Self-assembly of a water soluble perylene and surfactant into fluorescent supramolecular ensembles sensitive to acetylcholinesterase activity†
G. Grisci,
E. Kozma,
W. Mróz,
K. Pagano,
L. Ragona* and
F. Galeotti*
CNR-Istituto per lo Studio delle Macromolecole (ISMAC), Via A. Corti 12, 20133 Milano, Italy. E-mail: f.galeotti@ismac.cnr.it; l.ragona@ismac.cnr.it; Tel: +39-02-23699357
First published on 28th June 2016
Abstract
The modulation of assembly/disassembly of perylene diimide derivatives (PDIs) represents an intriguing challenge for fluorescence sensing purposes. Herein, an aminoacid-functionalized PDI (PDI-Thr) is selected as a conformationally-sensitive probe which transduces the modification of its aggregation state into an optical response. Photoluminescence measurements reveal that the emission of PDI-Thr in aqueous solution can be finely modulated by a cationic surfactant, myristoylcholine chloride (MyrCh). Fluorescence sensing studies illustrate that the PDI-Thr/MyrCh ensembles exhibit an emission intensity variation in response to the enzymatic activity of acetylcholinesterase, which is able to reverse the surfactochromic effect by catalyzing the hydrolysis of MyrCh. Detailed NMR investigations elucidate the supramolecular mechanism behind the sensing behavior. This study sheds light on the synergistic combination of electrostatic and hydrophobic interactions occurring between PDI-Thr and MyrCh, leading to the fluorescent ensemble which is responsible for the sensing performance. The discussed results outline that the development of fluorophore/surfactant ensembles based on water soluble PDIs have great potential for the label-free sensing of a wide range of analytes and enzymes.
Introduction
Over the past few decades, perylene-3,4:9,10-tetracarboxylic acid diimides (PDIs) have emerged as a pivotal class of fluorescent dyes characterized by transient properties, from monomeric to bulk material, due to their tendency to give supramolecular interactions. Thanks to their unmatched combination of favorable properties such as high chemical and thermal photostability, unique spectroscopical and electrochemical behavior, PDI-based materials have proved to be useful for a broad variety of applications, including organic solar cells,1–3 field effect transistors,4,5 OLEDs,6,7 and white hybrid LEDs.8 In addition, the simplicity of tailoring these dyes into building blocks able to self-assemble into supramolecular architectures opens the path to comprehensive novel applications in the sensing and biosensing fields.Typically, PDI organization into supramolecules is dictated by various non-covalent and solvent-dependent interactions, such as hydrogen and van der Waals bonding, π–π stacking, solvophobic and electrostatic interactions. In the aqueous medium, the self-association behavior of PDI derivatives is especially determined by the ratio between the hydrophilic/hydrophobic components, along with the different steric effects of the substituents. In fact, PDIs normally display poor solubility and very weak fluorescence in aqueous solution because of their strong tendency to give π–π intermolecular interactions, which consistently decrease both the solubility and emission efficiency. However, recent studies on PDI structures specifically designed to prevent aggregation have demonstrated that fluorescence quantum yields of 0.9 are possible for this chromophore even in water.9 The incorporation of hydrophilic residues (e.g. carboxylates, sulfonates, ammonium salts, aminoacids, oligoethylenglycol chains, sugars, DNA fragments) at the imide or bay positions of the aromatic planar core can be effective in preventing the π–π interactions by steric hindrance or electrostatic repulsion. In this way, the high emission efficiency of the disassembled state can be preserved and, at the same time, the problem of water-insolubility can be overcome, hence extending the field of applications of PDI to the biomedical area.10 Nevertheless, other attractive forces (e.g. hydrogen bonds) may still occur making predominant the interactions leading to aggregation, which turns-off the emission of the system.
