Mussel inspired locomotive: the moisture induced actuation in a poly(vinyl alcohol) film containing melanin-like dopamine nano spheres†
Francis O. Obiweluozor‡
ab,
Amin GhavamiNejad‡*a,
Chan Hee Park*ab and
Cheol Sang Kim*ab
aDepartment of Bionanosystem Engineering, Graduate School, Chonbuk National University, Jeonju 561-756, Republic of Korea. E-mail: chskim@jbnu.ac.kr; biochan@jbnu.ac.kr; ghavaminejad@jbnu.ac.kr
bDivision of Mechanical Design Engineering, Chonbuk National University, Jeonju City, Republic of Korea
First published on 4th July 2016
Abstract
Smart responsive materials are gaining scientific interest, especially those driven by green sources. Moisture induced locomotion is a process of energy conversion that has applications in areas capable of converting chemical potential energy in water gradients to mechanical work. Here we describe for the first time the development of a moisture-responsive polymeric film of polydopamine nanospheres (PDNs) embedded in a polyvinyl alcohol (PVA) matrix. PVA film is a hydroxyl rich polymer known for its high water absorption property. However, after adding PDNs to this matrix, the desorption property increases due to the intramolecular hydrogen bonding between the functional groups of PDNs and the hydroxyl groups of PVA chains. Therefore, anisotropic adsorption and desorption of water vapor, which is responsible for the fast locomotion of PVA@PDNs, were observed.
Introduction
Recent developments in multifunctional stimuli-responsive systems, such as smart polymers that reversibly change their shape, size, or mechanical properties in response to external stimuli have attracted considerable interest due to their potential applications as smart devices, sensors, environmental monitors, ultrasensitive artificial skins, actuators for biomedical and mechanical purposes, organic transistor-based pressure sensing matrices and so on.1–8 Smart actuators are materials that can reversibly change their magnitude under certain stimuli or convert various types of energy to mechanical deformation.9,10 In recent years, a wide variety of cutting edge techniques, including electrostatic or piezoelectric actuation,11 shape memory alloy,12 optical tweezers13 and pneumatic systems14 have been successfully adopted for the development of smart actuators. However, most of these devices exhibit slower responses and lower stress generation. They also generally require external energy-supply systems, which limits their applications.In this regard, pursuing new stimuli responsive signals has become a crucial task of both scientific and industrial importance for manipulation at a longer range.15 Inspired by nature, moisture responsive materials are being investigated, with mechanisms similar to plants in the wake of seed dispersion or spore release16 as well as the opening of pine cones. During the alteration of environmental moisture content, i.e. relative humidity, a particular part of biological systems reversibly absorbs or releases moisture. During this process, a mechanical deformation takes place, with the goal of performing a specific function such as directed complex motions.16 Most researchers had delved into this area of study due to ecofriendly nature of water vapor which tend to exist in most moist surfaces like human palm. More research in this area could bridge the gap between man machine interaction systems (MMIS).17
Poly(vinyl alcohol) (PVA) is a synthetic water-soluble, biodegradable and biocompatible polymer with good mechanical properties and outstanding gas barrier properties against oxygen.17,18 More so, PVA is a good film forming polymer which has applications in various industrial sectors, for instance, as a membrane and as packaging materials.19–23 On the other hand, melanin is a well-known biopolymer that is found in various organisms and has many distinct functions, including the protection of humans and animals from ultraviolet injury, thermoregulation, antibiotic function, free radical quenching, and some nervous system involvement.24–28 Little effort has been applied to exploit the mechanical properties in melanin related materials in comparison to other biopolymers such as cellulose, chitin, or collagen.29 In general, the predominant forms of melanin in humans include brown black eumelanin and yellow-reddish pheomelanin.30 Although it's hard to characterize the fine chemical structure of melanin, it is well known that there are several functional groups in melanin, such as –NH– and –OH. Therefore, if melanin nanoparticles (PDNs) are incorporated in a PVA matrix with a large number of –OH groups, due to the strong hydrogen bonding between melanin and PVA, nanoparticles can be homogenously dispersed in the composite.27,31 We report here for the first time a PVA film incorporated with PDNs that is capable of fast and perpetual motion driven by a humidity gradient. The composite material (PVA@PDNs) utilizes the chemical potential of even very small humidity gradients for rapid and non-uniform exchange of water with the surroundings to propel itself through a series of swift and regular mechanical deformations, when exposed to a moisture source. To optimize the film performance, films of varying thickness containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of PVA and PDNs were chosen as the best humidity responsive film. This is as a result of balance in the functional groups between the PVA and PDNs, which creates a functional group equilibrium that drives mechanical deformation in the system. The composite film could perform multiform locomotion depending on the aspect ratio. Films that were cut into a strip could perform a spiral locomotion with a displacement of 0.7 mm s−1. This material could also transport a load 250% more than its weight.
