwald ripening, contributed to three individual and sequential processes that dominate the formation of the hierarchical hollow microspheres.
3.7 Phase conversion of γ-BaB2O4 hollow microspheres to β-BaB2O4 hollow microspheres
As is known, the low-temperature phase γ-BaB2O4 can be transformed by annealing to β-BaB2O4 at 590–650 °C, and then to the high-temperature phase α-BaB2O4 at 870–940 °C.31,32 Accordingly, the present γ-BaB2O4 hollow microspheres were calcined at 500 °C, 600 °C and 700 °C for 3.0 h in air so as to see the thermostability of the samples, as shown in Fig. 6. When calcined at 500 °C, no distinct change in the product composition was observed (Fig. 6(a1)), and the calcined γ-BaB2O4 hollow microspheres (Fig. 6(b) and (b1)) exhibited a relatively broader size distribution, ca. 88% of them having a diameter within the range of 10.0–16.0 µm (average size: 13.7 µm) (Fig. 6(b2)), in contrast with those hydrothermally synthesized at 180 °C for 12.0 h (Fig. 1(a)). When calcined at 600 °C, in addition to the majority phase of γ-BaB2O4 (JCPDS no. 35-0181), rhombohedral β-BaB2O4 (JCPDS no. 80-1489) began to appear (Fig. 6(a2)). Meanwhile, the product morphology did not demonstrate a significant change (Fig. 6(c) and (c1)), whereas ca. 90% of the calcined microspheres exhibited a diameter of 10.0–16.0 µm (average size: 13.4 µm) (Fig. 6(c2)). With the temperature increased to 700 °C, the product was confirmed as the pure phase of rhombohedral β-BaB2O4 (Fig. 6(a3)), and 91.0% of the as-obtained microspheres (Fig. 6(d) and (d1)) revealed a diameter of 9.0–15.0 µm (average size: 11.2 µm) (Fig. 6(d2)). The TEM images in Fig. 6(e) and (e1) clearly indicated that the constituent nanorods, which were dissociated from the calcined microspheres during the TEM sample preparation owing to the ultrasonic dispersion, possessed rather rough surfaces, attributed to the phase conversion taking place during the high-temperature annealing. In addition, the high-resolution TEM (HRTEM) image was also recorded from the top end of an individual nanorod (Fig. 6(f)). Corresponding with the red dashed rectangular region, the interplanar spacing of 0.311 nm detected from the legible lattice fringes along the radial direction of the selected nanorod (Fig. 6(f1)) was quite analagous to the standard value (0.313 nm) of the (220) planes for rhombohedral β-BaB2O4, indicating the preferential growth of the constituent nanorod perpendicular to the (220) planes. The high quality HRTEM image clearly evidenced the high crystallinity of the as-obtained β-BaB2O4 microspheres.As confirmed above, with the temperature increasing from 500 °C to 600 °C and 700 °C, the average diameter of the calcined microspheres decreased from 13.7 µm to 13.4 µm, and to 11.2 µm, respectively. Notably, in contrast with that of the hydrothermally synthesized hierarchical γ-BaB2O4 hollow microspheres (Fig. 1(b) and (b2)), the average size of the calcined microspheres tended to be smaller, due to the sintering during the high-temperature annealing. Taking the well-preserved morphology and slight change in the average size in the course of the phase conversion into consideration, both the hydrothermally synthesized γ-BaB2O4 hollow microspheres and calcined β-BaB2O4 microspheres exhibited excellent thermostability.
