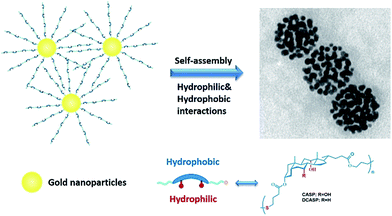
Main chain poly(bile acid) directed plasmonic nanospheres with amphiphilic binding pockets and photo-triggered destruction†
Jinzhi Suna,
Weina Li*a,
Lin Xiaoa,
Guanghui Yub and
Jinsheng Shi*a
aCollege of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University, Qingdao, 266109, China. E-mail: wnli@qau.edu.cn
bCollege of Animal Science and Technology, Qingdao Agricultural University, Qingdao, 266109, China
First published on 16th June 2016
Abstract
Amphiphilic block copolymer-directed self-assembly of gold nanoparticles (GNPs) with fine-tunable optical and plasmonic properties has attracted considerable attention. In this paper, we developed a series of sulfide-bridged main chain poly(bile acid)s and investigated biologically sourced amphiphilic homopolymer-directed plasmonic nanospheres and their properties. With the functional sulfide groups, the amphiphilic homopolymers were conveniently grafted to the surface of GNPs by noncovalent Au–S bonds. Directed by the biologically amphiphilic poly(bile acid)s, the modified GNPs assembled into nanospheres with a tunable size. With the organized arrangement of GNPs, the assemblies exhibited excellent optical–thermal conversion phenomena, which can disrupt the ensembles of GNPs and provide an alternative efficient strategy for the controlled release of encapsulated functional molecules. Inherited from the unique facial amphiphilicity and concave skeleton of the bile acids, the assemblies possessed both hydrophilic and hydrophobic cavities, which can encapsulate both hydrophilic and hydrophobic molecules simultaneously. These amphiphilic biologically sourced main chain polymers may provide a new avenue for the directed assembly of GNPs and functional materials.
Introduction
Gold nanoparticles (GNPs) with unique physicochemical properties, including strong localized surface plasmon resonances (LSPR), large surface-area-to-volume ratios, excellent biocompatibility and chemical stability,1 have attracted considerable attention in the fields of catalysis,2 sensors,3 drug delivery,4 bioimaging5 etc. Changing the size and shape of individual GNPs and fine-tuning the spatial arrangement and the interparticle distance have been demonstrated as efficient strategies to modulate their optical and plasmonic properties.6 Recently, constructing well-ordered 1D, 2D and 3D nanostructures to tailor the spatial arrangement of GNPs has aroused widespread interest in nanoscience and nanotechnology.7 In this respect, the bottom-up assembly approach offers a competitive and promising alternative to construct the hierarchical architectures of GNPs with superior performance. It has been well-demonstrated that amphiphilic polymers with outstanding controllable supramolecular assembly properties can serve as protective and stabilizing agents, as well as assembly induced agents when modified to the surface of the GNPs.8 Thereby, the assembly technologies of amphiphilic polymers can also be efficient strategies to construct the ordered nanostructures of GNPs through polymer-directed assembly. By surrounding the GNPs with a layer of amphiphilic poly(styrene-block-acrylic acid) (PS-b-PAA), Taton and coworkers developed a facile method to spontaneously assemble individual nanoparticles into plasmonic chains.7c Plasmonic vesicles with organized monolayer GNPs as the shell have also been fabricated by Duan and coworkers through the assembly of amphiphilic GNPs with hydrophilic poly(ethylene glycol) (PEG) and hydrophobic poly(methyl methacrylate) (PMMA) coatings, which can be disrupted by stimulus mechanisms pertaining to either the nanoparticles or the graft polymers.9 These multifunctional vesicles with unique structural and optical properties could provide a promising platform for biosensing, multimodality bioimaging, and theragnostic nanomedicine.10 Nie and coworkers fabricated various plasmonic superstructures, such as unimolecular micelles, vesicles and tubular structures, through the assembly of GNPs modified with amphiphilic block copolymers poly(ethyl oxide)-b-polystyrene (PEO-b-PS) by tuning the lengths of the graft polymer and the sizes of the nanoparticle cores.11 Until now, directing the spatial arrangement of GNPs by amphiphilic block copolymers has been verified to be an efficient strategy and various superstructures can be constructed conveniently, while amphiphilic homopolymers, especially biologically sourced amphiphilic polymers, were rarely considered to direct the assembly of GNPs.Bile acids are a class of naturally occurring compounds that play important roles in a wide variety of biological processes in animal life.12 With multiple features, including excellent biological activity, a rigid steroidal skeleton and a unique facial amphiphilicity, they act as excellent building blocks in the design of biological materials13 and supramolecular chemistry systems.14 A wide variety of chemical systems based on bile acids, such as adaptable foldamers,15 supramolecular assemblies,16 elastomer-like materials,17 and gelation materials18 have been fully investigated and are potentially applicable in the biomedical and pharmaceutical fields. Recently, Li and coworkers synthesized a series of main chain poly(bile acid)s by an efficient metal-free topochemical approach and demonstrated that these biologically amphiphilic polymers can retain the characteristics of bile acid building blocks and exhibited excellent assembly properties.19 This type of polymers may provide a new avenue to construct various polymer-based functional assembly systems.
