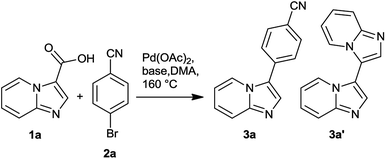
Ligand-free Pd-catalysed decarboxylative arylation of imidazo[1,2-a]pyridine-3-carboxylic acids with aryl bromides†
Uttam B. Karaleab,
Saradhi Kalaria,
Jala Shivakumarc,
Vitthal B. Makaneab,
Dattatraya A. Babarab,
Ritesh P. Thakared,
Bathini Nagendra Babuc,
Sidharth Choprad and
Haridas B. Rode*ab
aDepartment of Natural Product Chemistry, CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad-500007, India. E-mail: haridas.rode@iict.res.in
bAcademy of Scientific and Innovative Research (AcSIR), New Delhi, India
cDepartment of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research, Balanagar, Hyderabad-500 037, India
dMicrobiology Division, Central Drug Research Institute, Lucknow-226 031, India
First published on 4th July 2016
Abstract
A facile ligand-free method for Pd(OAc)2 catalysed decarboxylative arylation of imidazo[1,2-a]pyridine-3-carboxylic acids with hetero(aryl) bromides has been developed. This method is applicable to a variety of (hetero)aryl bromides as coupling partners. Electron withdrawing and donating groups on imidazo[1,2-a]pyridine-3-carboxylic acids are well tolerated. It represents the first general protocol for ligand-free Pd(OAc)2 catalysed decarboxylative arylation of imidazo[1,2-a]pyridine-3-carboxylic acids with (hetero)aryl halides. A few of the compounds synthesized using this protocol showed antibacterial activity against Staphylococcus aureus.
Introduction
Imidazopyridines have attracted much attention due to their unique biological properties recently.1 Imidazo[1,2-a]pyridine, in particular, is an important scaffold and has shown various biological activities including antiviral, analgesic, anthelmintic, antifungal, antibacterial, antiprotozoal, anxiolytic etc.2 A preclinical candidate Q203 has been developed for its antimycobacterial potential and has shown significant activity against the multidrug resistant strains of Mycobacterium tuberculosis.3 The marketed drugs zolpidem, necopidem, olprinone, saripidem, alpidem contain imidazo[1,2-a]pyridine scaffold.4 As a result, modifications of imidazo[1,2-a]pyridine have been a focus of drug discovery research. Chemically diverse set of compounds can be obtained in one step within a reasonably short duration of time using palladium catalyzed cross-coupling reactions. The modification of imidazo[1,2-a]pyridines using Suzuki-type cross-coupling5 and Stille cross-coupling6 have been reported. However, these reactions showed limited substrate scope, and commercial availability of heteroaromatic boronic acids or stannanes limits its applicability. Further, the strategy for regioselective palladium-catalyzed arylation of imidazo[1,2-a]pyridine with aryl/heteroaryl bromide has been described and depends on triphenyl phosphine as ligand or Pd(PPh3)4.2c,7 Recently palladium catalyzed oxidative cross-coupling reaction to obtain 3-aryl imidazo[1,2-a]pyridine has been demonstrated albeit with generation of two regioisomers in few cases.8 The direct arylation of imidazo[1,2-a]pyridines has also been reported.9 These methods make use of aryl bromides or tosylate or mesylate. However, they have either limited substrate scope with respect to substituents on imidazo[1,2-a]pyridines core9a,b,e or limited to C-2 substituted9c,d imidazo[1,2-a]pyridines.The decarboxylative arylation reaction makes use of carboxylic acids and aryl halides. In general, aromatic acids are stable solids, thereby easy to handle, making such strategy an important synthetic method.10 Only two methods have been reported for the decarboxylative arylation of imidazo[1,2-a]pyridine-3-carboxylic acid till date. First method uses Pd(0)bis(carbene) complex, prepared from NHC ligand precursor and palladium precursor,11 and has limited substrate scope.12 The second method uses aryl chloride with 5 mol% Pd(OAc)2 and S-Phos as ligand in DMA/H2O medium.10 This method requires phosphine ligand and many a times it become difficult to separate such ligands from the product, jeopardizing its applicability. Hence, a ligand-free catalytic approach becomes the method of choice in such scenario. Ligandless catalytic process are the most economical and efficient ones and hence are widely used in industries.13 Hence, there is a need for a ligandless facile synthesis of C-3 substituted imidazo[1,2-a]pyridines. Herein, we report a method for synthesis of C-3 aryl/heteroaryl imidazo[1,2-a]pyridines from aryl/heteroaryl bromides and imidazo[1,2-a]pyridine-3-carboxylic acids with palladium acetate in the absence of ligand. This facile protocol offers good tolerability towards various aryl/heteroaryl bromides and also applicable to various imidazo[1,2-a]pyridine-3-carboxylic acids. Till date there is no report on ligand-free palladium(II) catalyzed decarboxylative arylation of imidazo[1,2-a] pyridine-3-carboxylic acid with aryl halides.
Results and discussion
For initial optimization of reaction conditions, the decarboxylative arylation between imidazo[1,2-a]pyridine-3-carboxylic acid (1a) with 4-bromobenzonitrile (2a) in the presence of catalytic amount of Pd(OAc)2 at 160 °C in DMA solvent was selected as a model reaction. The results are summarized in Table 1.| Entry | 1a(equiv.) | Base (equiv.) | Pd(OAc)2 (equiv.) | 3ab(%) |
|---|---|---|---|---|
| a Reaction conditions: 1a, 2a (0.1 g, 1 equivalent), base, Pd(OAc)2 in 7 mL DMA at 160 °C for 24 h under N2 atmosphere.b Isolated yields.c Starting material 1a was present after 24 h of reaction time.d Traces of decarboxylative homodimerization product 3a′ observed.e 0.5 equivalent of TBAB was used.f 0.5 equivalent of TBAI was used.g NMP was used as solvent.h DMF was used as solvent. | ||||
| 1 | 1.2 | Ag2CO3 (2) | 0.10 | 42 |
| 2c | 1.2 | Ag2CO3 (1) | 0.10 | 17 |
| 3 | 1.2 | AgOAc (2) | 0.10 | 50 |
| 4 | 1.2 | KOAc (2) | 0.10 | 62 |
| 5 | 1.2 | Cu(OAc)2 (2) | 0.10 | 25 |
| 6 | 1.2 | KOAc (2) | 0.05 | 84 |
| 7 | 1.2 | KOAc (2) | 0.02 | 71 |
| 8c | 1.2 | KOAc (2) | 0.01 | 67 |
| 9 | 1.2 | KOAc (2) | 0.02 | 71 |
| 10 | 1.3 | KOAc (2) | 0.02 | 80 |
| 11 | 1.4 | KOAc (2) | 0.02 | 85 |
| 12d | 1.5 | KOAc (2) | 0.02 | 84 |
| 13d | 1.6 | KOAc (2) | 0.02 | 83 |
| 14e | 1.4 | KOAc (2) | 0.02 | 80 |
| 15f | 1.4 | KOAc (2) | 0.02 | 75 |
| 16g | 1.4 | KOAc (2) | 0.02 | 60 |
| 17h | 1.4 | KOAc (2) | 0.02 | 77 |
The screening of various bases with 10 mol% Pd(OAc)2 resulted in low to moderate yield of 3a (entry 1 to 5). Of note the use of Ag2CO3 as base resulted in byproducts formation along with 3a whilst the use of AgOAc slightly suppressed the byproduct formation. Furthermore, use of KOAc (2 equivalent) produced 3a in moderate yields with negligible amounts of byproducts. We were pleased to observe that the catalyst loading could be reduced to 5 to 2 mol% with high yields of 3a along with complete suppression of byproducts (entry 6, 7). This is in agreement with the observation that the low catalytic loading reduces colloid formation of Pd(0) species and hence increases the efficiency of reaction as observed in Heck reaction involving ligand free Pd(OAc)2 as catalyst and aryl bromide as coupling partner.14 Further reduction in Pd(OAc)2 (1 mol%) did not result in completion of the reaction in 24 h. Next, we turned our attention to the stoichiometry of reactants wherein 1a (from 1.2 to 1.6 equivalent) was employed with 2a (1 equivalent), Pd(OAc)2 (2 mol%), KOAc (2 equivalents) in DMA (entry 9 to 13). The 1.4 equivalent of 1a resulted in high yield, 85%, of 3a. The use of 1.5 and 1.6 equivalent of 1a furnished eventually similar yields of 3a although with traces of decarboxylative homodimerization (3a′). Furthermore, neither additives such as TBAB and TBAI (entry 14 and 15) nor the use of NMP or DMF as solvents (entry 16 and 17) showed any improvement in the yield of 3a. This led to the identification of optimized reaction condition as illustrated in Table 1, entry 11.