1 ratio of PVA and PDNs were chosen as the best humidity responsive film. This is as a result of balance in the functional groups between the PVA and PDNs, which creates a functional group equilibrium that drives mechanical deformation in the system. The composite film could perform multiform locomotion depending on the aspect ratio. Films that were cut into a strip could perform a spiral locomotion with a displacement of 0.7 mm s−1. This material could also transport a load 250% more than its weight.
Experimental
Materials
3,4-Dihydroxyphenethylamine hydrochloride (dopamine monomer), polyvinyl alcohol (Wako pure chemicals industries ltd, no. 160-08295), ethanol and ammonium hydroxide were purchased from Sigma-Aldrich, South Korea. All aqueous solutions were prepared with ultrapure water purified with a Milli-Q UV-Plus water purification system (Millipore, Bedford, MA). The water had a resistivity of >1018 MU cm−1.Preparation and characterization of PVA@PDNs composite film
First, synthesis of PDNs was carried out in a water–alcohol mixed solvent according to the literature.32 The alcohol (methanol, ethanol, or 2-propanol) was mixed with water and the volume of the mixed solvent was fixed at 65 mL in a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. Ammonia aqueous solution (NH4OH, 1 mL, 28–30%) was added to the alcohol/water mixtures under mild stirring at room temperature for 30 min. Finally, dopamine hydrochloride was directly added to the mixed solution. The color of this solution immediately turned to pale brown and gradually changed to dark brown. The reaction was allowed to proceed for 30 h. The product was centrifuged and washed three times with water and anhydrous ethanol then dried in vacuum overnight. An equal ratio of polyvinyl alcohol and PDNs was mixed in aqueous solution after dip sonication of PDNs. The resultant dark brown composite was cast onto a silicon glass. Film was obtained after drying in air over 2 days, then cut into various shapes.
9. Ammonia aqueous solution (NH4OH, 1 mL, 28–30%) was added to the alcohol/water mixtures under mild stirring at room temperature for 30 min. Finally, dopamine hydrochloride was directly added to the mixed solution. The color of this solution immediately turned to pale brown and gradually changed to dark brown. The reaction was allowed to proceed for 30 h. The product was centrifuged and washed three times with water and anhydrous ethanol then dried in vacuum overnight. An equal ratio of polyvinyl alcohol and PDNs was mixed in aqueous solution after dip sonication of PDNs. The resultant dark brown composite was cast onto a silicon glass. Film was obtained after drying in air over 2 days, then cut into various shapes.
Characterization
Results and discussion
Herein, we fabricated a dynamic polymer composite film consisting of melanin-like polydopamine nano spheres (PDNs) embedded in a flexible polyvinyl alcohol (PVA) matrix labeled PVA@PDNs that would be responsive to water vapor absorption and desorption under ambient conditions of 25% to 50% humidity. Pure PVA film is known for its high moisture absorption property with minimal amounts of desorption due to the H-bond interaction of hydroxyl groups with water molecules. However, when combined with PDNs, intermolecular hydrogen bonding occurs between the catecholic OH-groups and NH-groups of PDNs with the hydroxyl groups of PVA chains. Recently, Wang et al. demonstrated that melanin nanoparticles significantly improved the thermal and mechanical properties of PVA due to the strong hydrogen bonding, suggesting that there is great potential in melanin nanoparticles for the reinforcement of polymer composites.27,31 These intermolecular hydrogen bonding networks between the PDNs and the PVA are sensitive to water vapor by means of hydrolysis and reforming of the network upon absorption and desorption, which changes the mechanical property of the composite (Fig. 1).The hydrogen bonding plays a significant role in modulating the intermolecular packing of PVA@PDNs. Therefore, when this film is placed on a moisture source, it exhibits fast, reversible, and dramatic mechanical deformation and recovery (movie S1†). The film always deflects away from the source of humidity as a result of the expansion force of PVA chains on the side facing the humidity source caused by water absorption. This causes the film to curl and uncurl consecutively and in different directions, thereby propelling quickly across the filter paper barrier. The motion of the film can be stopped when it is removed from the source of humidity and reinitiated when placed back. The locomotion can be turned on and off in this manner for an unlimited number of times without apparent fatigue.