In this article, we developed a series of sulfide-bridged main chain poly(bile acid)s and investigated biologically sourced amphiphilic homopolymer-directed assembly of GNPs (Scheme 1), as well as the properties of the plasmonic assemblies. By introducing alkene and thiol groups to the alternative terminal of the bile acid skeleton, the sulfide-bridged main chain polymers can be synthesized efficiently by a thiol–ene click reaction. With the functional sulfide groups, the amphiphilic polymers were conveniently grafted to the surface of the GNPs by noncovalent Au–S bonds. Directed by the biologically amphiphilic poly(bile acid)s, the modified GNPs assembled into nanospheres with a tunable size. The assemblies can retain the characteristics of both the amphiphilic poly(bile acid)s and GNPs. With the organized arrangement of GNPs, the assemblies exhibited excellent optical–thermal conversion phenomena, which can disrupt the ensembles of the polymer modified GNPs and provide an efficient alternative strategy for the controlled release of encapsulated functional molecules. Inherited from the unique facial amphiphilicity and concave skeleton of the bile acids, the assemblies possessed both hydrophilic and hydrophobic cavities, which can encapsulate both hydrophilic and hydrophobic molecules simultaneously. These amphiphilic biologically sourced main chain polymers may provide a new avenue for the directed assembly of GNPs and functional materials.
Experimental details
General information
HAuCl4·4H2O and sodium citrate tribasic dehydrate (≥99%) were purchased from Sinopharm Chemical Reagent Company. Sodium borohydride (NaBH4, 96%), cholic acid (98%), deoxycholic acid (98%) and other chemicals were purchased from Alfa Aesar and used as received without further purification. All of the solvents (analytically pure) were purchased from Sinopharm Chemical Reagent Company and purified by standard methods before use.1H and 13C NMR were recorded on a JEOL ECA300 NMR spectrometer. Electrospray ionization mass spectrometry (ESI-MS) was measured on a Bruker ESQUIRE-LC spectrometer. Fourier transform infrared spectroscopy (FTIR) was measured on an AVATAR 360 ESP Fourier-transform spectrophotometer using KBr pellets. Scanning electron microscopy images were obtained from an FESEM (Hitachi-S4800, Japan). Transmission electron microscopy (TEM) observations were conducted on an HT7700 transmission electron microscope. UV-visible absorption spectra were recorded by using a TU-1900 spectrometer (Purkinje General, China), using quartz cuvettes with a 1 cm path length. Infrared thermographic images of micelle dispersions were obtained using a Fluke TiR32 Thermal Imager.
Poly(bile acid) preparation
To a stirred solution of 3 (1.0 mmol) in 0.1 mL of freshly distilled tetrahydrofuran (THF), 4-dimethylaminopyridine (2.6 mg, 0.01 mmol) was added. The resulting reaction mixture was irradiated at 365 nm for 2 h until compound 3 completely disappeared. The reaction mixture was then added dropwise to methanol and a white precipitate was obtained, which was further purified by redissolution and reprecipitation.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700, Mw/Mn 1.2; IR (KBr pellets), ν (cm−1): 3668–3229 (–OH stretching), 3049–2758 (alkyl CH, CH2 stretching), 1724 (C
700, Mw/Mn 1.2; IR (KBr pellets), ν (cm−1): 3668–3229 (–OH stretching), 3049–2758 (alkyl CH, CH2 stretching), 1724 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching); 1H NMR (300 MHz, CDCl3): δ 0.69 (br, 3nH, 18-CH3), 0.91 (br, 3nH, 19-CH3), 0.99 (br, 3nH, 21-CH3), 3.85 (br, 1nH, 7β-CH), 3.98 (br, 1nH, 12β-CH), 4.16 (m, 2nH, COOCH2), 4.57 (br, 1nH, 3β-CH).
O stretching); 1H NMR (300 MHz, CDCl3): δ 0.69 (br, 3nH, 18-CH3), 0.91 (br, 3nH, 19-CH3), 0.99 (br, 3nH, 21-CH3), 3.85 (br, 1nH, 7β-CH), 3.98 (br, 1nH, 12β-CH), 4.16 (m, 2nH, COOCH2), 4.57 (br, 1nH, 3β-CH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching); 1H NMR (300 MHz, CDCl3): δ 0.67 (br, 3nH, 18-CH3), 0.92 (br, 3nH, 19-CH3), 0.98 (br, 3nH, 21-CH3), 3.98 (br, 1nH, 12β-CH), 4.17 (m, 2nH, COOCH2), 4.72 (br, 1nH, 3β-CH).