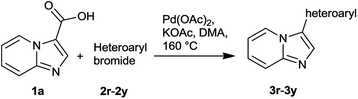
The substrate scope and generality of decarboxylative arylation was investigated under optimized condition and results are summarized in Table 2. The electron deficient aryl bromides reacted with 1a to produce coupled products in moderate to good yield (3i–3p). High reactivity was observed for most of the electron deficient aryl bromides (2i–2k, 2n–2p Table 2, ESI†) with exception of 3-bromobenzaldehyde (2m) which took 24 h to complete the reaction. The ortho-substituted aryl bromides furnished 3l in 68% yield. The longer reaction time in this case may be attributed to the steric hindrance caused by ortho-substituent. The trisubstituted 2-bromo-4,6-dimethoxybenzaldehyde successfully furnished 3q in modest yield. Furthermore, electron rich aryl bromides successfully furnished 3b–3h in moderate to high yields. In general, the electron rich aryl bromides required longer reaction time compared to electron deficient aryl bromides. 2e reacted sluggishly with 1a (5 days reaction time) resulting in complex reaction mixture allowing to isolate 3e in 20%. The reaction of bromo naphthalenes with 1a produced 3g, 3h in high yield (91% and 95% respectively). The reaction tolerated variety of electron withdrawing and donating groups as shown in Table 2.
| a Reaction conditions: 1a (1.4 equivalents), 2b–2q (1 equivalent), KOAc (2 equivalents), 2 mol% Pd(OAc)2 in DMA at 160 °C under N2 atmosphere. Aryl bromide structures are shown in ESI.b Isolated yield.c For 14 h.d For 5 h.e For 20 h.f For 5 days.g For 24 h.h For 3.5 h.i For 18 h.j For 8 h.k For 12 h.l Aryl chlorides (1 equivalent) were used instead of aryl bromides with 1a (1.4 equivalents), KOAc (2 equivalents), 2 mol% Pd(OAc)2 in DMA at 160 °C under N2 atmosphere. |
|---|
 |
The scope of the decarboxylative arylation was extended to aryl chlorides and is shown in Table 2 (condition l). Compound 3a, 3j and 3n were obtained in 44%, 58% and 85% respectively. In this protocol when 4-chloroanisole, 2-chloronaphthalene, 1-chloronaphthalene, and 4-chlorobezotrifluoride were treated with 1a, the decarboxylative arylated products 3d, 3g, 3h and 3i were obtained in lower yields along with the protodecarboxylation of 1a. The protodecarboxylation was the only product isolated when 1a was treated with chlorobenzene or 3-chlorobenzaldehyde or 4-chlorotoluene, indicating limitation for some of the aryl chlorides in this protocol.
Further, the scope of reaction was studied with respect to heteroaryl bromides (Table 3). The decarboxylative arylation between 1a with bromides of quinoline, isoquinoline, indole, furan, thiophene, quinoxaline, and pyridine successfully furnished diverse molecules (Table 3, 3r–3y). These hybrid-chemotypes are important as they contain various heterocycles which are known to be the privileged scaffolds in drug discovery.15 The heteroatom in these privileged motifs often take part in various interactions with biological targets/proteins.16 The coupling of 3-bromoquinoline, 5-bromoisoquinoline and 4-bromo indole with 1a resulted 3r, 3s and 3t in high yields whereas 5-bromo indole gave 3u in 50% yield. Presence of electron withdrawing groups like ester on furan (3v) was tolerated. The 2-bromothiophene and 6-bromoquinoxaline could produce 3w, 3x respectively in moderate yield. The 3-bromopyridine successfully coupled with 1a to produce 3y. The protocol tolerated free NH group in indole substrate (3t, 3u).
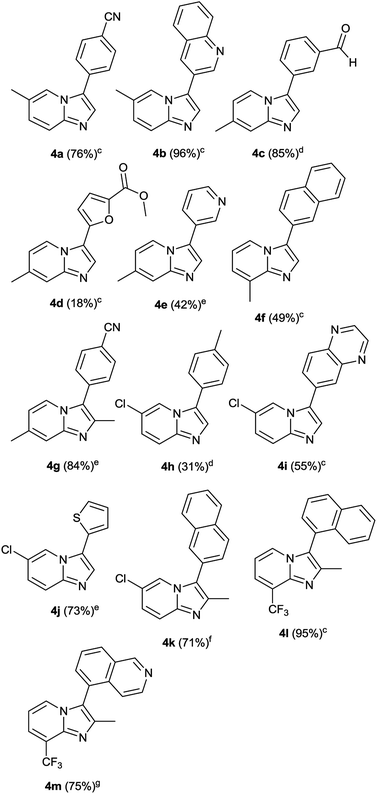
Upon having successfully studied scope of the method with respect to various aryl/heteroaryl bromides, we next turned our attention to study the scope of method with respect to various imidazo[1,2-a]pyridine-3-carboxylic acids. The results of this study is summarized in Table 4. Various imidazo[1,2-a]pyridine-3-carboxylic acids 1b–1i were synthesized according to the reported method or modifications thereof.17 Details of their synthesis has been described in the ESI.† Imidazo[1,2-a]pyridine-3-carboxylic acids with methyl substituent at various positions underwent decarboxylative arylation with good yields (4a, 4c, 4f and 4g). In addition, electron withdrawing groups like chloro, trifluoromethyl on imidazo[1,2-a]pyridine-3-carboxylic acid were well tolerated in the reaction (4h, 4k and 4l). Furthermore, we were pleased to find that the reaction is applicable to substituted imidazo[1,2-a]pyridine-3-carboxylic acids with various heteroaryl bromides producing 4b, 4d, 4e, 4i, 4j and 4m in various yields.
The scope of the ligand-free Pd-catalysed decarboxylative arylation reaction was further extended to different heterocyclic acids as shown in Scheme 1. The 2-thiophenecarboxylic acid, 2,4-disubstituted thiazole-5-carboxylic acid underwent decarboxylative arylation with 2a and produced 5a (21%) and 5b (59%) respectively. Further, N-substituted indole 2-carboxylic acid produced 2-arylated N-substituted indole 5c (43%) with minor amount of 3-arylated N-substituted indole 5c′ (7%). When benzofuran-2-carboxylic acid was used in the protocol, the expected 2-arylated benzofuran 5d was obtained in 34% yield along with 12% of 2,3-diarylated benzofuran 5d′.
The previous study on decarboxylative arylation of 1a with aryl chloride in the presence of palladium acetate, S-Phos are reported by Wu et al. and proposed via Pd(0) species.10 The generation of Pd(0) species was proposed through decarboxylative homocoupling of 1a, although formation of homodimer 3a′ in the presence of aryl chloride was not observed. To understand the decarboxylative arylation process, we submitted 1a under standard condition without aryl bromide and isolated 74% homodimer 3a′, while in the absence of aryl chloride, Wu et al. isolated homodimer 3a′ in 98%10 yield. Additionally, we observed 3a′ only in the cases where excess (1.5 equivalent and more; entry 12 and 13 in Table 1) of 1a with 2a (1 equivalent) was used indicating the formation of 3a′ may be a consequence of excess amount of 1a. Based on these observations a plausible mechanism for decarboxylative arylation is proposed and is shown in Scheme 2.
The catalytic cycle begins with formation of Pd(0) species I in the reaction. Formation of Pd(0) species from Pd(OAc)2 catalysed Heck reaction in ligand-free condition at high temperature has been reported by de Vries et al.13b,14 The Pd(0) initially is in soluble palladium cluster form13b which further can form insoluble palladium black. Hence such ligandless systems work well with low catalyst loading so as to avoid the insoluble palladium black formation due to aggregation.9b,13b,18 We observed that the yield of arylated product decreased in case of 10 mol% Pd(OAc)2 as compared to 5 mol% Pd(OAc)2 (entry 4 and 6, Table 1) which probably is due to the formation of palladium black. The next step in plausible mechanism is the oxidative addition of aryl bromide to I resulting in intermediate II. This palladium(II) complex coordinates with carboxylate and forms intermediate III which upon decarboxylation collapses in intermediate IV. Finally, reductive elimination converts Pd(II) to Pd(0) with release of 3-aryl imidazo[1,2-a]pyridine.
Finally compounds were screened on bacteria panel consisting of E. coli, S. aureus, P. aeruginosa, K. pneumoniae and A. baumannii. The MIC of active compounds was determined and was defined as the lowest concentration of a compound that inhibited visible growth of bacteria after 24 h. Compound 3i, 4l, 4f and 3h showed MIC of 50, 50, 25 and 50 μM against S. aureus respectively. Modifications of 4f are in progress to obtain more potent analogues and the results pertaining to this will be published in due course.