The modulation of assembly/disassembly of PDI derivatives represents an intriguing challenge for fluorescence sensing purposes. One interesting option for tuning the self-association behavior of water soluble PDIs is to let them form supramolecular ensembles with surfactants. This approach, mainly explored for the development of pyrene-based sensors, has been recently followed for realizing a fluorescence “switching off–on” probe for metal ions, based on the assembly of an aminoacid-functionalized PDI with the cationic surfactant cetyltrimethylammonium bromide (CTAB).11 In fact, the surfactant/PDI interaction can lead to highly emissive supramolecules, where the chromophores are kept in a quasi-isolated state by the presence of the surfactant molecules. The intrinsic properties of surfactants allow to build a synergistic combination of electrostatic and hydrophobic interactions with conformational-sensitive probes whose perturbation in the aggregation state can be recorded as “surfactochromic” variations of their optical response.12 This surfactochromic output can be sufficiently strong not only for detecting simple chemicals or toxic metals but even for monitoring biological processes.13
Acetylcholinesterase (AChE) is a hydrolytical enzyme which catalyzes the breakdown of acetylcholine (ACh), a small ammonium salt acting as neurotransmitter in human body.14 Because AChE is involved in various physiological functions, ranging from the cognitive processes (e.g. learning and memory) to the neuromuscular ones (e.g. regulation of muscle contraction),15 an imbalance in ACh levels could lead to pathological conditions. In particular, damages or abnormalities in cerebral cholinergic pathways have been reported to play a key role in Alzheimer's disease (AD), a neurodegenerative disorder culminating in dementia.16,17 According to this assumption known as “cholinergic hypothesis”, AChE has been successfully identified as the main target of the therapeutic symptom-treatment to contrast AD cognitive impairments and improve life quality of patients.17 Therefore, sensing of AChE activity and screening for its potential inhibitors are of great importance.
Driven by the need of simplifying as much as possible the time-consuming and complex experimental procedures, in the last ten years various researchers have proposed new optical probes (e.g. conjugated oligo/poly-electrolytes) for simple “mix-and-detect” continuous assays to monitor AChE activity.18,19 A further improvement for this approach has derived from the use of the commercially available myristoylcholine (MyrCh) as AChE substrate, which does not require any labelling step.20,21
By exploiting a similar working principle, our group has recently demonstrated that MyrCh-mediated tuning of the optical response of a fluorescent oligothiophene probe can be retained even after anchoring the sensing moiety to a flat surface, providing the proof-of-concept of a solid biosensor to monitor AChE activity.22 The sensing response was primarily based on a spectral variation, while its intensity change was limited, hampering the possibility of using portable and fixed wavelength fluorometers as detection systems.
Pushed by the idea of developing a more versatile fluorescent probe with similar features, but of simpler realization and easier signal detection, herein we studied by a combination of techniques (UV, Fluorescence, IR and NMR) the optical properties of a PDI derivative functionalized with the aminoacid L-threonine (Thr), previously synthesized in our laboratory (Scheme 1).23 We show that the water soluble PDI-Thr, in the presence of MyrCh, acts as a conformational-sensitive probe which transduces the modification of its aggregation state into an optical response, which is clearly observable by the naked eye by exposing the solution under UV light and even perceivable under ambient light.
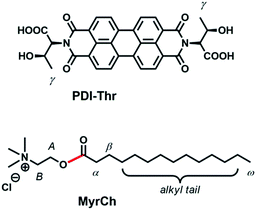 | ||
| Scheme 1 Structures of the aminoacid-functionalized perylene derivative PDI-Thr and of the AChE-cleavable surfactant MyrCh (cleavable bond evidenced in red). | ||
We demonstrate that AChE-catalyzed hydrolysis process can trigger the breakdown of such complexes, reversing the “surfactochromic” effect and confirming the non-covalent and reversible nature of the interaction between the chromophore and the surfactant. The results reported here outline an appealing strategy, based on the supramolecular approach, to monitor AChE activity in continuous by setting up a label-free “mix-and-detect” colorimetric/fluorometric assay, which may support the screening of AChE inhibitors in AD drug discovery.
Experimental
Materials and methods
MyrCh (as chloride salt) and AChE (from Electrophorus electricus – electric eel) were purchased from Sigma Aldrich. All the other chemicals and solvents were supplied by Sigma Aldrich and TCI and were used as received, without further purification. The phosphate buffer (PB) solution, 0.1 M Na2HPO4–NaH2PO4 pH = 8, was freshly prepared before use with ultrapure water (resistivity about 18.2 MΩ cm at 25 °C) obtained with a Millipore Direct-Q®3 system. PDI-Thr and PDI-Asp were prepared in our laboratory by following an already described procedure.23 In synthesis, perylene-3,4:9,10-tetracarboxylic acid dianhydride (0.5 mmol), L-threonine or L-aspartic acid (1.05 mmol) and 1 g of imidazole were added into a Schlenk tube and heated at 120 °C for 4 hours. The reaction mixture was cooled and poured into water and filtered. The filtrate was acidified with 2.0 M HCl and the precipitate was filtered and washed with water several times, then with methanol and finally dried under vacuum to give the final product (yield: PDI-Thr 89%, PDI-Asp 85%).Characterization techniques
Results and discussion
Design and optimization of the sensing material
We have recently designed and synthesized water soluble aminoacid-based PDIs (PDI-AAs) whose self-association behavior could be finely modulated by varying the kind of amino-acid side chain.23 Specifically, in that study, PDI derivatives containing Thr or Asp functionalities showed good fluorescence quantum yields (ΦF) in diluted aqueous buffer samples and a marked concentration-dependent aggregation behavior, with a consequent gradual photoluminescence (PL) quenching when concentration raised over 10 μM. Now we have investigated further the aggregation state of the two PDI-AAs in water by measuring with NMR the self-diffusion coefficients of the soluble molecular species and deriving an indication of their size. 2D diffusion ordered spectroscopy (DOSY) experiments indicated that PDI-Thr, at 25 °C, in the concentration range 0.1–0.5 mM forms aggregates of ∼40 units. In the same experimental conditions, PDI-Asp molecules form smaller aggregates, ranging from 6 (0.1 mM PDI-Asp) to 10 units (0.5 mM PDI-Asp) (Fig. S1†). The bigger size of the aggregates observed for PDI-Thr nicely matches the optical data, showing a stronger PL quenching for PDI-Thr than for PDI-Asp at the same concentration (Fig. S2†).With the aim of taking advantage of this aggregation phenomenon for sensing purposes, we decided to further study PDI-Thr, characterized by a higher homo-aggregate size than PDI-Asp in the same concentration range (i.e. 0.1–0.5 mM) and by a good ΦF (∼25%) in the isolated state.
The surfactant myristoylcholine chloride (MyrCh) was selected to induce a perturbation in PDI-Thr aggregation tendency. In fact, given the cationic nature of MyrCh, we expected that its polar head could interact with the PDI-Thr aminoacidic residues, which are in a dissociated and negatively charged form at pH 8.
As shown in Fig. 1 (bottom), the addition of increasing amounts of MyrCh to an aqueous solution (PB, pH 8) of PDI-Thr kept at constant concentration of 0.1 mM, readily triggers a PL switch-on by surfactochromic effect. By exposing the solutions to a UV source (λ = 365 nm), this effect is visible to the naked eye starting from MyrCh concentration of 1.5 mM. However, before this concentration is reached, another effect is clearly evident by observing the same samples under ambient light, as shown in Fig. 1 (top). At the beginning the solutions look hot pink, and, by increasing MyrCh concentration, no color variation is observable in the first three samples (0 to 0.1 mM). Above this threshold, at MyrCh 0.2 and 0.5 mM, the solutions look pale pink. The presence of solid material on the bottom of these two vials suggests that, in these experimental conditions, the supramolecular aggregate is scarcely soluble and the solution color intensity is consequently decreased. Moving from MyrCh 0.8 to 8 mM, the solution color initially comes back to hot pink, and then shifts to orange. To shed light on these naked-eye observations, a deeper investigation of the dye/surfactant supramolecular interaction was required; to this aim we analyzed the solutions by different spectroscopical techniques.
 | ||
| Fig. 1 Solutions of PDI-Thr (0.1 mM) after addition of increasing amounts of MyrCh, viewed under ambient light (top) and under UV lamp (bottom; λ = 365 nm). | ||
Optical characterization of the probe
The optical behavior of the samples reported in Fig. 1 was explored by UV-vis absorption and PL emission spectroscopy.As shown by the spectra in Fig. 2A, by increasing the concentration of MyrCh, the absorption plot of 0.1 mM PDI-Thr samples in PB at pH 8 substantially changes. We can obtain information on the aggregation state of PDI-Thr from the “evolution” of these spectra. In fact, when PDI molecules are in a non-aggregated form, which is the case of highly soluble derivatives in diluted organic solutions, the absorption spectra typically show three well-defined vibronic structures in the 450–550 nm range (as in Fig. 2C, light green line), which are attributed to the S0–S1 transition of the PDI chromophore. These structures are ascribable to breathing vibrations of the perylene core which couples with the S0–S1 transition polarized along the long axis of the molecule.30 Upon π–π stacking, the relative intensity between the 0 → 0 (around 550 nm) and 0 → 1 (around 500 nm) vibronic absorption bands is reversed and this specific spectral feature is often used to estimate the degree of aggregation of PDI molecules in solution.31
The absorption spectra of 0.1 mM PDI-Thr in Fig. 2A are clearly split into two different groups. The solutions in which MyrCh concentration is equal or higher than 0.80 mM show well-resolved vibronic absorption bands, with three peaks at around 465 nm, 495 nm and 530 nm, whose intensity increases with MyrCh concentration. As indicated by the A0→0/A0→1 values ranging from 1.30 to 1.50, these spectra tend to the vibrational progression typical of the molecularly dissolved PDI (A0→0/A0→1 ≈ 1.6). At MyrCh concentration lower than 0.10 mM, the spectral features become less defined, with the A0→0/A0→1 values falling below the unit, symptomatic of a predominantly assembled state.