O stretching); 1H NMR (300 MHz, CDCl3): δ 0.67 (br, 3nH, 18-CH3), 0.92 (br, 3nH, 19-CH3), 0.98 (br, 3nH, 21-CH3), 3.98 (br, 1nH, 12β-CH), 4.17 (m, 2nH, COOCH2), 4.72 (br, 1nH, 3β-CH).Synthesis of GNPs
The GNPs were prepared using methods reported previously.20 Nanoparticles with a diameter of 5 nm were synthesized through a one-step sodium borohydride reduction method. A sodium citrate solution (2 mL, 38.8 mM) was added to 50 mL of a 1 wt% HAuCl4 aqueous solution under continuous stirring. Then a NaBH4 solution (0.5 mL, 0.145 wt%) was added and the mixture continued to be stirred for 30 minutes in order to form GNPs with a uniform size. For the synthesis of the 20 and 40 nm nanoparticles, a sodium citrate reduction method was used. Using the 20 nm nanoparticles as an example, a HAuCl4 aqueous solution (50 mL, 1 wt%) was heated to reflux under stirring. Then, a sodium citrate solution (1 mL, 1 wt%) was added quickly and the reaction mixture continued to be heated for 30 min.By varying the concentration of HAuCl4, nanoparticles with different diameters of 20 nm and 40 nm were obtained accordingly. The obtained nanoparticles were collected by centrifugation before using.
Surface modification of GNPs and self-assembly of poly(bile acid) modified GNPs
Surface modification of the obtained GNPs was performed by a ligand-exchange method.11b Typically, 3.0 mg of CASP or DCASP was firstly dissolved in 5 mL of THF. Then 5 mL of the GNPs (∼2 mg mL−1) was added dropwise into the above solution. The mixture was sonicated for 30 min under room temperature and then was kept for 6 h to allow the ligand to sufficiently exchange. The poly(bile acid) modified nanoparticles were collected and further purified by centrifugation in THF for 2–4 cycles.The self-assembly of CASP-Au or DCASP-Au was triggered by slowly adding deionized water into a THF solution of CASP-Au or DCASP-Au (1 mg mL−1) until the Tyndall effect occured. Then the mixed solution was dialyzed against deionized water to remove the organic solvents. The obtained nanostructures were then observed by electron microscopes.
Photothermal effect
To study the photothermal effect, the assembled poly(bile acid) modified GNPs were dispersed in deionized water, which was irradiated with an 808 nm NIR laser (BWT Beijing Ltd, 3.5 W cm−2). Real-time thermal imaging of the assemblies was recorded using a thermal camera (SAT Infrared Technology Co. Ltd, HM360). The quantification analysis was performed by SAT software.Results and discussion
The synthetic procedures for the cholic acid (CA) and deoxycholic acid (DCA) derivatives with alkene and thiol groups alternately at both ends are shown in Scheme S1.† Firstly, alkene-functionalized (deoxy)cholanoate (1a and 1b) was synthesized through the esterification of bile acid in dimethyl formamide (DMF) solvents. The following esterification of the C3-linked hydroxyl groups of the bile acid derivatives (2a and 2b) was carried out with relatively high yield and this procedure did not require tedious protection and deprotection of the C7, C12-linked hydroxyl groups. Then, the intermediate 2a or 2b was reacted with hexamethyldisilathiane [(TMSi)2S] in the presence of tetrabutyl ammonium fluoride (TBAF) to get thiol-functionalized 3a and 3b.21 The thiol group could be conveniently introduced by this (trimethylsilyl)thioxy-dehalogenation reaction. More importantly, our synthetic thiol-functionalized bile acid derivatives are stable and not easily oxidized into disulfides, as evidenced by the ESI-MS peaks at m/z 586.8 and 570.9 (Fig. S1†), which correspond to the monomers [3a + Cl]− and [3b + Cl]− respectively, recorded from the samples even after having been kept for over one month under ambient conditions. So the obtained thiols can be further purified by flash chromatography on a silica gel.The thiol–ene click reaction with distinct characteristics, including an inherently rapid reaction rate and formation of homogeneous polymer structures, has been widely used in many fields, such as polymer synthesis, optical materials and biomaterials etc.22 In our systems, to obtain the sulfide-linked main chain poly(bile acid)s, photopolymerization of the bile acid monomers with both alkene and thiol functional groups was carried out. According to the optimization of the polymerization conditions, 4-dimethylaminopyridine (DMAP) was selected as the catalyst. Nonpolar THF, which can dissolve both bile acid monomers and catalysts, was chosen as the reaction solvent. The irradiation time under 365 nm light was optimized to 2 h to increase the molecular weight of the polymer. The crude product was then added dropwise to the methanol solvent and white precipitates were obtained, which were further purified by redissolution and reprecipitation procedures. This methodology provides another strategy for the synthesis of main chain poly(bile acid)s, which has long been a great challenge in the field of bile acid chemistry.17
The thiol–ene click reaction of the bile acid derivatives was also characterized by spectroscopic methods. As shown by the FTIR spectra in Fig. 1, both the alkene and thiol functionalized monomers exhibited characteristic stretching vibration bands of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at ca. 1648 cm−1 and olefinic C–H at ca. 3087 cm−1.23 After the photo-induced polymerization, all of these peaks disappeared. The 1H NMR results can provide more accurate evidence for the photo-induced polymerization. Fig. 2 shows the 1H NMR spectra of CASP and DCASP and their monomers 3a and 3b. The multiple resonance signals of the alkene protons (HC
C at ca. 1648 cm−1 and olefinic C–H at ca. 3087 cm−1.23 After the photo-induced polymerization, all of these peaks disappeared. The 1H NMR results can provide more accurate evidence for the photo-induced polymerization. Fig. 2 shows the 1H NMR spectra of CASP and DCASP and their monomers 3a and 3b. The multiple resonance signals of the alkene protons (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) of 3a and 3b at 5.90 and 5.28 ppm almost disappeared in the spectra of the polymers CASP and DCASP (Fig. 2), which revealed that most of the alkene and thiol reactive groups in the monomers have been transformed to sulfide groups. It is further revealed by the gel permeation chromatography (GPC) tests that main chain polymers with molecular weights of up to 15 kDa and polydispersity indices (PDI) approaching 1.2 were obtained (Fig. S2†). The sulfide-bridged main chain poly(bile acid)s exhibited good solubility in most of the common solvents (e.g. DMF, dimethyl sulphoxide (DMSO), THF, CH2Cl2, CHCl3, and CHCl3–MeOH).