Conclusion
In summary, we have successfully developed a facile ligand-free palladium catalysed decarboxylative arylation reaction of imidazo[1,2-a]pyridine-3-carboxylic acids with various aryl and heteroaryl bromides. This method provides a broad substrate scope and excellent functional group tolerance. The protocol is applied to generate heteroaryl-hetero(aryl) motifs and few of these motifs showed antibacterial activity against S. aureus. The efforts are underway to modify these motifs to obtain more potent and drug like compounds. Further, generation of new heteroaryl-hetero(aryl) motif will find importance in drug discovery.Experimental
In a typical procedure, the mixture of 2 (0.1 g, 1 equivalent), 1 or heterocyclic acid [2-thiophenecarboxylic acid or 2,4-disubstituted thiazole-5-carboxylic acid or N-substituted indole 2-carboxylic acid or benzofuran-2-carboxylic acid] (1.4 equivalent), Pd(OAc)2 (2 mol%), KOAc (2 equivalent) in 7 mL DMA were stirred at 160 °C. The progress of reaction was monitored through TLC. Upon completion of the reaction, the mixture was diluted with 200 mL of water and extracted with ethyl acetate (2 × 70 mL). The ethyl acetate layer was washed with water (200 mL), brine (100 mL) and dried with sodium sulfate. The organic solvent evaporated under vacuum and residue was purified by column chromatography to afford pure 3-substituted imidazo[1,2-a]pyridines 3 or 4 or 5.4-(Imidazo[1,2-a]pyridin-3-yl)benzonitrile (3a)12
Yellow solid. Yield: 102 mg (85%). Mp = 177 °C; 1H NMR (400 MHz, CDCl3): δ 8.37 (dt, J = 7.0, 1.1 Hz, 1H), 7.82–7.78 (m, 3H), 7.74–7.68 (m, 3H), 7.31–7.25 (m, 1H), 6.91 (td, J = 6.9, 1.1 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 147.2, 134.3, 134.1, 133.2 (×2), 127.8 (×2), 125.4, 124.1, 123.3, 118.8, 118.6, 113.6, 111.4; HRMS (ESI-MS): calc. for C14H10N3 [(M + H)+]: 220.0869, found: 220.0891.3-(p-Tolyl)imidazo[1,2-a]pyridine (3b)12
Yellow solid. Yield: 110 mg (90%). Mp = 78–80 °C; 1H NMR (500 MHz, CDCl3): δ 8.31 (d, J = 7.0 Hz, 1H), 7.69–7.64 (m, 2H), 7.45 (d, J = 8.1 Hz, 2H), 7.33 (d, J = 8.1 Hz, 1H), 7.21–7.16 (m, 1H), 6.80 (td, J = 6.8, 1.1 Hz, 1H), 2.44 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 146.0, 138.3, 132.4, 128.2, 126.4, 125.9, 124.2, 123.5, 118.4, 112.5, 21.5; HRMS (ESI-MS): calc. for C14H13N2 [(M + H)+]: 209.1073, found: 209.1062.3-Phenylimidazo[1,2-a]pyridine (3c)12
Brown liquid. Yield: 115 mg (92%) 1H NMR (400 MHz, CDCl3): δ 8.30 (d, J = 7.0 Hz, 1H), 7.67 (s, 1H), 7.65 (d, J = 9.1 Hz, 1H), 7.57–7.45 (m, 4H), 7.38 (t, J = 7.5 Hz, 1H), 71.6 (t, J = 7.5 Hz, 1H), 6.77 (t, J = 6.7 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 146.0, 132.4, 129.2, 128.1, 127.9, 125.6, 124.2, 123.3, 118.1, 112.5; HRMS (ESI-MS): calc. for C13H10N2Na [(M + Na)+]: 217.0736, found: 217.0729.3-(4-Methoxyphenyl)imidazo[1,2-a]pyridine (3d)9b
Brown solid. Yield: 75 mg (63%). Mp = 116 °C; 1H NMR (500 MHz, CDCl3): δ 8.25 (dt, J = 7.0, 1.1 Hz, 1H), 7.66 (d, J = 10.0 Hz, 1H), 7.63 (s, 1H), 7.50–7.45 (m, 2H), 7.20–7.15 (m, 1H), 7.08–7.03 (m, 2H), 6.78 (td, J = 6.8, 1.1 Hz, 1H), 3.88 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 159.8, 145.9, 132.1, 129.8, 125.7, 124.1, 123.4, 121.7, 118.3, 114.8, 112.5, 55.6; HRMS (ESI-MS): calc. for C14H13N2O [(M + H)+]: 225.1022, found: 225.1013.4-(Imidazo[1,2-a]pyridin-3-yl)-N,N-dimethylaniline (3e)9b
Yellow solid. Yield: 23 mg (20%). Mp = 128 °C; 1H NMR (500 MHz, CDCl3): δ 8.27 (dt, J = 7.0, 1.1 Hz, 1H), 7.64 (d, J = 10.0 Hz, 1H), 7.60 (s, 1H), 7.44–7.38 (m, 2H), 7.17–7.12 (m, 1H), 6.87–6.81 (m, 2H), 6.76 (td, J = 6.8, 1.1 Hz, 1H), 3.03 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 150.5, 145.6, 131.4, 129.4, 126.3, 123.8, 123.6, 118.1, 116.7, 112.8, 112.3, 40.5; HRMS (ESI-MS): calc. for C15H15N3Na [(M + Na)+]: 260.1158, found: 260.1171.3-(3-Methoxyphenyl)imidazo[1,2-a]pyridine (3f)9e
White solid. Yield: 95 mg (79%). Mp = 110 °C; 1H NMR (500 MHz, CDCl3): δ 8.37 (dt, J = 7.0, 1.1 Hz, 1H), 7.70 (s, 1H), 7.68 (dt, J = 9.1, 1.1 Hz, 1H), 7.44 (t, J = 8.0 Hz, 1H), 7.23–7.18 (m, 1H), 7.17–7.14 (m, 1H), 7.11–7.07 (m, 1H), 6.99–6.93 (m, 1H), 6.82 (td, J = 6.9, 1.1 Hz, 1H), 3.87 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.4, 146.3, 132.7, 130.7, 130.5, 125.8, 124.4, 123.7, 120.4, 118.4, 113.9, 113.7, 112.7, 55.5; HRMS (ESI-MS): calc. for C14H13N2O [(M + H)+]: 225.1022, found: 225.1032.3-(Naphthalen-2-yl)imidazo[1,2-a]pyridine (3g)9e
Brown liquid. Yield: 108 mg (91%). 1H NMR (500 MHz, CDCl3): δ 8.43 (d, J = 6.9 Hz, 1H), 8.01 (s, 1H), 8.97 (d, J = 8.4 Hz, 1H), 7.92–7.88 (m, 2H), 7.81 (s, 1H), 7.75 (d, J = 9.0 Hz, 1H), 7.64 (d, J = 9.0 Hz, 1H), 7.56–7.51 (m, 2H), 7.24 (t, J = 8.0 Hz, 1H), 6.85 (t, J = 6.8 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 146.1, 133.7, 133.0, 132.5, 129.2, 128.1, 128.0, 126.9 (overlapped), 126.7, 126.6, 125.9, 125.8, 123.5, 118.3, 113.0; HRMS (ESI-MS): calc. for C17H13N2 [(M + H)+]: 245.1073, found: 245.1085.3-(Naphthalen-1-yl)imidazo[1,2-a]pyridine (3h)9e
Yellow semisolid. Yield: 100 mg (84%). 1H NMR (400 MHz, CDCl3): δ 8.02–7.94 (m, 2H), 7.79 (s, 1H), 7.74 (d, J = 9.1 Hz, 1H), 7.70 (d, J = 6.9 Hz, 1H), 7.61–7.56 (m, 2H), 7.56–7.48 (m, 2H), 7.46–7.38 (m, 1H), 7.21 (t, J = 8.0 Hz, 1H), 6.68 (t, J = 6.8 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 145.9, 134.0, 133.8, 132.1, 129.7, 129.2, 128.8, 127.0, 126.4, 126.3, 125.7, 125.2, 124.4, 124.1, 123.7, 118.1, 112.3; HRMS (ESI-MS): calc. for C17H12N2Na [(M + Na)+]: 267.0893, found: 267.0909.3-(4-(Trifluoromethyl)phenyl)imidazo[1,2-a]pyridine (3i)9b