To demonstrate the capability of the film to perform mechanical work, silver rods weighing 250% times the weight of the film was attached as cargo. The loaded film is shown in movie S2.† In addition to the fast response of the film at 50% humidity, we found that even a very small amount of humidity gradient on a bare finger can trigger the response of this material. Thin film fixed on a support deflects away when approached with a bare finger whereas it acquires stress instantaneously when placed on a palm and folds up or moves swiftly away from the palm due to absorption of moisture vapor from the skin (movie S3†). The reversibility of water absorption and desorption was confirmed by weight measurement experiments under humidity exposure and non-exposure (Fig. 2).
 | ||
| Fig. 2 PVA@PDNs film showing variation of weight change with relative humidity of air. The film was not saturated with water vapor in this experiment before the weight loss was recorded. | ||
Desorption was monitored in situ, after the film was exposed to a high humidity source (RH-50%) for 5 min and excess water on the surface was eliminated. As the film exchanged water with the environment, the resultant weight was recorded on a micro balance. Without the high surface-area-to-volume ratio that promotes water desorption, equilibrium will not be established between the water absorbed in the film and the surroundings, which consequently propels the ceaseless motion of the film. The dynamic equilibrium can be shifted and re-established on a minute time scale simply by the variation of relative humidity, which is reflected in the weight of the film. In the first cycle, 8 wt% absorbed water was desorbed. However, in the 2nd and 3rd cycles, 15 wt% absorbed water was found to be desorbed at a later time. In these cycles of hydration and dehydration experiments, we concluded that the optimal water content for rapid locomotion is 8–15%; lower water content is insufficient for swift locomotion while at higher water content, the film fast locomotion is mitigated by the film coiling up on itself.
In order to better investigate the difference between PVA and PVA@PDNs films in terms of absorption–desorption rate, the time-dependent ATR-FTIR spectroscopy of the films were studied (Fig. 3a) after soaking the films in D2O vapor and keeping them in air for up to 3 min. After D2O exposure, an intense peak at ∼2500 cm−1 was observed for the PVA film, which belongs to the absorption band of D2O. As expected, after keeping the film in the air, the band at ∼3330 cm−1 increased due to the adsorption of water vapor from the surroundings without noticeable changes in the D2O peak, indicating a fast absorption capability of OH− group in the pure PVA film. However, the ATR-FTIR spectrum for the PVA@PDNs film showed a different behavior compared to the PVA film. After exposing the PVA@PDNs film that was soaked in D2O to the air, the band at ∼3330 cm−1 increased with a concomitant decrease of the band at ∼2500 cm−1, indicating the rapid desorption of D2O and concomitant adsorption of water molecules from the surroundings in PVA@PDNs. This result clearly proves our hypothesis that after applying humidity, there are absorption–desorption processes in the PVA@PDN film due to the intermolecular hydrogen bonding between the hydroxyl groups of PVA and the functional groups of PDNs. However, only absorption takes place in the pure PVA film. The FTIR-ATR result for the PVA@PDNs also shows that the –OH stretching band of pure PVA shifted from 3320 to 3334 cm−1. Furthermore, the amide I region, N–H shearing band, and catecholic C–OH stretching band of the PDNs (Fig. SI1†) are also shifted from 1614, 1510, and 1285 to 1604, 1505 and 1265 cm−1 respectively. These phenomena demonstrate the existence of hydrogen bonding between the hydroxyl groups of PVA and the functional groups of PDNs.