CH2) of 3a and 3b at 5.90 and 5.28 ppm almost disappeared in the spectra of the polymers CASP and DCASP (Fig. 2), which revealed that most of the alkene and thiol reactive groups in the monomers have been transformed to sulfide groups. It is further revealed by the gel permeation chromatography (GPC) tests that main chain polymers with molecular weights of up to 15 kDa and polydispersity indices (PDI) approaching 1.2 were obtained (Fig. S2†). The sulfide-bridged main chain poly(bile acid)s exhibited good solubility in most of the common solvents (e.g. DMF, dimethyl sulphoxide (DMSO), THF, CH2Cl2, CHCl3, and CHCl3–MeOH).
 | ||
| Fig. 2 1H NMR spectra of the monomers 3a and 3b in CDCl3 and their corresponding polymers CASP and DCASP in CDCl3. The signals with the asterisk represent the solvent residual peaks. | ||
GNPs with different sizes (20 and 40 nm) were synthesized using the sodium citrate reduction method with various amounts of reducing agent added.20a The 5 nm nanoparticles were synthesized through the sodium borohydride reduction method (see the Experimental section for synthetic details). Their average size was measured from the TEM images (Fig. 3a–c) and all of the nanoparticles exhibited standard UV-vis absorption spectra1c (Fig. 3d).
 | ||
| Fig. 3 TEM images of the GNPs with a diameter of 5 nm (a), 20 nm (b) and 40 nm (c), and (d) the UV-vis spectra of GNPs with different sizes. | ||
Our sulfide-bridged amphiphilic poly(bile acid)s with multiple thioether bonds have the ability to be modified onto the surface of the GNPs via covalent Au–S bonds.1e By using the ligand exchange approach, the main chain amphiphilic polymers CASP and DCASP were grafted onto nanoparticles of different sizes (details of the procedure are presented in the Experimental section). The nanoparticles modified with the amphiphilic poly(bile acid)s were redispersed and centrifuged in THF for 2–4 cycles to remove the free polymers. The concentration of residual free polymers remaining in the final THF solution was estimated to be below 10−8 M. The GNPs modified with CASP and DCASP are denoted as CASP-Au-D and DCASP-Au-D, where D represents the diameter of the GNPs. The average grafting number of the poly(bile acid)s was estimated to be about 52 chains of CASP and 80 chains of DCASP per 5 nm GNPs (20 nm GNPs: 254 chains of CASP and 278 chains of DCASP; 40 nm GNPs: 372 chains of CASP and 405 chains of DCASP).9a The CASP and DCASP grafted GNPs are well-dispersed in good solvents for the corresponding polymers, including THF, DMF, and CHCl3. Moreover, these modified nanoparticles can retain the characteristic amphiphilic features of the poly(bile acid)s. This implies that the modified nanoparticles can adapt to the environment by self-adjusting their assembly behaviour.
Self-assembly behaviour of CASP-Au and DCASP-Au
The self-assembly behaviour of CASP and DCASP was first investigated to further understand the characteristics of these sulfide-bridged poly(bile acid)s. The nonpolar solvent THF was chosen to dissolve both polymers (1 mg mL−1) and a transparent solution was obtained. Under ultrasonication, deionized water was added dropwise until the Tyndall effect occured (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 1
VH2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The representative SEM images (Fig. 4) show that self-supporting nanospheres with diameters in the range of 200–1000 nm were obtained for both polymers, indicating that both polymers possess similar assembly properties although CASP and DCASP have different numbers of hydroxyl groups on the polymer chains. This result also suggested that this type of amphiphilic sulfide-bridged poly(bile acid) has the ability to induce the self-assembly of GNPs.