Yellow solid. Yield: 100 mg (85%). Mp = 152 °C; 1H NMR (500 MHz, CDCl3): δ 8.36 (dt, J = 7.0, 1.1 Hz, 1H), 7.81–7.74 (m, 3H), 7.74–7.67 (m, 3H), 7.29–7.21 (m, 1H), 6.88 (td, J = 7.0, 1.1 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 146.8, 133.7, 133.1, 130.2, 129.9, 128.0, 126.4, 124.5, 123.3, 118.7, 113.2; HRMS (ESI-MS): calc. for C14H10F3N2 [(M + H)+]: 263.0791, found: 263.0821.1-(4-(Imidazo[1,2-a]pyridin-3-yl)phenyl)ethanone (3j)9b
Yellow solid. Yield: 95 mg (80%). Mp = 164 °C; 1H NMR (400 MHz, CDCl3): δ 8.40 (d, J = 6.8 Hz, 1H), 8.10 (d, J = 8.2 Hz, 2H), 7.80 (s, 1H), 7.75–7.65 (m, 3H), 7.28–7.21 (m, 1H), 6.88 (t, J = 6.8 Hz, 1H), 2.66 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 197.1, 146.8, 136.1, 133.9, 133.7, 129.3, 127.2, 124.8, 124.7, 123.4, 118.4, 113.1, 26.6; HRMS (ESI-MS): calc. for C15H13N2O [(M + H)+]: 237.1022, found: 237.1032.Methyl-4-(imidazo[1,2-a]pyridin-3-yl)benzoate (3k)9b
Yellow solid. Yield: 60 mg (51%); mp = 145 °C; 1H NMR (400 MHz, CDCl3): δ 8.40 (dt, J = 7.0, 1.1 Hz, 1H), 8.21–8.15 (m, 2H), 7.80 (s, 1H), 7.71 (dt, J = 9.1, 1.0 Hz, 1H), 7.68–7.64 (m, 2H), 7.29–7.22 (m, 1H), 6.88 (td, J = 7.0, 1.1 Hz, 1H), 3.97 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 166.7, 146.7, 133.9, 133.7, 130.7, 129.4, 127.4, 125.0, 123.5, 118.6, 113.2, 52.4; HRMS (ESI-MS): calc. for C15H13N2O2 [(M + H)+]: 253.0972, found: 253.0985.Methyl-2-(imidazo[1,2-a]pyridin-3-yl)benzoate (3l)9b
Yellow semisolid. Yield: 80 mg (68%). 1H NMR (500 MHz, CDCl3): δ 8.10 (dd, J = 8.0, 1.1 Hz, 1H), 7.76–7.70 (m, 2H), 7.66 (td, J = 7.6, 1.4 Hz, 1H), 7.62 (s, 1H), 7.57 (td, J = 7.6, 1.4 Hz, 1H), 7.48 (dd, J = 7.6, 1.4 Hz, 1H), 7.24–7.20 (m, 1H), 6.77 (t, d, J = 6.8 Hz, 1H), 3.56 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 167.2, 145.6, 133.0, 132.7, 132.2, 131.3, 131.2, 129.4, 129.1, 124.7, 124.1, 124.0, 117.9, 112.4, 52.4; HRMS (ESI-MS): calc. for C15H13N2O2 [(M + H)+]: 253.0972, found: 253.0983.3-(Imidazo[1,2-a]pyridin-3-yl)benzaldehyde (3m)9b
Yellow solid. Yield: 100 mg (83%). Mp = 76 °C; 1H NMR (500 MHz, CDCl3): δ 10.11 (s, 1H), 8.35 (dt, J = 7.0, 1.1 Hz, 1H), 8.10 (t, J = 1.6 Hz, 1H), 7.93 (dt, J = 7.6, 1.4 Hz, 1H), 7.85 (dt, J = 7.6, 1.4 Hz, 1H), 7.79 (s, 1H), 7.75–7.69 (m, 2H), 7.30–7.22 (m, 1H), 6.88 (td, J = 7.0, 1.1 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 191.9, 146.6, 137.4, 133.8, 133.2, 130.6, 130.2, 129.7, 128.4, 125.0, 124.5, 123.2, 118.5, 113.3; HRMS (ESI-MS): calc. for C14H11N2O [(M + H)+]: 223.0866, found: 223.0878.3-(4-Nitrophenyl)imidazo[1,2-a]pyridine (3n)9b
Yellow solid. Yield: 95 mg (80%). Mp = 185 °C; 1H NMR (500 MHz, CDCl3): δ 8.42 (d, J = 7.0 Hz, 1H), 8.40–8.36 (m, 2H), 7.86 (s, 1H), 7.78–7.75 (m, 2H), 7.74 (d, J = 9.1 Hz, 1H), 7.34–7.27 (m, 1H), 6.94 (td, J = 7.0, 1.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 147.4, 147.0, 136.0, 134.7, 127.7, 125.6, 124.9, 123.8, 123.4, 118.9, 113.8; HRMS (ESI-MS): calc. for C13H10N3O2 [(M + H)+]: 240.0768, found: 240.0776.2-Fluoro-5-(imidazo[1,2-a]pyridin-3-yl)benzonitrile (3o)19
Brown solid. Yield: 50 mg (42%). Mp = 217–219 °C; 1H NMR (500 MHz, DMSO-d6): δ 8.61 (dt, J = 7.0, 1.1 Hz, 1H), 8.27 (dd, J = 6.1, 2.3 Hz, 1H), 8.11–8.06 (m, 1H), 7.86 (s, 1H), 7.73–7.65 (m, 2H), 7.38–7.33 (m, 1H), 7.00 (td, J = 7.0, 1.1 Hz, 1H); 13C NMR (100 MHz, DMSO-d6): δ 161.7 (d, J = 256.4 Hz), 145.9, 135.3 (d, J = 8.5 Hz), 133.6, 132.7, 126.7 (d, J = 3.2 Hz), 125.3, 124.4, 122.4, 120.5, 117.6 (d, J = 19.8 Hz), 117.5, 113.8, 113.2; HRMS (ESI-MS): calc. for C14H9FN3 [(M + H)+]: 238.0775, found: 238.0774.3-(2,4-Difluorophenyl)imidazo[1,2-a]pyridine (3p)
White solid. Yield: 75 mg (63%). Mp = 95 °C; 1H NMR (500 MHz, CDCl3): δ 7.94 (dt, J = 7.0, 1.1 Hz, 1H), 7.73–7.66 (m, 2H), 7.52–7.45 (m, 1H), 7.26–7.21 (m, 1H), 7.08–6.98 (m, 2H), 6.84 (td, J = 7.0, 1.1 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 163.4 (dd, J = 251.4, 11.7 Hz), 160.3 (dd, J = 251.5, 11.7 Hz), 146.5, 134.0, 132.4 (AB q, J = 4.7 Hz), 124.7, 124.2 (d, J = 3.7 Hz), 119.3, 118.3, 113.5 (dd, J = 15.2, 3.3 Hz), 112.7, 112.4 (dd, J = 21.6, 3.4 Hz), 105.0 (t, J = 25.6 Hz); HRMS (ESI-MS): calc. for C13H9F2N2 [(M + H)+]: 231.0728, found: 231.0734.6-(Imidazo[1,2-a]pyridin-3-yl)-2,3dimethoxybenzaldehyde (3q)
Yellow semisolid. Yield: 60 mg (52%). 1H NMR (400 MHz, CDCl3) δ: 9.68 (s, 1H), 7.93 (dt, J = 6.8, 1.0 Hz, 1H), 7.72 (dt, J = 9.1, 1.0 Hz, 1H), 7.70 (s, 1H), 7.61 (s, 1H), 7.29–7.23 (m, 1H), 6.93 (s, 1H), 6.84 (dt, J = 6.8, 1.0 Hz, 1H), 4.02 (s, 3H), 3.96 (s, 3H); 13C NMR (125 MHz, CDCl3) δ: 189.9, 154.4, 150.2, 146.2, 134.9, 128.8, 126.7, 125.1, 123.3, 120.5, 118.3, 113.4, 113.1, 109.6, 56.6, 56.4; HRMS (ESI-MS): calc. for C16H15N2O3 [(M + H)+]: 283.1077, found: 283.1095.3-(Imidazo[1,2-a]pyridin-3-yl)quinoline (3r)9b
Brown solid. Yield: 90 mg (76%). Mp = 140 °C; 1H NMR (500 MHz, CDCl3): δ 9.13 (d, J = 2.2 Hz, 1H), 8.39 (d, J = 6.9 Hz, 1H), 8.33 (d, J = 2.1 Hz, 1H), 8.17 (d, J = 8.5 Hz, 1H), 7.89 (d, J = 8.1 Hz, 1H), 7.87 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.74 (d, J = 9.1 Hz, 1H), 7.64 (t, J = 7.2 Hz, 1H), 7.31–7.24 (m, 1H), 6.90 (t, J = 6.8 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 149.9, 147.5, 146.8, 133.9, 133.8, 130.1, 129.5, 127.9 (×2), 127.6, 125.0, 123.1, 122.8, 122.6, 118.6, 113.2; HRMS (ESI-MS): calc. for C16H11N3Na [(M + Na)+]: 268.0845, found: 268.0858.5-(Imidazo[1,2-a]pyridin-3-yl)isoquinoline (3s)