3.8 Hierarchical porous structure of the γ-BaB2O4 and β-BaB2O4 hollow microspheres
N2 adsorption–desorption isotherms were recorded to examine the porous structures within the hierarchical γ-BaB2O4 and β-BaB2O4 hollow microspheres, as shown in Fig. 7. Both adsorption–desorption isotherms of the γ-BaB2O4 (Fig. 7(a)) and β-BaB2O4 (Fig. 7(b)) hollow microspheres demonstrated a type IV isotherm with H3-type hysteresis loops, revealing the existence of dominant slit pores and channels with a relatively uniform shape and size within the structures. Meanwhile, the occurrence of the narrow hysteresis loops at high relative pressures of P/P0 = 0.8–1.0 (γ-BaB2O4, Fig. 7(a)) and P/P0 = 0.9–1.0 (β-BaB2O4, Fig. 7(b)) indicated abundant mesopores and especially macropores contained within the hollow microspheres.33,34 In particular, more constitutional macropores were present within the calcined loose β-BaB2O4 microspheres. The present hysteresis loops observed at high relative pressures were very similar to those of hierarchical porous Mg2B2O5 superstructures6 and (Ni, Mg)3Si2O5(OH)4 solid-solution nanotubes.35 The Barrett–Joyner–Halenda (BJH) pore size distribution (PSD) profiles of the γ-BaB2O4 (Fig. 7(a1)) and β-BaB2O4 (Fig. 7(b1)) hollow microspheres demonstrated pore diameters of 3–110 nm and 3–150 nm, respectively. This reconfirmed the presence of as-obtained γ-BaB2O4 (Fig. 7(a)) and β-BaB2O4 (Fig. 7(b)) hollow microspheres with abundant hierarchical porous structures, ranging from mesopores to macropores. | ||
| Fig. 7 Nitrogen adsorption–desorption isotherms (a and b) and the corresponding pore diameter distribution profiles (a1 and b1) of the γ-BaB2O4 hollow microspheres (a and a1) hydrothermally synthesized at 180 °C for 12.0 h (i.e. Fig. 1(b)) and β-BaB2O4 hollow microspheres (b and b1) calcined at 700 °C for 3.0 h (i.e. Fig. 6(d)). | ||
The hierarchical γ-BaB2O4 hollow microspheres (Fig. 7(a)) exhibited a multipoint Brunauer–Emmett–Teller (BET) specific surface area of 46.25 m2 g−1, a pore volume of 0.306 cm3 g−1, and an average pore diameter of 17.075 nm. In contrast, the calcined β-BaB2O4 hollow microspheres revealed a much smaller specific surface area (2.44 m2 g−1) and pore volume (0.025 cm3 g−1), and an average pore diameter of 3.240 nm. The distinct decrease in the porosity characteristic parameters was probably attributed to the abrupt reduction or even disappearance of the multitudes of macropores (>50 nm) (Fig. 7(a1) and (b1)) on one hand and further growth of the constituent nanorods during the high-temperature annealing on the other hand.48 The BET surface area of the present hierarchical γ-BaB2O4 hollow microspheres was somehow smaller than that of hierarchical porous MgBO2(OH) microspheres (57.22 m2 g−1),6 but larger than those of porous Ca(BO2)2 microspheres (42.7 m2 g−1),12 flower-like Bi2MoO6 hollow spheres (40.0 m2 g−1),36 CoFe2O4 microspheres (32.3 m2 g−1),37 and SrCO3 submicron spheres (40.2 m2 g−1),38 suggesting potential applications of the present hollow microspheres in catalysis, adsorption, etc.
3.9 FT-IR and UV-vis properties of the γ-BaB2O4 and β-BaB2O4 hollow microspheres
Fig. 8(a1) and (b) show the FT-IR spectra of the hierarchical γ-BaB2O4 hollow microspheres hydrothermally synthesized at 180 °C for 12.0 h. The vibrational bands are concentrated in the wavenumber range of 1650–600 cm−1. According to the FT-IR results of borates,39 the band at 3440 cm−1 was due to the O–H stretching of the absorbed water, the absorption peak at 1577 cm−1 was owing to the H–O–H bending of the lattice water, the bands at 1385 cm−1 and 1084 cm−1 were attributed to the asymmetric stretching of B(3)–O, and the band at 923 cm−1 corresponded to the symmetric stretching of B(3)–O. In addition, the