2). The representative SEM images (Fig. 4) show that self-supporting nanospheres with diameters in the range of 200–1000 nm were obtained for both polymers, indicating that both polymers possess similar assembly properties although CASP and DCASP have different numbers of hydroxyl groups on the polymer chains. This result also suggested that this type of amphiphilic sulfide-bridged poly(bile acid) has the ability to induce the self-assembly of GNPs.
 | ||
Fig. 4 SEM images of CASP (a) and DCASP (b) assemblies in THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 1 VH2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) systems with a concentration of 1 mg mL−1. 2) systems with a concentration of 1 mg mL−1. | ||
Then, we further investigated the self-assembly behaviour of CASP-Au and DCASP-Au using a similar method to that of CASP and DCASP. By adding deionized water into the THF solution of CASP-Au or DCASP-Au (1 mg mL−1), the Tyndall effect occured (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 1
VH2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Then the solution was dialysed against water to remove the organic solvent. Deionized water is a poor solvent for the hydrophobic side of the poly(bile acid)s, which can minimize the overall free energy of the poly(bile acid) modified GNP systems and result in the assembly of modified GNPs into well-dispersed assemblies. The representative TEM images in Fig. 5 show the spherical assemblies of CASP-Au and DCASP-Au. It is revealed that the dimensions of the nanospheres can be adjusted by varying the size of the GNP cores. Monodispersed spheres with an average size of about 150 nm were obtained for CASP-Au-5 and DCASP-Au-5 (Fig. 5a and d), while the size of the spherical assemblies of CASP-Au-20 and DCASP-Au-20 increased up to about 300 nm (Fig. 5b and e). As for CASP-Au-40 and DCASP-Au-40, nanospheres with a size of 150–300 nm were obtained (Fig. 5c and f). Besides, the assembly of CASP-Au-5/20 and DCASP-Au-5/20 can lead to densely packed GNPs, while for CASP-Au-40 and DCASP-Au-40, only spherical assemblies with limited clusters of GNPs were obtained. This may be due to the molecular length of the poly(bile acid)s not being long enough to well-tune the assembly of the GNPs with diameters larger than 20 nm.9 Moreover, due to their assembly behaviour, the amphiphilic CASP and DCASP exhibited a similar effect in directing the assembly of the GNPs, although they are different in terms of the number of hydroxyl groups in the main chain. In the assemblies of the GNPs, it is expected that the GNPs are located in the protected layer of the poly(bile acid)s. Both the hydrophilic and hydrophobic interactions, as well as the rigid skeleton of the bile acid building blocks, contribute to the formation of the well-dispersed GNP assemblies. However, the presence of multiple sulfide groups in the main chain of the poly(bile acid)s makes the interactions between the GNPs and polymers become much more complex. There may exist multiple interactions between one polymer chain and one nanoparticle, unlike the ideal interaction state displayed in Scheme 1. This may also be the reason why we only obtained the spherical assemblies of GNPs instead of well-organized plasmonic vesicles. To further develop this systems, we have been working with another main chain poly(bile acid) with only one interaction group with the GNPs.
2). Then the solution was dialysed against water to remove the organic solvent. Deionized water is a poor solvent for the hydrophobic side of the poly(bile acid)s, which can minimize the overall free energy of the poly(bile acid) modified GNP systems and result in the assembly of modified GNPs into well-dispersed assemblies. The representative TEM images in Fig. 5 show the spherical assemblies of CASP-Au and DCASP-Au. It is revealed that the dimensions of the nanospheres can be adjusted by varying the size of the GNP cores. Monodispersed spheres with an average size of about 150 nm were obtained for CASP-Au-5 and DCASP-Au-5 (Fig. 5a and d), while the size of the spherical assemblies of CASP-Au-20 and DCASP-Au-20 increased up to about 300 nm (Fig. 5b and e). As for CASP-Au-40 and DCASP-Au-40, nanospheres with a size of 150–300 nm were obtained (Fig. 5c and f). Besides, the assembly of CASP-Au-5/20 and DCASP-Au-5/20 can lead to densely packed GNPs, while for CASP-Au-40 and DCASP-Au-40, only spherical assemblies with limited clusters of GNPs were obtained. This may be due to the molecular length of the poly(bile acid)s not being long enough to well-tune the assembly of the GNPs with diameters larger than 20 nm.9 Moreover, due to their assembly behaviour, the amphiphilic CASP and DCASP exhibited a similar effect in directing the assembly of the GNPs, although they are different in terms of the number of hydroxyl groups in the main chain. In the assemblies of the GNPs, it is expected that the GNPs are located in the protected layer of the poly(bile acid)s. Both the hydrophilic and hydrophobic interactions, as well as the rigid skeleton of the bile acid building blocks, contribute to the formation of the well-dispersed GNP assemblies. However, the presence of multiple sulfide groups in the main chain of the poly(bile acid)s makes the interactions between the GNPs and polymers become much more complex. There may exist multiple interactions between one polymer chain and one nanoparticle, unlike the ideal interaction state displayed in Scheme 1. This may also be the reason why we only obtained the spherical assemblies of GNPs instead of well-organized plasmonic vesicles. To further develop this systems, we have been working with another main chain poly(bile acid) with only one interaction group with the GNPs.