Fig. 3b shows the changes in the catecholic C–OH stretching band of PDNs, PVA@PDNS, and PVA@PDNS after applying humidity. As shown, after mixing with PVA, the catecholic peak shifted from 1285 to 1264 cm−1 due to hydrogen bonding. However, after the sample was exposed to humidity for 3 min, the C–OH stretching vibration of the catechol groups significantly decreased in intensity, indicating that the catechol groups lost their hydrogens bonds and were converted to a quinone structure.33 This result strongly suggests that the intermolecular hydrogen bonding network between the PDNs and the PVA is also sensitive to water vapor via processes that involve the breaking of hydrogen bonds by water molecules. This exothermic process uses energy to break the hydrogen bonds and this energy can also continuously drive the film away from the source of humidity.
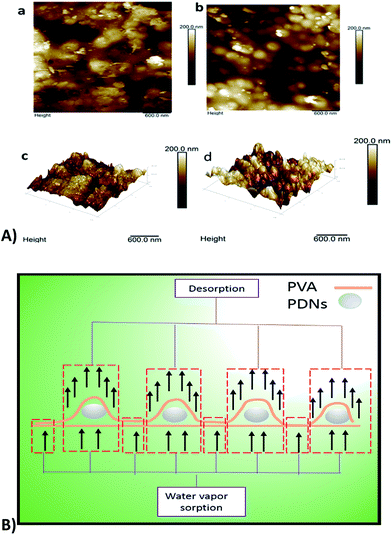
In an effort to draw correlations between the structural changes during the absorption–desorption process, atomic force microscopy (AFM) analysis was conducted. Comparing the images before and after humidity sorption, it could be clearly observed that the absorption of water vapor alters the surface morphology with the formation of ridges (Fig. 4A) and the increase of the roughness from 22.5 to 33.1 nm. Furthermore, in contrast to the surface in (Fig. 4Aa and c), there are more circular protrusions after the sample was exposed to humidity (Fig. 4Ab and d). This is as a result of the replacement of hydrogen bonding with water molecules around the PDNs, which increases the roughness and makes the particles more evident. After applying humidity, there are absorption–desorption processes around the PDN particles due to the intermolecular hydrogen bonding between the hydroxyl groups of PVA and the functional groups of PDNs. However, only absorption takes place at the areas of the film without PDNs (Fig. 4B).
The strong hydrogen bonding interaction between PDNs and PVA is also supported by the results of the TGA experiments. Thermogravimetric analysis was performed at the temperature range of 10 and 800 °C to examine the thermal stability of the PDNs, PVA films and PVA@PDNs composite films. As illustrated in (Fig. 5), pristine PDNs started to lose their weight at about 80 °C and beyond 800 °C, 50% of the original PDN content was left. The weight loss at around 200 °C is ascribed to the loss of hydroxyl groups and the degradation around 380 °C was a result of loss of the amide groups and alkyl spacers.34 The pure PVA film shows a degradation transition at 100 °C which is ascribed to loss of the moisture absorbed in the pure PVA. Furthermore, this film almost shows a complete degradation with a residue of less than 2% at 800 °C. The degradation transition behavior of PVA@PDNs is slightly delayed in comparison to the pure PVA and the addition of PDNs in the PVA matrix leads to a residue of 32%, which is higher than the expected residue of 25% for this sample. Hence, it can be proposed that strong hydrogen bonding between the hydroxyl groups of PVA and the functional groups of PDNs improve the thermal stability of the nanocomposite. Similar phenomena have been reported by GhavamiNejad et al. after the interaction of PNIPAM with graphene oxide sheets.35
In order to examine the effect of the shape and aspect ratio on the locomotion of the film, a film was cut into a triangular shape. The result shows different patterns or mechanisms of locomotion for a 39 s time frame (Fig. 6a). The first row shows an increase in time by 1 s, the second row shows a time increase of 2 s while the third row shows an increase in time by 5 s. The pictures show that the shape of the film determines how fast the film responds to the humidity gradient. It was observed that the film cut in a triangular shape undergoes 10 stages of locomotive cycles as shown in (Fig. 6b). When the PVA@PDNs film is placed on a moist surface, the side in contact with the substrate absorbs moisture faster than the upper side, which results in asymmetric swelling and the film curling away from the substrate. Stage (1) was a very fast process, and is illustrated in (Fig. 4b). So, the film curls up into a cone shape in (1) and due to mechanical instability caused by moisture absorption and desorption, the film eventually unfolds (2). As the film unfolds, one corner begins rolling up (3). Concomitantly, the other side rises, forming (4). Due to loss of the center of gravity, the film falls over (5). The end with a high surface area in contact with the substrate coils up (6). The other flange under the assembly pulls out (7). This pushes the film in the other direction (8), and due to asymmetric swelling, the unfolding film continues to curl up to start a new cycle (9), and the remaining side then rolls up (10). Interestingly, when a film of PVA@PDNs was cut into strips (3 cm × 0.5 cm), the mechanism of locomotion changed literally to a twisting motion with a displacement of 0.7 mm s−1 (Fig. 6c; movie S4 and Fig. SI2†) comprising of 5 stages. Briefly, the film coils up from both ends (1, 2) and topples over on the faster end (3) prompting the other side to twist facing down (4). The motion is repeated after the film (5) is flattened.