Brown solid. Yield: 85 mg (73%). Mp = 133 °C; 1H NMR (500 MHz, CDCl3) δ 9.38 (d, J = 1.2 Hz, 1H), 8.51 (d, J = 6.0 Hz, 1H), 8.13 (d, J = 8.2 Hz, 1H), 7.86 (dd, J = 7.1, 1.2 Hz, 1H), 7.82 (s, 1H), 7.80–7.73 (m, 3H), 7.39 (d, J = 6.0 Hz, 1H), 7.30–7.25 (m, 1H), 6.78 (td, J = 6.8, 1.2 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 153.3, 146.2, 144.1, 134.8, 134.2, 133.0, 129.1, 127.3, 125.7, 124.9, 123.8, 122.1, 118.3, 118.0, 112.9; HRMS (ESI-MS): calc. for C16H12N3 [(M + H)+]: 246.1026, found: 246.1034.3-(1H-Indol-4-yl)imidazo[1,2-a]pyridine (3t)
Brown solid. Yield: 60 mg (50%). Mp = 198–200 °C; 1H NMR (500 MHz, CDCl3): δ 8.55 (bs, 1H), 8.22 (dt, J = 7.0, 1.1 Hz, 1H), 7.85 (s, 1H), 7.72 (dt, J = 9.1, 1.0 Hz, 1H), 7.50 (dt, J = 7.5, 1.0 Hz, 1H), 7.36–7.30 (m, 2H), 7.29 (t, J = 2.8 Hz, 1H), 7.24–7.19 (m, 1H), 6.77 (td, J = 6.8, 1.1 Hz, 1H), 6.43–6.39 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 145.9, 136.4, 132.7, 126.8, 125.2, 124.8, 124.4, 122.3, 121.0, 120.6, 118.0, 112.2, 111.7, 102.2; HRMS (ESI-MS): calc. for C15H11N3Na [(M + Na)+]: 256.0845, found: 256.0867.3-(1H-Indol-5-yl)imidazo[1,2-a]pyridine (3u)
Brown semisolid. Yield: 60 mg (50%). 1H NMR (500 MHz, CDCl3): δ 9.27 (bs, 1H), 8.36 (d, J = 7.0, Hz, 1H), 7.83 (s, 1H), 7.71 (s, 1H), 7.67 (d, J = 9.1 Hz, 1H), 7.54 (d, J = 8.3 Hz, 1H), 7.38–7.30 (m, 2H), 7.22–7.15 (m, 1H), 6.78 (td, J = 6.8, 0.9 Hz, 1H), 6.66–6.60 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 145.6, 135.9, 131.7, 128.6, 127.3, 125.7, 124.1, 123.7, 122.6, 120.9, 120.3, 117.9, 112.4, 112.1, 102.7; HRMS (ESI-MS): calc. for C15H12N3 [(M + H)+]: 234.1026, found: 234.1056.Methyl-5-(imidazo[1,2-a]pyridin-3-yl)furan-2-carboxylate (3v)20
Yellow solid. Yield: 34 mg (40%). Mp = 133–135 °C; 1H NMR (500 MHz, CDCl3): δ 8.85 (dt, J = 7.0, 1.1 Hz, 1H), 8.02 (s, 1H), 7.72 (dt, J = 9.1, 1.0 Hz, 1H), 7.35–7.19 (m, 2H), 7.00 (td, J = 6.9, 1.0 Hz, 1H), 6.72 (d, J = 3.6, 1.0 Hz, 1H), 3.94 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 159.1, 149.0, 146.9, 143.2, 134.5, 125.9, 125.8, 119.9, 118.3, 116.7, 114.0, 107.4, 52.1; HRMS (ESI-MS): calc. for C13H10N2NaO3 [(M + Na)+]: 265.0584, found: 265.0607.3-(Thiophen-2-yl)imidazo[1,2-a]pyridine (3w)21
Yellow oil. Yield: 92 mg (75%). 1H NMR (400 MHz, CDCl3): δ 8.40 (d, J = 4.9, Hz, 1H), 7.77 (s, 1H); 7.68 (d, J = 9.1 Hz, 1H), 7.44 (dd, J = 5.2, 1.2 Hz, 1H), 7.29 (dd, J = 3.6, 1.1 Hz, 1H), 7.25–7.22 (m, 1H), 7.22–7.18 (m, 1H), 6.87 (t, J = 6.8, 1.1 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 146.2, 133.5, 129.9, 127.8, 125.9 (×2), 124.5, 123.8, 119.2, 118.0, 112.9; HRMS (ESI-MS): calc. for C11H9N2S [(M + H)+]: 201.0481, found: 201.0476.6-(Imidazo[1,2-a]pyridin-3-yl)quinoxaline (3x)
Yellow solid. Yield: 62 mg (50%). Mp = 143–145 °C; 1H NMR (400 MHz, CDCl3): δ 8.91 (d, J = 1.8, Hz, 1H), 8.89 (d, J = 1.8 Hz, 1H), 8.58 (d, J = 7.0 Hz, 1H), 8.34 (d, J = 1.9 Hz, 1H), 8.26 (d, J = 8.7 Hz, 1H), 8.03 (dd, J = 8.7, 2.0 Hz, 1H), 7.93 (s, 1H), 7.76 (d, J = 9.1 Hz, 1H), 7.33–7.27 (m, 1H), 6.93 (td, J = 6.8, 1.1 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 147.0, 145.9, 145.2, 143.5, 142.5, 134.2, 131.3, 130.8, 130.1, 126.5, 125.3, 124.5, 123.5, 118.6, 113.5; HRMS (ESI-MS): calc. for C15H11N4 [(M + H)+]: 247.0978, found: 247.0991.3-(Pyridin-3-yl)imidazo[1,2-a]pyridine (3y)9b
Brown semisolid. Yield: 60 mg (49%). 1H NMR (400 MHz, CDCl3): δ 8.84 (d, J = 2.2 Hz, 1H), 8.65 (dd, J = 1.6, 1H), 8.28 (d, J = 6.0 Hz, 1H), 7.90–7.84 (m, 1H), 7.75 (s, 1H), 7.71 (d, J = 8.2 Hz, 1H), 7.45 (dd, J = 4.9, 3.0 Hz, 1H), 7.28–7.22 (m, 1H), 6.88 (td, J = 6.8, 1.0 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 149.3, 148.9, 146.7, 135.2, 133.4, 125.7, 125.0, 124.0, 123.0, 122.3, 118.5, 113.2; HRMS (ESI-MS): calc. for C12H9N3Na [(M + Na)+]: 218.0689, found: 218.0684.4-(6-Methylimidazo[1,2-a]pyridin-3-yl)benzonitrile (4a)
White solid. Yield: 100 mg (78%). Mp = 152–154 °C; 1H NMR (400 MHz, CDCl3): δ 8.13 (s, 1H), 7.82–7.76 (m, 2H), 7.73 (s, 1H), 7.71–7.65 (m, 2H), 7.60 (d, J = 15.8 Hz, 1H), 7.12 (dd, J = 9.2, 1.6 Hz, 1H), 2.34 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 146.2, 134.3, 134.0, 133.1 (×2), 128.5, 127.7 (×2), 123.7, 123.3, 120.8, 118.7, 117.9, 111.7, 18.5; HRMS (ESI-MS): calc. for C15H12N3 [(M + H)+]: 234.1026, found: 234.1033.3-(6-Methylimidazo[1,2-a]pyridin-3-yl)quinoline (4b)
Brown solid. Yield: 120 mg (96%). Mp = 138–140 °C; 1H NMR (500 MHz, CDCl3): δ 9.12 (d, J = 2.2 Hz, 1H), 8.32 (d, J = 2.0 Hz, 1H), 8.18 (d, J = 8.4 Hz, 1H), 8.15 (s, 1H), 7.91 (d, J = 7.9 Hz, 1H), 7.81 (s, 1H), 7.80–7.76 (m, 1H), 7.69–7.60 (m, 2H), 7.13 (dd, J = 9.2, 1.4 Hz, 1H), 2.34 (d, J = 0.8 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 150.0, 147.5, 145.9, 134.1, 133.6, 130.1, 129.6, 128.3, 128.0, 127.9, 127.6, 123.1, 123.0, 122.3, 120.7, 117.9, 18.5; HRMS (ESI-MS): calc. for C17H14N3 [(M + H)+]: 260.1182, found: 260.1194.3-(7-Methylimidazo[1,2-a]pyridin-3-yl)benzaldehyde (4c)
Yellow solid. Yield: 110 mg (85%). Mp = 80–82 °C; 1H NMR (500 MHz, CDCl3): δ 10.10 (s, 1H), 8.23 (d, J = 7.0 Hz, 1H), 8.07 (s, 1H), 7.89 (dt, J = 7.6, 1.2 Hz, 1H), 7.82 (dt, J = 7.7, 1.9, 1.2 Hz, 1H), 7.70 (s, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.45 (s, 1H), 6.69 (dd, J = 7.0, 1.5 Hz, 1H), 2.43 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 191.9, 147.2, 137.3, 135.9, 133.6, 133.2, 130.9, 130.2, 129.4, 128.2, 123.9, 122.5, 116.9, 115.8, 21.4; HRMS (ESI-MS): calc. for C15H13N2O [(M + H)+]: 237.1022, found: 237.1033.Methyl-5-(7-methylimidazo[1,2-a]pyridin-3-yl)furan-2-carboxylate (4d)