 | ||
| Fig. 5 Representative TEM images of the spherical assemblies of (a) CASP-Au-5, (b) CASP-Au-20, (c) CASP-Au-40, (d) DCASP-Au-5, (e) DCASP-Au-20, and (f) DCASP-Au-40. | ||
Accompanied by the assembly of the GNPs, the interparticle plasmonic coupling, which is extremely sensitive to interparticle distances and changes in the local environment around the particles, showed an obvious change.1a In the UV-vis spectra (Fig. S3†), the local surface plasmon resonance (LSPR) peak shows a red shift from 519 nm to 541 nm for the assemblies of CASP-Au-20 and DCASP-Au-20 (Fig. S3b†). In the case of the 5 nm and 40 nm GNPs (Fig. S3a and c†), the LSPR peaks of the plasmonic nanospheres show an 11 nm and 27 nm red shift respectively, relative to their corresponding LSPR bands of the discrete nanoparticles in deionized water.9b,11c,28
Photothermal effect and thermal-sensitive assembly behaviour
Due to the plasmonic coupling of the GNP cores, the assemblies of the GNPs exhibited distinct absorption in the near-infrared range (NIR) and could simultaneously convert the absorbed light into heat, thus offering the possibility for applications in photothermal therapy. To illustrate this probability, we evaluated the representative photothermal effect of the CASP-Au assemblies in distilled water. Before that, it was confirmed by the 1H NMR data that the chemical structure of CASP was not affected by the NIR laser irradiation (Fig. S4†). The change in the solution temperature was recorded and quantified by real-time thermal imaging utilizing a thermal camera. Fig. 6 shows the colour of the light path changing from blue to bright yellow, demonstrating the increase in solution temperature. When exposed to the NIR laser at 808 nm (3.5 W cm−2), the temperature of the CASP-Au-5/20/40 assemblies increased gradually with the prolonged irradiation time, leading to a rise in the solution temperature to > 40 °C within 70 s and > 50 °C within 180 s (Fig. 6a). On the other hand, no obvious colour changes were observed for the unmodified GNPs corresponding to the relatively smaller increase in the temperature. The temperature profiles plotted against a function of irradiation time by using GNPs with different sizes and the different assemblies of CASP-Au-5/20/40 are displayed in Fig. 6b. With the assistance of the amphiphilic poly(bile acid)s, the discrete GNPs can be well reorganized into plasmonic nanostructures and thus displayed a much stronger photothermal effect, which is especially attractive for the development of plasmonic nanomaterials with great application potentials in photothermal therapy.To further detect the potential of our plasmonic nanomaterials in remotely controlled drug delivery systems, we also examined the NIR laser-induced self-assembly behaviour of CASP-Au-20. By irradiating the assemblies of CASP-Au-20 with an 808 nm NIR laser (3.5 W cm−2) for 120 s, the nanospheres collapsed into flattened clusters of GNPs and the spherical morphology disappeared (Fig. 7). This phenomenon confirmed that the localized heating generated by the assembled GNPs was sufficient to induce the collapse of the poly(bile acid) shell.
 | ||
| Fig. 7 TEM images of the assemblies of CASP-Au-20 (a) and CASP-Au-20 nanospheres after 120 s of irradiation with an 808 nm laser (b). | ||
Hydrophilic and hydrophobic binding pockets
Notably, it has been demonstrated by Li and co-workers that the supramolecular structures of bile acid derivatives possess both hydrophilic and hydrophobic pockets simultaneously,24 which have the ability to encapsulate hydrophilic and hydrophobic functional materials at the same time. In our systems, we expected that the hydrophilic and hydrophobic pockets could still co-exist in the CASP and DCASP induced GNP assemblies. Thus hydrophilic thioflavin T (ThT)25 and hydrophobic tetraphenylethylene derivatives (TPE),26 which are always used as probes to reveal the existence of hydrophilic and hydrophobic microenvironments, were chosen to prove our hypotheses. Fig. 8a shows the fluorescence change of ThT in the absence and presence of the assemblies of CASP and CASP-Au-20. Because of the nonradiative energy decay originating from free rotations of the molecular bonds, the ThT aqueous solution shows a diminished emission. In the presence of the CASP nanospheres, an obvious enhancement of 489 nm fluorescence is observed, which demonstrates the existence of hydrophilic microenvironments to accommodate the ThT molecules and thus restricts the rotational freedom of the ThT molecule bond. This restriction is responsible for the fluorescence enhancement. On the contrary, the fluorescence intensity of the ThT molecule in the presence of the CASP-Au-20 assemblies decreased, which may be due to the close contact of the ThT molecule with the GNP cores, thereby leading to fluorescence quenching.27 This phenomenon verified that the CASP-Au-20 assemblies can accommodate the hydrophilic ThT molecules and thus demonstrated the existence of hydrophilic binding pockets. Similarly, experiments with a hydrophobic TPE probe revealed a similar enhancement phenomenon (Fig. 8b), and verified the existence of hydrophobic pockets in our CASP directed GNP nanospheres. These results suggest that the poly(bile acid) directed GNP nanospheres with embedded amphiphilic binding sites will have the potential to encapsulate different functional molecules. The plasmonic nanomaterials we developed by using the amphiphilic main chain poly(bile acid)s may provide a useful new material for drug carriers with synergistic effects of photothermal treatment and the ability of various functional chemicals to be loaded in the amphiphilic binding pockets.Conclusions
In summary, biologically sourced amphiphilic poly(bile acid) directed plasmonic nanospheres with a tunable size have been constructed. The hierarchical nanostructures exhibited characteristics of both GNPs and poly(bile acid)s, and displayed an excellent photo-thermal conversion effect and possessed hydrophilic and hydrophobic binding pockets simultaneously. This poly(bile acid)-directed assembly of GNPs may provide a new avenue for the spatial arrangement of NPs and may broaden the potential usage of main chain poly(bile acid)s.Acknowledgements
We gratefully acknowledge the financial support from the National Science Foundation of China (21403122), Natural Science Foundation of Shandong Province, China (BS2014CL016), Basic Research Program of Qingdao (14-2-4-121-jch), and High-level Science Foundation of Qingdao Agricultural University (6631405).Notes and references
- (a) C. J. Murphy, T. K. San, A. M. Gole, C. J. Orendorff, J. X. Gao, L. Gou, S. E. Hunyadi and T. Li, J. Phys. Chem. B, 2005, 109, 13857–13870 CrossRef CAS PubMed; (b) N. Lewinski, V. Colvin and R. Drezek, Small, 2008, 4, 26–49 CrossRef CAS PubMed; (c) K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668–677 CrossRef CAS; (d) M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed; (e) M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801–802 RSC.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS PubMed.