out-of-plane bending of B(3)–O was located in the range of 730–635 cm−1, and the band at 722 cm−1 might be ascribed to the existence of the (B3O6)3− group.40,41 This further evidenced the high purity of the hierarchical γ-BaB2O4 hollow microspheres, similar to the previously reported results in the literature.42Fig. 8(a2) and (c) display the FT-IR spectra of the β-BaB2O4 hollow microspheres calcined at 700 °C for 3.0 h. The absorption band at 3450 cm−1 might be due to the O–H stretching vibration of the absorbed water. The absorption peaks at 963 and 1425 cm−1 were owing to the stretching vibration of extra-ring B–O bonds. The peak at 1247 cm−1 was ascribed to the B–O stretching in the (BO3)3− unit, a component of the (B3O6)3− ring.43 The strong absorption band observed close to 700 cm−1 corresponded to the bending vibrations of O–B–O. These FT-IR results of the β-BaB2O4 hollow microspheres were similar to those previously reported in literature.18
Fig. 8(d) demonstrates the UV-vis spectra of the hierarchical γ-BaB2O4 and β-BaB2O4 hollow microspheres dispersed in DI water. Obviously, both the hydrothermally synthesized γ-BaB2O4 hollow microspheres and the calcined β-BaB2O4 microspheres exhibited low absorption within the whole wavelength range of 200–900 nm (Fig. 8(d1) and (d2)). Meanwhile, the transmittance spectra (Fig. 8(d3) and (d4)) demonstrated that both the γ-BaB2O4 hollow microspheres and the calcined β-BaB2O4 microspheres exhibited good transparent characteristics from the ultraviolet to the visible regions. This was quite similar to the transparent nature of β-BaB2O4 nanospindles,18 β-BaB2O4 nanorods,19 β-BaB2O4 network-like nanostructures,44 and magnesium borate nanowhiskers.45
3.10 Adsorption performance of the hierarchical γ-BaB2O4 hollow microspheres
The hierarchical γ-BaB2O4 hollow microspheres were used as an adsorbent for the removal of Pb2+ ions from mimic waste water. Fig. 9(a) shows the variation of the adsorption rate of Pb2+ ions with time, where the initial concentration of the solution was 50 mg L−1. It was obvious that the adsorption process proceeded quickly during the first 10 min, and equilibrium was achieved within 1.0 h. This indicated that the adsorption efficiency reached 78.14% within 10 min, and ultimately remained relatively constant at 80.83%. Meanwhile, the variation of the adsorption capacity at any instant (i.e. qt) as a function of time is shown in Fig. 9(a1). The high adsorption rate and adsorption capacity of Pb2+ onto the hierarchical γ-BaB2O4 hollow microspheres were probably attributed to the intrinsically and relatively large specific surface area as well as the surface alkalinity conditions. The maximum adsorption capacity (qm) for Pb2+ was ca. 101.0 mg g−1.Compared with some other adsorbents for Pb2+ removal in the literature (Table 1), it was apparent that the hierarchical γ-BaB2O4 hollow microspheres exhibited higher adsorption abilities than urchin-like α-FeOOH hollow spheres,46 organic silica hollow spheres47 and BiOBr microspheres.48 Notably, however, the qm of the γ-BaB2O4 hollow microspheres was smaller than hierarchical porous MgO microrods,49 porous Ca(BO2)2 microspheres,12 and TiO2 hollow spheres.50 However, these hierarchical porous structures all originated from the hydrothermal synthesis of the corresponding metal-bearing hydrated precursors, followed by thermal conversion at high temperatures of 400–750 °C.12,49,50 Taking the facile hydrothermal formation (no need for subsequent high-temperature calcination) and cost-effectiveness (cheaper than titania) into consideration, the current hierarchical γ-BaB2O4 hollow microspheres on the whole demonstrated satisfactory adsorption performance for the removal of Pb2+ ions from mimic waste water.