The observed absorption peak inversion is specifically highlighted in Fig. 2B. In 0.05 and 0.1 mM MyrCh samples, the surfactant is not enough concentrated to affect significantly the self-aggregation tendency of PDI-Thr. The spectra of samples 0.20 and 0.50 mM in MyrCh, corresponding to the pale pink solutions of Fig. 1 top, show low absorption intensities (in accordance with the formation of insoluble hetero-aggregates) and A0→0/A0→1 values just above the unit, indicating an intermediate situation between the isolated and the assembled state.
Hence, the evolution of the absorption spectra suggests that the addition of MyrCh above 0.1 mM has a disaggregating effect on 0.1 mM PDI-Thr solution.
In Fig. 2C, the absorption spectra of PDI-Thr alone in aqueous PB pH 8 and in DMSO are directly compared with those in PB at moderate (1.5 mM) and high (8.0 mM) concentration of MyrCh. It is clearly evident that the main effect of the addition of MyrCh to PDI-Thr aqueous solutions is to decrease the amount of aggregation state to the low levels which are encountered in DMSO. The PL analysis essentially confirmed these findings, as summarized by the spectra in Fig. 2D and by the integration plot in the inset. At low MyrCh concentration, the PL intensity of the system is initially quenched by the formation of insoluble hetero-aggregates. Then, as soon as MyrCh concentration is increased over the critical value of 1.0 mM, the surfactochromic effect takes place and the PL intensity starts to rise up in a surfactant concentration-dependent fashion. Upon MyrCh addition, the PL spectrum of PDI-Thr exhibits an order of magnitude increase at MyrCh 1.0 mM and a 24-fold increase at 8.0 mM, which is a promising basis for using this system as a probe to detect targeted analytes.
NMR monitoring of the supramolecular assembly
In order to rationalize the observed optical behavior, we investigated by 1H NMR the assembly/disassembly of homo and hetero supramolecular structures. The analysis of the chemical shift perturbation of MyrCh resonances upon 0.1 mM PDI-Thr addition indicated that, when MyrCh and PDI-Thr were mixed in a stoichiometric ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, no effect was observed, while significant variations were measured at MyrCh
2, no effect was observed, while significant variations were measured at MyrCh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PDI-Thr ratios higher than 10. Specifically, the highest chemical shift perturbations were observed for protons belonging to the non-polar alkyl tail (ten –CH2– and ω –CH3), while the groups belonging to MyrCh polar head (N–CH3 and B –CH2–) and those adjacent to carbonyl carbon (α –CH2– and β –CH2–) underwent to slight chemical shift changes, as reported in Fig. 3A (see Fig. S3† for the complete 1H NMR spectrum assignment of MyrCh). Protons of A –CH2– were not considered in this analysis because their signal at 4.56 ppm, close to water resonance, was not clearly detectable in the whole concentration range under study. The observed chemical shift change of ω –CH3 group at different MyrCh concentrations (1.2–5.0 mM), in the absence and in the presence of 0.1 mM PDI-Thr, are reported in Fig. 3B, as an example.
PDI-Thr ratios higher than 10. Specifically, the highest chemical shift perturbations were observed for protons belonging to the non-polar alkyl tail (ten –CH2– and ω –CH3), while the groups belonging to MyrCh polar head (N–CH3 and B –CH2–) and those adjacent to carbonyl carbon (α –CH2– and β –CH2–) underwent to slight chemical shift changes, as reported in Fig. 3A (see Fig. S3† for the complete 1H NMR spectrum assignment of MyrCh). Protons of A –CH2– were not considered in this analysis because their signal at 4.56 ppm, close to water resonance, was not clearly detectable in the whole concentration range under study. The observed chemical shift change of ω –CH3 group at different MyrCh concentrations (1.2–5.0 mM), in the absence and in the presence of 0.1 mM PDI-Thr, are reported in Fig. 3B, as an example.