- K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739–2779 CrossRef CAS PubMed.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC.
- E. K. Lim, T. Kim, S. Paik, S. Haam, Y. M. Huh and K. Lee, Chem. Rev., 2015, 115, 327–394 CrossRef CAS PubMed.
- B. Gao, M. J. Rozin and A. R. Tao, Nanoscale, 2013, 5, 5677–5691 RSC.
- (a) J. Kao, K. Thorkelsson, P. Bai, B. J. Rancatore and T. Xu, Chem. Soc. Rev., 2013, 42, 2654–2678 RSC; (b) L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869–10939 CrossRef CAS PubMed; (c) Y. J. Kang, K. J. Erickson and T. A. Taton, J. Am. Chem. Soc., 2005, 127, 13800–13801 CrossRef CAS PubMed; (d) K. Niikura, N. Iyo, Y. Matsuo, H. Mitomo and K. Ijiro, ACS Appl. Mater. Interfaces, 2013, 5, 3900–3907 CrossRef CAS PubMed; (e) K. Niikura, N. Iyo, T. Higuchi, T. Nishio, H. Jinnai, N. Fujitani and K. Ijiro, J. Am. Chem. Soc., 2012, 134, 7632–7635 CrossRef CAS PubMed; (f) C. Fan, T. Bian, L. Shang, R. Shi, L.-Z. Wu, C.-H. Tung and T. Zhang, Nanoscale, 2016, 8, 3923–3925 RSC; (g) K. J. M. Bishop, Angew. Chem., Int. Ed., 2016, 55, 1598–1600 CrossRef CAS PubMed.
- (a) H. Xu, J. Xu, X. Jiang, Z. Zhu, J. Rao, J. Yin, T. Wu, H. Liu and S. Liu, Chem. Mater., 2007, 19, 2489–2494 CrossRef CAS; (b) R. J. Hickey, J. Koski, X. Meng, R. A. Riggleman, P. Zhang and S.-J. Park, ACS Nano, 2014, 8, 495–502 CrossRef CAS PubMed; (c) R. J. Hickey, X. Meng, P. Zhang and S.-J. Park, ACS Nano, 2013, 7, 5824–5833 CrossRef CAS PubMed; (d) Y. Li, A. E. Smith, B. S. Lokitz and C. L. McCormick, Macromolecules, 2007, 40, 8524–8526 CrossRef CAS; (e) R. J. Hickey, M. Seo, Q. Luo and S. J. Park, Langmuir, 2015, 31, 4299–4304 CrossRef CAS PubMed; (f) R. J. Hickey, A. S. Haynes, J. M. Kikkawa and S.-J. Park, J. Am. Chem. Soc., 2011, 133, 1517–1525 CrossRef CAS PubMed.
- (a) J. Song, L. Cheng, A. Liu, J. Yin, M. Kuang and H. Duan, J. Am. Chem. Soc., 2011, 133, 10760–10763 CrossRef CAS PubMed; (b) J. Song, J. Zhou and H. Duan, J. Am. Chem. Soc., 2012, 134, 13458–13469 CrossRef CAS PubMed; (c) K. Rahme, L. Chen, R. G. Hobbs, M. A. Morris, C. O’Driscoll and J. D. Holmes, RSC Adv., 2013, 3, 6085–6094 RSC.
- (a) J. Song, P. Huang, H. Duan and X. Chen, Acc. Chem. Res., 2015, 48, 2506–2515 CrossRef CAS PubMed; (b) K. Sindhu, A. Rajaram, K. J. Sreeram and R. Rajaram, RSC Adv., 2014, 4, 1–11 RSC.
- (a) J. He, Z. Wei, L. Wang, Z. Tomova, T. Babu, C. Wang, X. Han, J. T. Fourkas and Z. Nie, Angew. Chem., Int. Ed., 2013, 52, 2463–2468 CrossRef CAS PubMed; (b) J. He, Y. Liu, T. Babu, Z. Wei and Z. Nie, J. Am. Chem. Soc., 2012, 134, 11342–11345 CrossRef CAS PubMed; (c) J. He, X. Huang, Y.-C. Li, Y. Liu, T. Babu, M. A. Aronova, S. Wang, Z. Lu, X. Chen and Z. Nie, J. Am. Chem. Soc., 2013, 135, 7974–7984 CrossRef CAS PubMed.