Conclusions
In summary, we feature herein a composite of PVA@PDNs with the ability to perform mechanical deformation by converting chemical potential energy in a humidity gradient to mechanical work. Due to anisotropic absorption and desorption in the film surface which propels this material into various shapes, locomotion occurs including folding, flipping and helical twisting. The material can transport a cargo 250% heavier than its weight and attain a displacement of 0.7 mm s−1. This material could be utilized to fabricate a control device that can monitor humidity changes, sensors and switches and serve as a power source for ultralow-power devices.Acknowledgements
This research was supported with grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (Project No. 2015-020449 Project No. 2016R1A2A2A07005160 and Project No. NRF-2015R1C1A1A02036404. The authors would also like to thank the staff of the CBNU central lab.Notes and references
- G. X. Bai, M. K. Tsang and J. H. Hao, Adv. Opt. Mater., 2015, 3, 431–462 CrossRef CAS.
- A. P. Blum, J. K. Kammeyer, A. M. Rush, C. E. Callmann, M. E. Hahn and N. C. Gianneschi, J. Am. Chem. Soc., 2015, 137, 2140–2154 CrossRef CAS PubMed.
- P. P. Guo, G. Y. Zhao, P. L. Chen, B. Lei, L. Jiang, H. T. Zhang, W. P. Hu and M. H. Liu, ACS Nano, 2014, 8, 3402–3411 CrossRef CAS PubMed.
- L. Isaacs, Acc. Chem. Res., 2014, 47, 2052–2062 CrossRef CAS PubMed.
- P. Theato, B. S. Sumerlin, R. K. O'Reilly and T. H. Epps, Chem. Soc. Rev., 2013, 42, 7055–7056 RSC.
- M. K. Tsang, G. X. Bai and J. H. Hao, Chem. Soc. Rev., 2015, 44, 1585–1607 RSC.
- H. B. Wei, N. Shi, J. L. Zhang, Y. Guan, J. Zhang and X. H. Wan, Chem. Commun., 2014, 50, 9333–9335 RSC.
- F. O. Obiweluozor, A. GhavamiNejad, S. Hashmi, M. Vatankhah-Varnoosfaderani and F. J. Stadler, Macromol. Chem. Phys., 2014, 215, 2125 CrossRef CAS.
- P. Brochu and Q. B. Pei, Macromol. Rapid Commun., 2010, 31, 10–36 CrossRef CAS PubMed.
- V. A. Ganesh, A. Baji and S. Ramakrishna, RSC Adv., 2014, 4, 53352–53364 RSC.
- S. Sridaran and S. A. Bhave, Opt. Express, 2011, 19, 9020–9026 CrossRef PubMed.
- A. Villanueva, C. Smith and S. Priya, Bioinspiration Biomimetics, 2011, 6 Search PubMed.
- S. Kawata, H. B. Sun, T. Tanaka and K. Takada, Nature, 2001, 412, 697–698 CrossRef CAS PubMed.
- R. V. Martinez, A. C. Glavan, C. Keplinger, A. I. Oyetibo and G. M. Whitesides, Adv. Funct. Mater., 2014, 24, 3003–3010 CrossRef CAS.