As known, surfactant molecules like MyrCh are able to self-organize into micellar supramolecules upon dissolution and this effect must be taken in account when analyzing PDI-Thr/MyrCh mixtures. Supramolecular ensembles formation is easily detectable by NMR; indeed the change of proximity of the different groups upon self-organization induces chemical shift changes, allowing a direct estimate of the critical micellar concentration (CMC). For surfactants in fast chemical exchange between monomers and micelles, the observed chemical shift (δobsd) can be expressed as the weighted mean of chemical shifts of the micelles (δmic) and monomers (δmon).32,33 Below CMC:
| δobsd = δmon |
| δobsd = (CMC/CT)(δmon − δmic) + δmic | (1) |
By plotting the chemical shift of α –CH2– protons, as a function of reciprocal MyrCh concentration, we obtained a CMC value of 0.2 mM for MyrCh in PB (pH 8) solution at 25 °C (Fig. 3C), in nice agreement with the one recently reported by Jia et al. in similar conditions.34
The same approach was extended to PDI-Thr/MyrCh mixtures to evaluate the critical aggregation concentration (CAC) above which PDI-Thr/MyrCh aggregates begin to form. The choice of MyrCh α –CH2– protons as probe to follow the PDI-Thr/MyrCh aggregation phenomenon was due to the fact that their chemical shift is slightly affected by the direct interaction with PDI-Thr (see Fig. 3A) and therefore mainly reflects changes in aggregation. In this case, by keeping the PDI-Thr concentration at 0.1 mM, the solutions with the MyrCh concentration range between 0.20 and 0.50 mM were not considered, due to the precipitation phenomenon. However, a CAC upper value of 0.8 mM could be estimated from α –CH2– chemical shift analysis.
From the above results, a scenario similar to that schematized by the cartoon in Fig. 4, can be inferred. PDI-Thr dissolved in PB at pH 8 at a concentration of 0.1 mM is in a highly aggregated, low emissive form. When very small amounts of the surfactant MyrCh are added to this solution, the system is slightly perturbed (MyrCh 0.05–0.10 mM) and no specific interaction is observed by NMR. When MyrCh concentration is increased to 0.20–0.50 mM, insoluble hetero-aggregates PDI-Thr/MyrCh are formed and the solutions become pale pink under ambient light. A possible explanation is that when the stoichiometry surfactant/dye is around 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, MyrCh molecules hinder the solvation sphere of PDI-Thr and the solubility is straightly decreased. We could ascertain by NMR and IR spectroscopy analysis (see Fig. S4†) that the precipitate composition is a mixture of both dye and surfactant. Interestingly, when MyrCh concentration is raised over the PDI-Thr/MyrCh CAC value (0.8), their interaction starts to evolve towards the formation of soluble hetero-aggregates. These hetero-aggregates are able to trigger the fluorescence switch-on of PDI, by inhibiting the π–π stacking-mediated PL self-quenching. The extent of this phenomenon increases with MyrCh concentration.
1, MyrCh molecules hinder the solvation sphere of PDI-Thr and the solubility is straightly decreased. We could ascertain by NMR and IR spectroscopy analysis (see Fig. S4†) that the precipitate composition is a mixture of both dye and surfactant. Interestingly, when MyrCh concentration is raised over the PDI-Thr/MyrCh CAC value (0.8), their interaction starts to evolve towards the formation of soluble hetero-aggregates. These hetero-aggregates are able to trigger the fluorescence switch-on of PDI, by inhibiting the π–π stacking-mediated PL self-quenching. The extent of this phenomenon increases with MyrCh concentration.
 | ||
| Fig. 4 Schematized graphical representation of PDI-Thr aggregates evolution upon increasing MyrCh concentration. | ||
PDI-Thr/MyrCh supramolecular ensembles appear as fluorescent systems, whose surfactochromic equilibrium can be finely tuned by the surfactant concentration. On this basis, the use of PDI-Thr/MyrCh system as a fluorescent probe for the sensing of AChE activity can be envisaged, given the ability of the enzyme to hydrolyze MyrCh and push-back the surfactochromic equilibrium.
Response to AChE activity
As a proof of concept, we monitored the evolution of PL response of the PDI-Thr/MyrCh system in the presence of AChE. MyrCh starting concentration was selected so that: (i) PDI-Thr/MyrCh system was in a switched-on condition; (ii) the surfactant concentration was slightly higher than CAC.Indeed, higher amounts of substrate (MyrCh) would require longer time for the enzymatic transformation, thus increasing the response time of the sensor. For these reasons, we selected 1.5 mM MyrCh as the substrate concentration for running this assay.