Yellow solid. Yield: 23 mg (18%). Mp = 124 °C; 1H NMR (500 MHz, CDCl3): δ 8.69 (d, J = 7.1 Hz, 1H), 7.92 (s, 1H), 7.44 (s, 1H), 7.29 (d, J = 3.6 Hz, 1H), 6.80 (dd, J = 7.1, 1.6 Hz, 1H), 6.65 (d, J = 3.6, Hz, 1H), 3.93 (s, 3H), 2.43 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 159.1, 149.2, 147.3, 143.0, 137.1, 134.2, 125.1, 120.0, 116.6, 116.1, 106.9, 52.1, 21.4; HRMS (ESI-MS): calc. for C14H13N2O3 [(M + H)+]: 257.0921, found: 257.0937.7-Methyl-3-(pyridin-3-yl)imidazo[1,2-a]pyridine (4e)
Yellow semisolid. Yield: 55 mg (42%). 1H NMR (500 MHz, CDCl3): δ 8.81 (s, 1H), 8.61 (d, J = 4.8, 1.5 Hz, 1H), 8.15 (d, J = 7.0 Hz, 1H), 7.86–7.81 (m, 1H), 7.66 (s, 1H), 7.47–7.38 (m, 2H), 6.68 (d, J = 7.0 Hz, 1H), 2.41 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 148.1, 147.8, 146.2, 135.2, 134.1, 132.0, 124.9, 123.0, 121.3, 120.9, 115.8, 114.9, 20.4; HRMS (ESI-MS): calc. for C13H11N3Na [(M + Na)+]: 232.0845, found: 232.0835.8-Methyl-3-(naphthalen-2-yl)imidazo[1,2-a]pyridine (4f)
Yellow solid. Yield: 61 mg (49%). Mp = 106 °C. 1H NMR (400 MHz, CDCl3): δ 8.31 (d, J = 6.9 Hz, 1H), 8.02 (s, 1H), 7.97 (d, J = 8.5 Hz, 1H), 7.92–7.85 (m, 2H), 7.80 (s, 1H), 7.66 (d, J = 8.5 Hz, 1H), 7.59–7.50 (m, 2H), 7.03 (d, J = 6.8 Hz, 1H), 6.76 (t, J = 6.8 Hz, 1H), 2.68 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 146.7, 133.7, 132.9, 132.3, 129.1, 128.1, 127.9, 127.0, 126.8 (×2), 126.6, 126.3, 126.0, 123.4, 121.4, 112.9, 17.2; HRMS (ESI-MS): calc. for C18H15N2 [(M + H)+]: 259.1230, found: 259.1237.4-(2,7-Dimethylimidazo[1,2-a]pyridin-3-yl)benzonitrile (4g)
White solid. Yield: 114 mg (84%). Mp = 169–171 °C; 1H NMR (400 MHz, CDCl3): δ 8.01 (d, J = 7.0 Hz, 1H), 7.83–7.78 (m, 2H), 7.60–7.55 (m, 2H), 7.35 (s, 1H), 6.63 (dd, J = 7.0, 1.6 Hz, 1H), 2.48 (s, 3H), 2.41 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 145.9, 142.4, 136.3, 135.6, 134.7, 133.0, 129.3, 122.1, 119.3, 118.8, 115.9, 115.3, 111.0, 21.4, 14.3; HRMS (ESI-MS): calc. for C16H14N3 [(M + H)+]: 248.1182, found: 248.1209.6-Chloro-3-(p-tolyl)imidazo[1,2-a]pyridine (4h)
Brown semisolid. Yield: 45 mg (32%). 1H NMR (500 MHz, CDCl3): δ 8.83 (dd, J = 1.9, 0.7 Hz, 1H), 7.67 (s, 1H), 7.62 (d, J = 9.2 Hz, 1H), 7.42 (d, J = 8.1 Hz, 2H), 7.35 (d, J = 7.9 Hz, 2H), 7.15 (dd, J = 9.5, 1.9 Hz, 1H), 2.44 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 144.3, 138.9, 134.5, 133.0, 130.2, 128.2, 126.5, 126.1, 125.7, 121.4, 121.1, 118.6, 118.3, 21.5; HRMS (ESI-MS): calc. for C14H12ClN2 [(M + H)+]: 243.0684, found: 243.0704.6-(6-Chloroimidazo[1,2-a]pyridin-3-yl)quinoxaline (4i)
Yellow solid. Yield: 75 mg (55%). Mp = 222 °C; 1H NMR (500 MHz, CDCl3): δ 9.92 (d, J = 1.7 Hz, 1H), 8.90 (d, J = 1.8 Hz, 1H), 8.57 (d, J = 1.3 Hz, 1H), 8.32 (d, J = 1.8 Hz, 1H), 8.28 (d, J = 8.7 Hz, 1H), 7.99 (dd, J = 8.7, 1.9 Hz, 1H), 7.93 (s, 1H), 7.70 (d, J = 9.5 Hz, 1H), 7.28–7.23 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 146.0, 145.5, 145.4, 143.5, 142.8, 135.0, 131.1, 130.7, 130.0, 127.0, 126.6, 125.1, 122.0, 121.4, 119.1; HRMS (ESI-MS): calc. for C15H10ClN4 [(M + H)+]: 281.0589, found: 281.0603.6-Chloro-3-(thiophen-2-yl)imidazo[1,2-a]pyridine (4j)
Green semisolid. Yield: 88 mg (62%). 1H NMR (400 MHz, CDCl3): δ 8.40 (dd, J = 2.0, 0.8 Hz, 1H), 7.77 (s, 1H), 7.63 (dd, J = 9.5, 0.8 Hz, 1H), 7.48 (dd, J = 5.2, 1.1 Hz, 1H), 7.30 (dd, J = 3.6, 1.1 Hz, 1H), 7.24–7.18 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 144.7, 134.6, 129.2, 128.1, 126.7, 126.6, 126.0, 121.8, 121.5, 119.8, 118.6; HRMS (ESI-MS): calc. for C11H7ClN2NaS [(M + Na)+]: 256.9911, found: 256.9927.6-Chloro-2-methyl-3-(naphthalen-2-yl)imidazo[1,2-a]pyridine (4k)
Brown semisolid. Yield: 100 mg (70%). 1H NMR (400 MHz, CDCl3): δ 8.16 (d, J = 1.3 Hz, 1H), 8.03 (d, J = 8.5 Hz, 1H), 7.96–7.89 (m, 3H), 7.62–7.56 (m, 3H), 7.53 (dd, J = 8.5, 1.7 Hz, 1H), 7.16 (dd, J = 9.5, 2.0 Hz, 1H); 2.53 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 142.8, 142.2, 133.7, 133.1, 129.3, 129.0, 128.2, 128.0, 127.0 (×2), 126.7, 126.1, 125.8, 122.3, 121.1, 120.7, 117.4, 14.0; HRMS (ESI-MS): calc. for C18H14ClN2 [(M + H)+]: 293.084, found: 293.0843.2-Methyl-3-(naphthalen-1-yl)-8-(trifluoromethyl)imidazo[1,2-a]pyridine (4l)
Yellow solid. Yield: 120 mg (95%). Mp = 95–98 °C. 1H NMR (500 MHz, CDCl3): δ 8.03 (d, J = 8.2 Hz, 1H), 7.98 (d, J = 8.2 Hz, 1H), 7.66 (d, J = 7.0 Hz, 1H), 7.63 (t, J = 7.2 Hz, 1H), 7.57–7.50 (m, 3H), 7.42 (t, J = 7.1 Hz, 1H), 7.31 (d, J = 8.4 Hz, 1H), 6.68 (t, J = 7.0 Hz, 1H), 2.44 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 143.7, 140.2, 134.1, 132.2, 130.1, 129.9, 128.9, 127.2, 127.1, 126.6, 125.8, 125.7, 124.9, 123.2 (q, J = 272.1 Hz, CF3), 122.6, 122.5, 120.9, 110.1, 14.2; HRMS (ESI-MS): calc. for C19H14F3N2 [(M + H)+]: 327.1104, found: 327.1139.5-(2-Methyl-8-(trifluoromethyl)imidazo[1,2-a]pyridin-3-yl)isoquinoline (4m)
Yellowish liquid. Yield: 118 mg (75%). 1H NMR (500 MHz, CDCl3): δ 9.38 (s, 1H), 8.47 (d, J = 5.5 Hz, 1H), 8.15 (d, J = 8.0 Hz, 1H), 7.82–7.75 (m, 2H), 7.67 (d, J = 6.9 Hz, 1H), 7.54 (d, J = 7.0 Hz, 1H), 7.15 (d, J = 5.8 Hz, 1H), 6.75 (t, J = 7.0 Hz, 1H), 2.40 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 153.4, 144.3, 144.2, 140.6, 134.9, 130.0, 129.6, 129.2, 127.4, 126.8, 125.1, 123.0, 123.1 (q, J = 272.1 Hz, CF3), 122.9, 119.4, 117.7, 110.6, 14.2; HRMS (ESI-MS): calc. for C18H12F3N3Na [(M + Na)+]: 350.0876, found: 350.0911.4-(Thiophen-2-yl)benzonitrile (5a)22
Yellow solid. Yield: 44 mg (21%). Mp = 79–81 °C; 1H NMR (400 MHz, CDCl3): δ 7.71–7.68 (m, 2H), 7.67–7.64 (m, 2H), 7.43–7.39 (m, 2H), 7.13 (dd, J = 5.1, 3.7 Hz, 1H); 13C NMR (100 MHz, CDCl3): 142.2, 138.8, 132.9 (×2), 128.7, 127.0, 126.2 (×2), 125.2, 119.0, 110.7; HRMS (ESI-MS): calc. for C11H7NNaS [(M + Na)+]: 208.0191, found: 208.0194.4-(2,4-Dimethylthiazol-5-yl)benzonitrile (5b)23
Yellow solid. Yield: 70 mg (59%). Mp = 69–70 °C; 1H NMR (400 MHz, CDCl3): 7.69 (d, J = 8.5, Hz, 2H), 7.51 (d, J = 8.5 Hz, 2H), 2.71 (s, 3H), 2.48 (s, 3H); 13C NMR (100 MHz, CDCl3): 165.0, 148.9, 137.3, 132.6 (×2), 129.6 (×2), 118.7, 111.1, 19.3, 16.5; HRMS (ESI-MS): calc. for C12H11N2S [(M + H)+]: 215.0637, found: 215.0645.4-(1-Phenyl-1H-indol-2-yl)benzonitrile (5c)24
Yellow solid. Yield: 70 mg (43%). Mp = 184–186 °C; 1H NMR (400 MHz, CDCl3): 7.76–7.72 (m, 1H), 7.57–7.53 (m, 2H), 7.50–7.47 (m, 2H), 7.43–7.46 (m, 1H), 7.37–7.40 (m, 2H), 7.31–7.34 (m, 1H), 7.27–7.30 (m, 2H), 7.27–7.26 (m, 1H), 7.21–7.25 (m, 1H), 6.95 (S, 1H); 13C NMR (100 MHz, CDCl3): 139.8, 138.5, 138.1, 137.1, 132.1 (×2), 129.7 (×2), 129.0 (×2), 128.0 (×2), 127.9, 123.6, 121.3, 121.1, 118.9, 110.9, 110.6, 105.8; HRMS (ESI-MS): calc. for C21H14N2Na [(M + Na)+]: 317.1049, found: 317.1059.4-(1-Phenyl-1H-indol-3-yl)benzonitrile (5c′)25
Yellow solid. Yield: 12 mg (7%). Mp = 105–107 °C; 1H NMR (400 MHz, CDCl3): 7.99–7.95 (m, 1H), 7.84–7.80 (m, 2H), 7.75–7.71 (m, 2H), 7.62–7.59 (m, 2H), 7.58–7.53 (m, 4H), 7.46–7.41 (m, 1H), 7.34–7.28 (m, 2H); 13C NMR (100 MHz, CDCl3): 140.3, 139.1, 137.1, 132.8 (×2), 130.0 (×2), 127.6 (×2), 127.4, 126.9, 126.5, 124.8 (×2), 123.5, 121.7, 119.9, 119.5, 117.4, 111.4, 109.3; HRMS (ESI-MS): calc. for C21H14N2Na [(M + Na)+]: 317.1049, found: 317.1043.4-(Benzofuran-2-yl)benzonitrile (5d)26
Yellow solid. Yield: 82 mg (34%). Mp = 121–125 °C; 1H NMR (400 MHz, CDCl3): 7.97–7.93 (m, 2H), 7.74–7.71 (m, 2H), 7.65–7.61 (m, 1H), 7.56–7.53 (m, 1H), 7.38–7.33 (m, 1H), 7.30–7.25 (m, 1H), 7.18 (d, J = 0.8 Hz, 1H); 13C NMR (100 MHz, CDCl3): 155.4, 153.7, 134.6, 132.7 (×2), 128.8, 125.7, 125.2 (×2), 123.6, 121.6, 118.9, 111.6 (×2), 104.5; HRMS (ESI-MS): calc. for C15H9NNaO [(M + Na)+]: 242.0576, found: 242.0586.4,4′-(Benzofuran-2,3-diyl)dibenzonitrile (5d′)26
Yellow solid. Yield: 43 mg (12%). Mp = 165–167 °C; 1H NMR (400 MHz, CDCl3): 7.83–7.78 (m, 2H), 7.72–7.68 (m, 2H), 7.64–7.58 (m, 5H), 7.49–7.40 (m, 2H), 7.34–7.29 (m, 1H); 13C NMR (100 MHz, CDCl3): 154.5, 149.1, 137.4, 134.3, 133.2 (×2), 132.6 (×2), 130.5 (×2), 129.1, 127.4 (×2), 126.5, 124.0, 120.1, 118.7, 118.6, 118.5, 112.3 (×2), 111.8; HRMS (ESI-MS): calc. for C22H12N2NaO [(M + Na)+]: 343.0842, found: 343.0868.Acknowledgements
This research was financially supported by Council of Scientific and Industrial Research of India through CSC0108 project and Open Source Drug Discovery Programme (HCP0001).Notes and references
- F. Couty and G. Evano, in Comprehensive Heterocyclic Chemistry III, ed. A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Elsevier, Oxford, 2008, vol. 11, pp. 409–499 Search PubMed.
- (a) C. Enguehard-Gueiffier and A. Gueiffier, Mini-Rev. Med. Chem., 2007, 7, 888–899 CrossRef CAS PubMed; (b) S. Feng, D. Hong, B. Wang, X. Zheng, K. Miao, L. Wang, H. Yun, L. Gao, S. Zhao and H. C. Shen, ACS Med. Chem. Lett., 2015, 6, 359–362 CrossRef CAS PubMed; (c) A. C. Humphries, E. Gancia, M. T. Gilligan, S. Goodacre, D. Hallett, K. J. Merchant and S. R. Thomas, Bioorg. Med. Chem. Lett., 2006, 16, 1518–1522 CrossRef CAS PubMed; (d) S. Ulloora, R. Shabaraya, S. Aamir and A. V. Adhikari, Bioorg. Med. Chem. Lett., 2013, 23, 1502–1506 CrossRef CAS PubMed; (e) C. Enguehard, J. L. Renou, H. Allouchi, J. M. Leger and A. Gueiffier, Chem. Pharm. Bull., 2000, 48, 935–940 CrossRef CAS PubMed; (f) Z. A. Kaplancikli, G. Turan-Zitouni, A. Ozdemir and G. Revial, J. Enzyme Inhib. Med. Chem., 2008, 23, 866–870 CrossRef CAS PubMed; (g) M. H. Fisher and A. Lusi, J. Med. Chem., 1972, 15, 982–985 CrossRef CAS PubMed; (h) Y. Rival, G. Grassy, A. Taudou and R. Ecalle, Eur. J. Med. Chem., 1991, 26, 13–18 CrossRef CAS; (i) C. Hamdouchi, J. de Blas, M. del Prado, J. Gruber, B. A. Heinz and L. Vance, J. Med. Chem., 1999, 42, 50–59 CrossRef CAS PubMed; (j) J. J. Kaminski and A. M. Doweyko, J. Med. Chem., 1997, 40, 427–436 CrossRef CAS PubMed; (k) M. Lhassani, O. Chavignon, J. M. Chezal, J. C. Teulade, J. P. Chapat, R. Snoeck, G. Andrei, J. Balzarini, E. De Clercq and A. Gueiffier, Eur. J. Med. Chem., 1999, 34, 271–274 CrossRef CAS; (l) Y. Maruyama, K. Anami and Y. Katoh, Arzneim. Forsch., 1978, 28, 2102–2107 CAS; (m) M. A. Ismail, R. K. Arafa, T. Wenzler, R. Brun, F. A. Tanious, W. D. Wilson and D. W. Boykin, Bioorg. Med. Chem., 2008, 16, 683–691 CrossRef CAS PubMed; (n) N. S. Rao, C. Kistareddy, B. Balaram and B. Ram, Pharma Chem., 2012, 4, 2408–2415 CAS.
- K. Pethe, P. Bifani, J. C. Jang, S. Kang, S. Park, S. Ahn, J. Jiricek, J. Y. Jung, H. K. Jeon, J. Cechetto, T. Christophe, H. Lee, M. Kempf, M. Jackson, A. J. Lenaerts, H. Pham, V. Jones, M. J. Seo, Y. M. Kim, M. Seo, J. J. Seo, D. Park, Y. Ko, I. Choi, R. Kim, S. Y. Kim, S. Lim, S. A. Yim, J. Nam, H. Kang, H. Kwon, C. T. Oh, Y. Cho, Y. Jang, J. Kim, A. Chua, B. H. Tan, M. B. Nanjundappa, S. P. S. Rao, W. S. Barnes, R. Wintjens, J. R. Walker, S. Alonso, S. Lee, J. Kim, S. Oh, T. Oh, U. Nehrbass, S. J. Han, Z. No, J. Lee, P. Brodin, S. N. Cho, K. Nam and J. Kim, Nat. Med., 2013, 19, 1157–1160 CrossRef CAS PubMed.