- X. X. Zhu and M. Nichifor, Acc. Chem. Res., 2002, 35, 539–546 CrossRef CAS PubMed.
- (a) S. Mukhopadhyay and U. Maitra, Curr. Sci., 2004, 87, 1666–1683 CAS; (b) Nonappa and U. Maitra, Org. Biomol. Chem., 2008, 6, 657–669 RSC.
- (a) J. Tamminen and E. Kolehmainen, Molecules, 2001, 6, 21–46 CrossRef CAS; (b) Y. Zhao, H. Cho, L. Widanapathirana and S. Zhang, Acc. Chem. Res., 2013, 46, 2763–2772 CrossRef CAS PubMed.
- Y. Zhao and Z. Q. Zhong, J. Am. Chem. Soc., 2005, 127, 17894–17901 CrossRef CAS PubMed.
- (a) Y. Li, G. Li, X. Wang, W. Li, Z. Su, Y. Zhang and Y. Ju, Chem.–Eur. J., 2009, 15, 6399–6407 CrossRef CAS PubMed; (b) Y.-G. Jia and X. X. Zhu, Chem. Mater., 2015, 27, 387–393 CrossRef CAS; (c) Y. Li, G. Li, X. Wang, C. Lin, Y. Zhang and Y. Ju, Chem.–Eur. J., 2008, 14, 10331–10339 CrossRef CAS PubMed; (d) S. Song, R. Dong, D. Wang, A. Song and J. Hao, Soft Matter, 2013, 9, 4209–4218 RSC; (e) G. Li, Y. Hu, J. Sui, A. Song and J. Hao, Langmuir, 2016, 32, 1502–1509 CrossRef CAS PubMed.
- (a) J. E. Gautrot and X. X. Zhu, Angew. Chem., Int. Ed., 2006, 45, 6872–6874 CrossRef CAS PubMed; (b) B. G. Bag and R. Majumdar, RSC Adv., 2014, 4, 53327–53334 RSC.
- (a) H. M. Willemen, T. Vermonden, A. T. M. Marcelis and E. J. R. Sudholter, Eur. J. Org. Chem., 2001, 2329–2335 CrossRef CAS; (b) V. Noponen, Nonappa, M. Lahtinen, A. Valkonen, H. Salo, E. Kolehmainen and E. Sievanen, Soft Matter, 2010, 6, 3789–3796 RSC.
- (a) W. Li, X. Li, W. Zhu, C. Li, D. Xu, Y. Ju and G. Li, Chem. Commun., 2011, 47, 7728–7730 RSC; (b) W. Li, T. Tian, W. Zhu, J. Cui, Y. Ju and G. Li, Polym. Chem., 2013, 4, 3057–3068 RSC; (c) W. Li, T. Tian, Y. Lan, W. Zhu, J. Li, M. Zhang, Y. Ju and G. Li, Polym. Chem., 2014, 5, 743–751 RSC.
- (a) G. Frens, Nature Physical Science, 1973, 241, 20–22 CrossRef CAS; (b) J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 55–75 RSC.
- J. Hu and M. A. Fox, J. Org. Chem., 1999, 64, 4959–4961 CrossRef CAS PubMed.
- (a) A. B. Lowe, Polym. Chem., 2010, 1, 17–36 RSC; (b) C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355–1387 RSC; (c) C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- C. D. Heidecke and T. K. Lindhorst, Chem.–Eur. J., 2007, 13, 9056–9067 CrossRef CAS PubMed.
- M. Zhang, X. Yin, T. Tian, Y. Liang, W. Li, Y. Lan, J. Li, M. Zhou, Y. Ju and G. Li, Chem. Commun., 2015, 51, 10210–10213 RSC.
- (a) E. Burakowska, J. R. Quinn, S. C. Zimmerman and R. Haag, J. Am. Chem. Soc., 2009, 131, 10574–10580 CrossRef CAS PubMed; (b) B. Stadler, R. Chandrawati, A. D. Price, S. F. Chong, K. Breheney, A. Postma, L. A. Connal, A. N. Zelikin and F. Caruso, Angew. Chem., Int. Ed., 2009, 48, 4359–4362 CrossRef CAS PubMed.
- R. T. Kwok, C. W. Leung, J. W. Lam and B. Z. Tang, Chem. Soc. Rev., 2015, 44, 4228–4238 RSC.
- E. Dulkeith, A. C. Morteani, T. Niedereichholz, T. A. Klar, J. Feldmann, S. A. Levi, F. C. van Veggel, D. N. Reinhoudt, M. Moller and D. I. Gittins, Phys. Rev. Lett., 2002, 89, 203002 CrossRef CAS PubMed.
- J. Song, B. Duan, C. Wang, J. Zhou, L. Pu, Z. Fang, P. Wang, T. T. Lim and H. Duan, J. Am. Chem. Soc., 2014, 136, 6838–6841 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedure for poly(bile acid)s. See DOI: 10.1039/c6ra11806b |
| This journal is © The Royal Society of Chemistry 2016 |