We prepared a PB mixture at pH 8 containing PDI-Thr 0.1 mM and MyrCh 1.5 mM, and then we added AChE in the required amount to obtain a final enzyme concentration of 50 U mL−1. The time course of the resulting sample was followed by recording PL spectra at room temperature at regular time intervals (Fig. 5A). Measurable fluorescence changes were observed within 10 min. As the incubating time proceeded, we observed the gradual decrease of the intensities of all the peaks, so that after 300 min the final maximum peak intensity (λ = 545 nm) was reduced of about 70% respect to the starting point. After this time, the plateau was reached, and no evident fluorescence variations were further detected.
The reason why several minutes are necessary to decrease the PL intensity to the final value depends on the relatively high substrate concentration employed in this assay (1.5 mM), selected on the basis of the spectroscopical data. The long response time, which may represent an issue for the development of a full-working sensor, could be shortened by increasing the enzyme concentration over the 50 U mL−1 value here employed.
The above results demonstrate that the addition of AChE to the PDI-Thr/MyrCh mixture is able to reverse the surfactochromic variations induced by MyrCh.
In order to characterize the effect of MyrCh hydrolysis on the evolution of the hetero-aggregates at a molecular level, NMR 1D and 2D DOSY experiments were performed. Indeed the combination of these experiments allows to follow the changes in PDI-Thr/MyrCh interactions and aggregation state during the progression of enzyme activity. The analysis of 1D NMR spectra recorded at different time upon enzyme addition showed the progressive decrease of MyrCh resonances (Fig. 5B) accompanied by the increase of choline resonance. The peak intensity analysis of α –CH2–, β –CH2– and ω –CH3 MyrCh resonances indicated a 35% substrate hydrolysis within the first 40 minutes, upon AChE addition. This observation confirms that the change in optical properties upon enzyme addition are associated to the AChE-catalyzed hydrolysis of MyrCh ester bond, as schematized in the upper part of Fig. 6. In addition, MyrCh NMR resonances gradually shifted back towards those observed in the absence of PDI-Thr, suggesting that MyrCh hydrolysis induces the progressive disassembly of PDI-Thr/MyrCh hetero-aggregates. As shown in Fig. 5B, this variation is evident in particular for those MyrCh protons whose chemical shift is affected by the direct interaction with PDI-Thr (ω –CH3 and the alkyl tail), in accordance to what summarized in Fig. 3A.
NMR diffusion measurements allowed us to monitor the MyrCh-triggered size evolution the PDI-Thr homo-aggregates and to observe the reverse phenomenon upon enzyme addition. Indeed the diffusion coefficient (D) deduced from DOSY experiments recorded on a 0.1 mM PDI-Thr sample at 37 °C was 2.8 × 10−10 m2 s−1, corresponding to self-aggregated species of roughly ten PDI-Thr units (Fig. 5C). When the sample was titrated with MyrCh 1.5 mM, an increase of D values was clearly observed, consistent with the onset of lower molecular weight species, highlighting the disaggregating ability of MyrCh. After 24 h incubation of the PDI-Thr/MyrCh sample with 14 U mL−1 of enzyme the equilibrium was reverted towards the self-assembled PDI-Thr homo-aggregates, corresponding to the switched-off system observed by fluorescence.
We further verified that choline, despite its positive charge, is unable to promote significant PDI-Thr disaggregation, as deduced from absorption and emission measurements (Fig. S5†). This observation supports the important role of the alkyl chain of MyrCh in the formation of the hetero-aggregates and is in agreement with the chemical shift perturbation observed for MyrCh tail upon PDI-Thr addition (Fig. 3A and B).
Altogether the recorded data can be interpreted as schematized in Fig. 6. As AChE starts to promote the hydrolytic breakdown of MyrCh, the PDI-Thr/MyrCh hetero-aggregates are slowly disassembled by the coulombic repulsive interactions between Thr residues and myristic acid fragments. PDI-Thr molecules recover the ability to self-arrange into low emitting homo-aggregates, thus inducing the system switch-off. The regression of the surfactochromic effect operated by AChE is readily measured by PL spectroscopy. It is worth noting here that the scale of PL intensity reduction resulting from the enzymatic activity is adequate to be detected by a standard fluorometer operating at 545 nm fixed wavelength, thus broadening the potential application of our sensing probe also to portable devices.