| Adsorbents | S BET | Pb2+ adsorption capacity (mg g−1) | Ref. |
|---|---|---|---|
| BiOBr microspheres | 59.3 | 6.5 | 48 |
| Organic silica hollow spheres | 259.9 | 75.6 | 47 |
| Urchin-like α-FeOOH hollow spheres | 96.9 | 80 | 46 |
| γ-BaB2O4 hollow microspheres | 46.25 | 101 | This work |
| Hierarchical porous MgO microrods | 50.2 | 124.4 | 49 |
| Porous Ca(BO2)2 microspheres | 42.7 | 140.2 | 12 |
| TiO2 hollow spheres | 334 | 323 | 50 |
As is known, adsorption kinetics models describing the pollutant uptake rate during the adsorption process are significant characteristics in defining the adsorption efficiency. Thus, such models were employed so as to better understand the adsorption mechanism of Pb2+ ions on the hierarchical γ-BaB2O4 hollow microspheres. The linearized forms of the pseudo-first-order and pseudo-second-order kinetic models are expressed as follows:
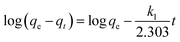 | (9) |
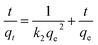 | (10) |
| Adsorption kinetics | q e, exp (mg g−1) | q e, cal (mg g−1) | k 1 (min−1) k2 (mg g−1 min−1) | R 2 |
|---|---|---|---|---|
| Pseudo-first-order models | 101.0 | 5.458 | k 1 = 0.006909 | 0.2567 |
| Pseudo-second-order models | 101.0 | 101.1 | k 2 = 0.01224 | 0.9959 |
To further ascertain the satisfactory adsorption efficiency of Pb2+ on the present adsorbent, the XRD pattern of the adsorbent containing the adsorbate were recorded (Fig. 9(d)). As shown, the diffraction peaks could be assigned to γ-BaB2O4 (JCPDS no. 35-0181) and Pb3(CO3)2(OH)2 (JCPDS no. 13-0131). It was assumed that, during the adsorption process, partially dissolved CO2 from the air reacted with Pb2+ and H2O, producing the new phase of Pb3(CO3)2(OH)2. This suggested the formation of Pb3(CO3)2(OH)2 via the following reaction:
| 3Pb2+ + 2CO2 + 4H2O → Pb3(CO3)2(OH)2 + 6H+ | (11) |
4. Conclusions
In summary, hierarchical γ-BaB2O4 hollow microspheres self-assembled from nanorods were synthesized by a facile EDTA-2Na assisted hydrothermal method (180 °C, 12.0 h), based on which β-BaB2O4 hollow microspheres with well-preserved spherical morphology and high crystallinity were obtained via the subsequent mild phase conversion (700 °C, 3.0 h). According to the effects of process parameters (e.g. reactant concentration, reaction time, temperature, and amount of the surfactant EDTA-2Na) on the hydrothermal product, a reasonable formation mechanism based on the EDTA-2Na assisted self-assembly was proposed. The formation mechanism of the γ-BaB2O4 hollow microspheres can be divided into three processes, including synthesis, hierarchical self-assembly and hollow structure formation. Both the hydrothermally synthesized hierarchical γ-BaB2O4 and calcined β-BaB2O4 hollow microspheres exhibited excellent thermostability. The hierarchical γ-BaB2O4 hollow microspheres possessed a specific surface area of 46.25 m2 g−1, a pore volume of 0.306 cm3 g−1, and an average pore diameter of 17.075 nm. The γ-BaB2O4 and β-BaB2O4 hollow microspheres revealed a unique transparent nature from the ultraviolet to the visible regions. Meanwhile, the as-obtained γ-BaB2O4 hollow microspheres demonstrated a satisfactory ability to adsorb heavy metal Pb2+ ions from mimic waste water when employed as an adsorbent. The present work deepened the fundamental understanding and realistic practice of surfactant-assisted nanocrystal growth and hydrothermal self-assembly of NPs, and also expanded the future potential applications of the 3D hierarchical porous or hollow nanoarchitectures of metal borates in related fields.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21276141) and the State Key Laboratory of Chemical Engineering, China (no. SKL-ChE-15A03).References
- B. Wang, H. B. Wu, L. Yu, R. Xu, T. T. Lim and X. W. Lou, Adv. Mater., 2012, 24, 1111–1116 CrossRef CAS PubMed.
- J. Y. Li, S. L. Xiong, J. Pan and Y. T. Qian, J. Phys. Chem. C, 2010, 114, 9645–9650 CAS.
- R. B. Rakhi, W. Chen, D. Cha and H. N. Alshareef, Nano Lett., 2012, 12, 2559–2567 CrossRef CAS PubMed.
- J. Elias, C. Levy-Clement, M. Bechelany, J. Michler, G. Y. Wang, Z. Wang and L. Philippe, Adv. Mater., 2010, 22, 1607–1612 CrossRef CAS PubMed.