- A. K. Bagdi, S. Santra, K. Monir and A. Hajra, Chem. Commun., 2015, 51, 1555–1575 RSC.
- (a) C. Enguehard, J. L. Renou, V. Collot, M. Hervet, S. Rault and A. Gueiffier, J. Org. Chem., 2000, 65, 6572–6575 CrossRef CAS PubMed; (b) S. C. Goodacre, L. J. Street, D. J. Hallett, J. M. Crawforth, S. Kelly, A. P. Owens, W. P. Blackaby, R. T. Lewis, J. Stanley, A. J. Smith, P. Ferris, B. Sohal, S. M. Cook, A. Pike, N. Brown, K. A. Wafford, G. Marshall, J. L. Castro and J. R. Atack, J. Med. Chem., 2006, 49, 35–38 CrossRef CAS PubMed; (c) M. Crawforth, S. C. Goodacre, D. J. Hallet, T. Harrison, A. P. Owens, M. Rowley and M. R. Teall, PCT Int Appl, WO2001038326A2, 2001; (d) T. Bilodeau, M. E. Fraley and Z. Wu, PCT Int Appl, WO2003092595A2, 2003.
- (a) A. M. Badger, P. E. Bender, K. M. Esser, D. E. Griswolds, H. Nabil, J. C. Lee, B. J. Votta and P. L. Simon, PCT Int Appl, WO 1991000092 A1, 1991; (b) Y. Kawai, S. Satoh, H. Yamasaki, N. Kayakiri, K. Yoshihara and T. Oku, PCT Int Appl, WO 1996034866 A1, 1996.
- J. Koubachi, S. El Kazzouli, S. Berteina-Raboin, A. Mouaddib and G. Guillaumet, Synlett, 2006, 3237–3242 CAS.
- S. H. Wang, W. J. Liu, J. H. Cen, J. Q. Liao, J. P. Huang and H. Y. Zhan, Tetrahedron Lett., 2014, 55, 1589–1592 CrossRef CAS.
- (a) J. Lee, J. Chung, S. M. Byun, B. M. Kim and C. Lee, Tetrahedron, 2013, 69, 5660–5664 CrossRef CAS; (b) H. Y. Fu, L. Chen and H. Doucet, J. Org. Chem., 2012, 77, 4473–4478 CrossRef CAS PubMed; (c) H. Cao, H. Y. Zhan, Y. G. Lin, X. L. Lin, Z. D. Du and H. F. Jiang, Org. Lett., 2012, 14, 1688–1691 CrossRef CAS PubMed; (d) S. Marhadour, M. A. Bazin and P. Marchand, Tetrahedron Lett., 2012, 53, 297–300 CrossRef CAS; (e) P. Y. Choy, K. C. Luk, Y. Wu, C. M. So, L. L. Wang and F. Y. Kwong, J. Org. Chem., 2015, 80, 1457–1463 CrossRef CAS PubMed.
- B. Mu, Y. Wu, J. Li, D. Zou, J. Chang and Y. Wu, Org. Biomol. Chem., 2016, 14, 246–250 CAS.
- (a) J. Y. Lee, P. Y. Cheng, Y. H. Tsai, G. R. Lin, S. P. Liu, M. H. Sie and H. M. Lee, Organometallics, 2010, 29, 3901–3911 CrossRef CAS; (b) K. J. Cavell, D. J. Stufkens and K. Vrieze, Inorg. Chim. Acta, 1981, 47, 67–76 CrossRef CAS.
- D. Nandi, Y. M. Jhou, J. Y. Lee, B. C. Kuo, C. Y. Liu, P. W. Huang and H. M. Lee, J. Org. Chem., 2012, 77, 9384–9390 CrossRef CAS PubMed.
- (a) J. G. de Vries, Can. J. Chem., 2001, 79, 1086–1092 CrossRef CAS; (b) A. H. M. de Vries, J. M. C. A. Mulders, J. H. M. Mommers, H. J. W. Henderickx and J. G. de Vries, Org. Lett., 2003, 5, 3285–3288 CrossRef CAS PubMed.
- J. G. de Vries, Dalton Trans., 2006, 421–429 RSC.
- (a) R. W. DeSimone, K. S. Currie, S. A. Mitchell, J. W. Darrow and D. A. Pippin, Comb. Chem. High Throughput Screening, 2004, 7, 473–494 CrossRef CAS; (b) N. S. Gray, L. Wodicka, A. M. Thunnissen, T. C. Norman, S. Kwon, F. H. Espinoza, D. O. Morgan, G. Barnes, S. LeClerc, L. Meijer, S. H. Kim, D. J. Lockhart and P. G. Schultz, Science, 1998, 281, 533–538 CrossRef CAS PubMed; (c) F. R. de Sa Alves, E. J. Barreiro and C. A. Fraga, Mini-Rev. Med. Chem., 2009, 9, 782–793 CrossRef PubMed; (d) M. E. Welsch, S. A. Snyder and B. R. Stockwell, Curr. Opin. Chem. Biol., 2010, 14, 347–361 CrossRef CAS PubMed; (e) B. R. Stockwell, Nature, 2004, 432, 846–854 CrossRef CAS PubMed; (f) R. Breinbauer, I. R. Vetter and H. Waldmann, Angew. Chem., Int. Ed., 2002, 41, 2879–2890 Search PubMed; (g) H. B. Rode, M. L. Sos, C. Grutter, S. Heynck, J. R. Simard and D. Rauh, Bioorg. Med. Chem., 2011, 19, 429–439 CrossRef CAS PubMed; (h) S. J. Haggarty, Curr. Opin. Chem. Biol., 2005, 9, 296–303 CrossRef CAS PubMed; (i) E. J. Barreiro, in Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation ed. S. Bräse, Royal Society of Chemistry, 2015, pp. 1–11 Search PubMed.
- J. A. Blair, D. Rauh, C. Kung, C. H. Yun, Q. W. Fan, H. Rode, C. Zhang, M. J. Eck, W. A. Weiss and K. M. Shokat, Nat. Chem. Biol., 2007, 3, 229–238 CrossRef CAS PubMed.
- (a) G. C. Moraski, L. D. Markley, P. A. Hipskind, H. Boshoff, S. Cho, S. G. Franzblau and M. J. Miller, ACS Med. Chem. Lett., 2011, 2, 466–470 CrossRef CAS PubMed; (b) F. Hongli, L. Xin, L. Xinsheng and C. Jintao, PCT Chin Appl, CN 103965190 A, 2014.
- (a) J. Roger, F. Pozgan and H. Doucet, Green Chem., 2009, 11, 425–432 RSC; (b) J. J. Dong, J. Roger, F. Pozgan and H. Doucet, Green Chem., 2009, 11, 1832–1846 RSC; (c) J. Roger and H. Doucet, Adv. Synth. Catal., 2009, 351, 1977–1990 CrossRef CAS; (d) J. Roger and H. Doucet, Tetrahedron, 2009, 65, 9772–9781 CrossRef CAS.
- B. Frett, N. McConnell, C. C. Smith, Y. Wang, N. P. Shah and H. Y. Li, Eur. J. Med. Chem., 2015, 94, 123–131 CrossRef CAS PubMed.
- H. Y. Fu, L. Q. Zhao, C. Bruneau and H. Doucet, Synlett, 2012, 2077–2082 CAS.
- G. P. Liu, X. F. Cong, J. H. He, S. Z. Luo, D. Wu and J. B. Lan, J. Chem. Res., 2012, 687–690 CrossRef.
- S. Shaaban, A. Jolit, D. Petkova and N. Maulide, Chem. Commun., 2015, 51, 13902–13905 RSC.
- X. X. He, Y. F. Li, J. Huang, D. S. Shen and F. S. Liu, J. Organomet. Chem., 2016, 803, 58–66 CrossRef CAS.
- U. Sharma, R. Kancherla, T. Naveen, S. Agasti and D. Maiti, Angew. Chem., Int. Ed., 2014, 53, 11895–11899 CrossRef CAS PubMed.
- R. Kancherla, T. Naveen and D. Maiti, Chem.–Eur. J., 2015, 21, 8723–8726 CrossRef CAS PubMed.
- T. Dao-Huy, M. Haider, F. Glatz, M. Schnurch and M. D. Mihovilovic, Eur. J. Org. Chem., 2014, 2014, 8119–8125 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12166g |
| This journal is © The Royal Society of Chemistry 2016 |