Conclusions
In summary, we have developed a novel fluorescent probe based on supramolecular ensembles of PDI-Thr and MyrCh. The probe is readily formed by self-assembly in aqueous solution at physiological pH and is sensitive to the aggregation changes. In fact, the addition of increasing concentrations of MyrCh to a non-luminescent PDI-Thr aqueous solution triggers the PL switch-on of the system by surfactochromic effect. By evaluating via NMR the critical concentration for the formation of the fluorescent PDI-Thr/MyrCh ensembles, we were able to finely tune the conditions for realizing a probe sensitive to AChE activity. By catalyzing the hydrolysis of MyrCh, the enzyme gradually restores the PDI-Thr aggregated state accompanied by the PL switch-off that can be monitored by fluorescence spectroscopy.The discussed results demonstrate the ability of PDI-Thr/MyrCh ensembles to transduce the modification of its aggregation state into an easily detectable optical response. This sensing system represents a step forward with respect to the oligothiophene-based probe previously proposed by our group,22 in terms of sensor versatility and ease of fabrication. A further advantage of PDI-Thr probe is that its optical response consists in a significant variation in PL intensity rather than in a change in PL spectrum, suitable to be measured by portable fluorescence detectors.
The only example of perylene-based probe for AChE reported in the literature so far is a turn-on sensor which requires the presence of different components, including a metal agent promoting the aggregation/disaggregation of perylene.35 In our approach, by contrast, the active probe formation requires the simple mixture of the fluorophore and surfactant components in the proper ratio.
In view of developing a sensing platform suitable for AChE activity assays, we are currently further simplifying the sensing system by direct functionalization of the PDI derivative with the surfactant. We expect that this approach will reduce the starting concentration of the enzyme substrate, resulting in a faster response, a key factor in view of the practical application as fluorescent probe for the screening of AChE inhibitors as anti-Alzheimer drugs.
Acknowledgements
This research was supported by Accordo Quadro Regione Lombardia/CNR (decreto 3667/2013) within the project “TIMES”. G. G. and K. P. acknowledge Fondazione Antonio de Marco for financial support.References
- W. Jiang, L. Ye, X. G. Li, C. Y. Xiao, F. Tan, W. C. Zhao, J. H. Hou and Z. H. Wang, Chem. Commun., 2014, 50, 1024–1026 RSC.
- X. Zhang, Z. H. Lu, L. Ye, C. L. Zhan, J. H. Hou, S. Q. Zhang, B. Jiang, Y. Zhao, J. H. Huang, S. L. Zhang, Y. Liu, Q. Shi, Y. Q. Liu and J. N. Yao, Adv. Mater., 2013, 25, 5791–5797 CrossRef CAS PubMed.
- E. Kozma and M. Catellani, Dyes Pigm., 2013, 98, 160–179 CrossRef CAS.
- S. H. Kim, Y. S. Yang, J. H. Lee, J.-I. Lee, H. Y. Chu, H. Lee, J. Oh, L.-M. Do and T. Zyung, Opt. Mater., 2003, 21, 439–443 CrossRef CAS.
- R. T. Weitz, K. Amsharov, U. Zschieschang, E. B. Villas, D. K. Goswami, M. Burghard, H. Dosch, M. Jansen, K. Kern and H. Klauk, J. Am. Chem. Soc., 2008, 130, 4637–4645 CrossRef CAS PubMed.
- L. Li, M. Guan, G. Cao, Y. Li and Y. Zeng, Appl. Phys. A: Mater. Sci. Process., 2010, 99, 251–254 CrossRef CAS.
- F. J. Cespedes-Guirao, S. Garcia-Santamaria, F. Fernandez-Lazaro, A. Sastre-Santos and H. J. Bolink, J. Phys. D: Appl. Phys., 2009, 42, 105106 CrossRef.
- E. Kozma, W. Mróz and F. Galeotti, Dyes Pigm., 2015, 114, 138–143 CrossRef CAS.
- T. Heek, C. Fasting, C. Rest, X. Zhang, F. Wurthner and R. Haag, Chem. Commun., 2010, 46, 1884–1886 RSC.
- D. Gorl, X. Zhang and F. Wurthner, Angew. Chem., Int. Ed., 2012, 51, 6328–6348 CrossRef PubMed.
- A. K. Dwivedi, M. Pandeeswar and T. Govindaraju, ACS Appl. Mater. Interfaces, 2014, 6, 21369–21379 CAS.
- J. J. Lavigne, D. L. Broughton, J. N. Wilson, B. Erdogan and U. H. Bunz, Macromolecules, 2003, 36, 7409–7412 CrossRef CAS.
- Y. Y. Lin, R. Chapman and M. M. Stevens, Anal. Chem., 2014, 86, 6410–6417 CrossRef CAS PubMed.
- D. M. Quinn, Chem. Rev., 1987, 87, 955–979 CrossRef CAS.
- T. Pradhan, H. S. Jung, J. H. Jang, T. W. Kim, C. Kang and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4684–4713 RSC.
- A. V. Terry and J. J. Buccafusco, J. Pharmacol. Exp. Ther., 2003, 306, 821–827 CrossRef CAS PubMed.
- A. Kumar, A. Singh and E. Ekavali, Pharmacol. Rep., 2015, 67, 195–203 CrossRef CAS PubMed.
- F. Feng, Y. Tang, S. Wang, Y. Li and D. Zhu, Angew. Chem., Int. Ed., 2007, 46, 7882–7886 CrossRef CAS PubMed.
- M. Wang, X. Gu, G. Zhang, D. Zhang and D. Zhu, Anal. Chem., 2009, 81, 4444–4449 CrossRef CAS PubMed.
- Y. G. Li, H. Bai, C. Li and G. Q. Shi, ACS Appl. Mater. Interfaces, 2011, 3, 1306–1310 CAS.
- E. K. Wujcik, N. J. Londoño, S. E. Duirk, C. N. Monty and R. I. Masel, Chemosphere, 2013, 91, 1176–1182 CrossRef CAS PubMed.
- G. Grisci, W. Mroz, U. Giovanella, K. Pagano, W. Porzio, L. Ragona, F. Samperi, S. Tomaselli, F. Galeotti and S. Destri, J. Mater. Chem. B, 2015, 3, 4892–4903 RSC.
- E. Kozma, G. Grisci, W. Mróz, M. Catellani, A. Eckstein-Andicsovà, K. Pagano and F. Galeotti, Dyes Pigm., 2016, 125, 201–209 CrossRef CAS.
- A. Jerschow and N. Müller, J. Magn. Reson., 1998, 132, 13–18 CrossRef CAS.
- T. L. Hwang and A. J. Shaka, J. Magn. Reson., Ser. A, 1995, 112, 275–279 CrossRef CAS.
- E. O. Stejskal and J. E. Tanner, J. Chem. Phys., 1965, 42, 288–292 CrossRef CAS.
- A. Einstein, Investigations on the Theory of Brownian Movement, Dover Publications, New York, 1956 Search PubMed.
- R. C. Reid, J. M. Prausnitz and B. E. Poling, The Properties of Gases and Liquids, McGraw-Hill, New York, 4th edn, 1987 Search PubMed.
- D. Li, G. Kagan, R. Hopson and P. G. Williard, J. Am. Chem. Soc., 2009, 131, 5627–5634 CrossRef CAS PubMed.
- M. Sadrai, L. Hadel, R. R. Sauers, S. Husain, K. Krogh-Jespersen, J. D. Westbrook and G. R. Bird, J. Chem. Phys., 1992, 96, 7988–7996 CrossRef CAS.
- Label 0 → 0 denotes transitions from the lowest energy vibrational level (0) of the ground electronic state (S0) of the molecule to the lowest energy vibrational level (0) of the first electronic excited state (S1). Label 0 → 1 corresponds to transitions from the lowest energy vibrational level (0) of the ground electronic state to the first excited vibrational level (1) of the first electronic excited state.
- S. Barhoum and A. Yethiraj, J. Chem. Phys., 2010, 132, 024909 CrossRef PubMed.
- X. Cui, S. Mao, M. Liu, H. Yuan and Y. Du, Langmuir, 2008, 24, 10771–10775 CrossRef CAS PubMed.
- L. Jia, J. Pan and J. X. Zhu, Anal. Methods, 2013, 5, 5431–5436 RSC.
- D. L. Liao, J. Chen, H. P. Zhou, Y. Wang, Y. X. Li and C. Yu, Anal. Chem., 2013, 85, 2667–2672 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 2D DOSY and PL comparison of PDI-Thr and PDI-Asp, MyrCh 1H NMR assignment, IR and NMR characterization of the insoluble hetero-aggregate, PDI-Thr spectroscopies in the presence of choline. See DOI: 10.1039/c6ra08869d |
| This journal is © The Royal Society of Chemistry 2016 |