- Q. W. Tian, M. H. Tang, Y. G. Sun, R. J. Zou, Z. G. Chen, M. Fang Zhu, S. P. Yang, J. L. Wang, J. H. Wang and J. Q. Hu, Adv. Mater., 2011, 23, 3542–3547 CrossRef CAS PubMed.
- Z. Q. Zhang, W. C. Zhu, R. G. Wang, L. L. Zhang, L. Zhu and Q. Zhang, J. Mater. Chem. A, 2014, 2, 19167–19179 CAS.
- Y. Zeng, H. B. Yang, W. Y. Fu, L. Qiao, L. X. Chang, J. J. Chen, H. Y. Zhu, M. H. Li and G. T. Zou, Mater. Res. Bull., 2008, 43, 2239–2247 CrossRef CAS.
- X. P. Chen, L. L. Zhang, Z. Q. Zhang, L. Zhu and W. C. Zhu, CrystEngComm, 2015, 17, 7856–7865 RSC.
- D. M. Mittleman, Nat. Photonics, 2013, 7, 666–669 CrossRef CAS.
- G. Y. Qu, Z. F. Hu, Y. P. Wang, Q. Yang and L. M. Tong, Adv. Funct. Mater., 2013, 23, 1232–1237 CrossRef CAS.
- T. Roger, S. Vezzoli, E. Bolduc, J. Valente, J. J. Heitz, J. Jeffers, C. Soci, J. Leach, C. Couteau, N. I. Zheludev and D. Faccio, Nat. Commun., 2015, 6, 7031–7035 CrossRef PubMed.
- Z. Q. Zhang, H. Zhang, L. Zhu, Q. Zhang and W. C. Zhu, Chem. Eng. J., 2016, 283, 1273–1284 CrossRef CAS.
- A. M. Chen, P. Gu and Z. M. Ni, Mater. Lett., 2012, 68, 187–189 CrossRef CAS.
- W. C. Zhu, X. L. Wang, X. Zhang, H. Zhang and Q. Zhang, Cryst. Growth Des., 2011, 11, 2935–2941 CAS.
- H. C. Pang, G. L. Ning, W. T. Gong, J. W. Ye, Y. Lin and X. N. Pan, New J. Chem., 2011, 35, 1449–1452 RSC.
- P. Becker, Adv. Mater., 1998, 10, 979–992 CrossRef CAS.
- N. Liu, D. Zhao, L. X. Yu, K. Z. Zheng and W. P. Qin, Colloids Surf., A, 2010, 363, 124–129 CrossRef CAS.
- R. Li, X. Y. Tao and X. D. Li, J. Mater. Chem., 2009, 19, 983–987 RSC.
- J. Zhang, G. P. He, R. H. Li and X. Chen, J. Alloys Compd., 2010, 489, 504–508 CrossRef CAS.
- Y. S. Yang, Q. Z. Wu, M. Wang, J. Long, Z. Mao and X. H. Chen, Cryst. Growth Des., 2014, 14, 4864–4871 CAS.
- L. N. Guo, Y. Z. Wang, Y. H. Wang, J. Zhang and P. Y. Dong, CrystEngComm, 2012, 14, 3131–3141 RSC.
- H. L. Qiu, G. Y. Chen, L. Sun, S. W. Hao, G. Han and C. H. Yang, J. Mater. Chem., 2011, 21, 17202–17208 RSC.
- W. C. Zhu, Z. Z. Liang, X. F. Liu, H. Zhang, Y. J. Zheng, X. L. Piao and Q. Zhang, Powder Technol., 2012, 226, 165–172 CrossRef CAS.
- L. Xu, J. M. Shen, C. L. Lu, Y. P. Chen and W. H. Hou, Cryst. Growth Des., 2009, 9, 3129–3136 CAS.
- B. Liu and H. C. Zeng, Small, 2005, 1, 566–571 CrossRef CAS PubMed.
- P. W. Voorhees, J. Stat. Phys., 1985, 38, 231–252 CrossRef.
- X.-Y. Yu, X.-Z. Yao, T. Luo, Y. Jia, J.-H. Liu and X.-J. Huang, ACS Appl. Mater. Interfaces, 2014, 6, 3689–3695 CAS.
- J. Li and H. C. Zeng, J. Am. Chem. Soc., 2007, 129, 15839–15847 CrossRef CAS PubMed.
- X. W. Lou, Y. Wang, C. Yuan, J. Y. Lee and L. A. Archer, Adv. Mater., 2006, 18, 2325–2329 CrossRef CAS.
- A. Q. Pan, H. B. Wu, L. Yu and X. W. D. Lou, Angew. Chem., 2013, 125, 2282–2286 CrossRef.
- D. Eimerl, L. Davis, S. Velsko, E. K. Graham and A. Zalkin, J. Appl. Phys., 1987, 62, 1968–1983 CrossRef CAS.
- O. Yamaguchi, K. Tominaga and K. Shimizu, Ceramurgia Int., 1980, 6, 103–105 CrossRef CAS.
- R. X. Chen, J. G. Yu and W. Xiao, J. Mater. Chem. A, 2013, 1, 11682–11690 CAS.
- F. Z. Mou, J. G. Guan, H. R. Ma, L. L. Xu and W. D. Shi, ACS Appl. Mater. Interfaces, 2012, 4, 3987–3993 CAS.
- W. C. Zhu, Y. Yang, S. Hu, G. L. Xiang, B. Xu, J. Zhuang and X. Wang, Inorg. Chem., 2012, 51, 6020–6031 CrossRef CAS PubMed.
- G. H. Tian, Y. J. Chen, W. Zhou, K. Pan, Y. Z. Dong, C. G. Tian and H. G. Fu, J. Mater. Chem., 2011, 21, 887–892 RSC.
- Q. Q. Xiong, J. P. Tu, S. J. Shi, X. Y. Liu, X. L. Wang and C. D. Gu, J. Power Sources, 2014, 256, 153–159 CrossRef CAS.
- W. C. Zhu, G. L. Zhang, J. Li, Q. Zhang, X. L. Piao and S. L. Zhu, CrystEngComm, 2010, 12, 1795–1802 RSC.
- L. Jun, S. P. Xia and S. Y. Gao, Spectrochim. Acta, Part A, 1995, 51, 519–532 CrossRef.
- T. Kobayashi, R. Ogawa and M. Kuwabara, Mater. Lett., 2003, 57, 1056–1061 CrossRef CAS.
- W. G. Zou, M. K. Lü, F. Gu, S. F. Wang, Z. L. Xiu and G. J. Zhou, Mater. Sci. Eng., B, 2006, 127, 134–137 CrossRef CAS.
- C. Babeela and T. C. S. Girisun, Opt. Mater., 2015, 49, 190–195 CrossRef CAS.
- J. Zhang, S. J. Liang and G. P. He, Chem. Lett., 2009, 38, 500–501 CrossRef CAS.
- Q. R. Zhao, X. Zhu, X. Bai, H. H. Fan and Y. Xie, Eur. J. Inorg. Chem., 2007, 1829–1834 CrossRef CAS.
- W. C. Zhu, R. G. Wang, S. L. Zhu, L. Zhang, X. L. Cui, H. Zhang, X. L. Piao and Q. Zhang, ACS Sustainable Chem. Eng., 2014, 2, 836–845 CrossRef CAS.
- B. Wang, H. B. Wu, L. Yu, R. Xu, T. T. Lim and X. W. Lou, Adv. Mater., 2012, 24, 1111–1116 CrossRef CAS PubMed.
- J. Hu, X. G. Wang, L. Q. Liu and L. M. Wu, J. Mater. Chem. A, 2014, 2, 19771–19777 CAS.
- X. Q. Wang, W. X. Liu, J. Tian, Z. H. Zhao, P. Hao, X. L. Kang, Y. H. Sang and H. Liu, J. Mater. Chem. A, 2014, 2, 2599–2608 CAS.
- L. L. Zhang, W. C. Zhu, H. Zhang, S. W. Bi and Q. Zhang, RSC Adv., 2014, 4, 30542–30550 RSC.
- N. Sutradhar, S. K. Pahari, M. Jayachandran, A. M. Stephan, J. Nair, B. Subramanian, H. C. Bajaj, H. M. Mody and A. B. Panda, J. Mater. Chem. A, 2013, 1, 9122–9131 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12015f |
| This journal is © The Royal Society of Chemistry 2016 |








