Effect of morphology on the oxygen evolution reaction for La0.8Sr0.2Co0.2Fe0.8O3−δ electrochemical catalyst in alkaline media
Abstract
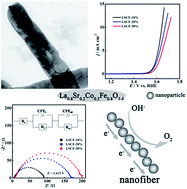
A series of perovskite oxides La0.8Sr0.2Co0.2Fe0.8O3−δ (LSCF-10%, LSCF-20% and LSCF-30%) with different morphologies (nanofibers, nanorods and nanoparticles) are synthesized as oxygen evolution reaction (OER) electrocatalysts by an electrospinning technique. In alkaline media, the LSCF-10% catalyst exhibits remarkable electrocatalytic activity and stability for OER, affording a low onset potential of 1.583 V and a stable current density of 10 mA cm−2 with a potential of 1.643 V. The excellent catalytic activity of OER for LSCF-10% is mainly attributed to the morphological characteristics of one-dimensional nanofibers. Compared with the nanorods and nanoparticles, one-dimensional nanofibers not only increase the specific surface area, but also enhance the efficient transport pathways for electrons and ions. Thus, the rational design of the morphology is beneficial to enhance the OER catalytic activities of the perovskite oxides.

 Please wait while we load your content...
Please wait while we load your content...