- M. Zirkl, A. Sawatdee, U. Helbig, M. Krause, G. Scheipl, E. Kraker, P. A. Ersman, D. Nilsson, D. Platt, P. Bodo, S. Bauer, G. Domann and B. Stadlober, Adv. Mater., 2011, 23, 2069–2074 CrossRef CAS PubMed.
- P. Fratzl and F. G. Barth, Nature, 2009, 462, 442–448 CrossRef CAS PubMed.
- C. M. Hassan and N. A. Peppas, Abstr. Pap. Am. Chem. Soc., 1998, 216, U908 Search PubMed.
- N. A. Peppas and S. L. Wright, Eur. J. Pharm. Biopharm., 1998, 46, 15–29 CrossRef CAS PubMed.
- J. B. Chang, J. H. Hwang, J. S. Park and J. P. Kim, Dyes Pigm., 2011, 88, 366–371 CrossRef CAS.
- P. C. Liu, J. H. Hsieh, C. Li, Y. K. Chang and C. C. Yang, Thin Solid Films, 2009, 517, 4956–4960 CrossRef CAS.
- J. L. Valentin, D. Lopez, R. Hernandez, C. Mijangos and K. Saalwachter, Macromolecules, 2009, 42, 263–272 CrossRef CAS PubMed.
- S.-H. Hwang, D. Kang, R. S. Ruoff, H. S. Shin and Y.-B. Park, ACS Nano, 2014, 8, 6739–6747 CrossRef CAS PubMed.
- Q. M. Bai, G. Z. Zhang, B. Xu, X. Q. Feng, H. Y. Jiang and H. J. Li, RSC Adv., 2015, 5, 91213–91217 RSC.
- G. A. Duff, J. E. Roberts and N. Foster, Biochemistry, 1988, 27, 7112–7116 CrossRef CAS PubMed.
- C. C. Felix, J. S. Hyde, T. Sarna and R. C. Sealy, J. Am. Chem. Soc., 1978, 100, 3922–3926 CrossRef CAS.
- A. GhavamiNejad, L. E. Aguilar, R. B. Ambade, S.-H. Lee, C. H. Park and C. S. Kim, Colloids Interface Sci. Commun., 2015, 6, 5–8 CrossRef CAS.
- Y. Wang, Z. Wang, P. M. Ma, H. Y. Bai, W. F. Dong, Y. Xie and M. Q. Chen, RSC Adv., 2015, 5, 72691–72698 RSC.
- A. GhavamiNejad, C. H. Park and C. S. Kim, Biomacromolecules, 2016, 17, 1213–1223 CrossRef CAS PubMed.
- W. B. Li, Y. J. Liu and J. S. Leng, J. Mater. Chem. A, 2015, 3, 24532–24539 CAS.
- K. Shanmuganathan, J. H. Cho, P. Iyer, S. Baranowitz and C. J. Ellison, Macromolecules, 2011, 44, 9499–9507 CrossRef CAS.
- S. Q. Xiong, Y. Wang, J. R. Yu, L. Chen, J. Zhu and Z. M. Hu, J. Mater. Chem. A, 2014, 2, 7578–7587 CAS.
- Y. L. Liu, K. L. Ai, J. H. Liu, M. Deng, Y. Y. He and L. H. Lu, Adv. Mater., 2013, 25, 1353–1359 CrossRef CAS PubMed.
- A. GhayamiNejad, A. R. Unnithan, A. Ramachandra, K. Sasikala, M. Samarikhalaj, R. G. Thomas, Y. Y. Jeong, S. Nasseri, P. Murugesan, D. M. Wu, C. H. Park and C. S. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 12176–12183 Search PubMed.
- V. K. Thakur, D. Vennerberg, S. A. Madbouly and M. R. Kessler, RSC Adv., 2014, 4, 6677–6684 RSC.
- A. GhavamiNejad, S. Hashmi, H. I. Joh, S. Lee, Y. S. Lee, M. Vatankhah-Varnoosfaderani and F. J. Stadler, Phys. Chem. Chem. Phys., 2014, 16, 8675–8685 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11987e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |