Silica-aerogel-powders “jammed” polyimide aerogels with excellent hydrophobicity and conversion to ultra-light polyimide aerogel†
Shuai Wua,
Ai Du‡
*a,
Youlai Xianga,
Mingfang Liua,
Tiemin Lia,
Jun Shena,
Zhihua Zhanga,
Conghang Lib and
Bin Zhou*a
aShanghai Key Laboratory of Special Artificial Microstructure Materials and Technology, Pohl Institute of Solid State Physics, Tongji University, Shanghai 200092, P. R. China. E-mail: zhoubin863@tongji.edu.cn; duai@tongji.edu.cn; Fax: +86 21 65986071; Tel: +86 21 65986071
bLaboratory of Space Mechanical and Thermal Integrative Technology, Shanghai Institute of Satellite Engineering, Shanghai 200240, P. R. China
First published on 8th June 2016
Abstract
Silica aerogel powders (SAp) are the scrap materials derived from the industrial production and use of silica-aerogel products and become the challenges to be disposed. In this paper, SAp play a role as the shrinkage inhibiter to fabricate the SAp “jammed” polyimide gels. Herein, the “jammed” effect means that the polyimide backbones have been “stretched up” by the micron-scale SAp. Namely, the strong chains-packing effect, which is always taking placing during the gelation process and results in a high shrinkage of the polyimide backbones, has been effectively prevented by a simple physical mixture of SAp. This “jammed” effect greatly reduces the shrinkage as low as 7.5%. Meanwhile, SAp are treated as the pore formers to make a conversion to polyimide aerogel with ultra-low density of about 0.02 g cm−3. In addition, incorporation of SAp also dramatically enhances the hydrophobicity (water contact angle up to 152°). Overall, SAp “jammed” polyimide aerogels have low thermal conductivity (0.028–0.031 W m−1 K−1), high mechanical strength, and high thermal stability. This combination of properties makes them more applicable as thermal insulators in harsh environments. Certainly, recycling of the scraped SAp by simple physical mixture with organic PI is also an advocated way for energy conservation.
Introduction
Aerogels are novel materials with three-dimensional open networks which were first produced by Kistler in 1931.1–3 Because of their many desirable properties, aerogels have achieved great success in a wide range of applications.4–7 Among all kinds of aerogels, silica aerogel is the most well-known and extensively studied. The low density and thermal conductivity make it promising candidate material for the thermal insulation application, such as insulation for cryogenic applications, space launch applications, solar collectors, as well as windows, refrigerators, water boilers, and vehicle heat accumulators.8,9 However, these applications are often limited by its weak mechanical properties.10–19 To improve the poor mechanic strength of monolithic silica aerogel, previous studies have been focused on cross-linking its skeletal framework with polymers such as epoxy,20,21 styrene,22 isocyanate,23–25 cyanoacrylates,26 polynorbornenen,27 polyurethanes,28 and polyimide.29–36In addition to pure silica aerogel and polymer reinforced silica aerogel, pure polymeric aerogels have also been prepared for their high mechanical strength.35 Among these polymers, polyimide exhibits significantly superior in thermal stability, which has a much higher decomposition temperature at 500–600 °C.29–36 Meanwhile, polyimide is also well-known for excellent dielectric properties, good chemical resistances, and high mechanical strength owing to its high degree of structural planarity and strong intermolecular forces.37
Generally, linear polyimide aerogels were produced through the physical interaction between polymer chains, which tend to exhibit a high shrinkage, poor thermal stability, and unsatisfactory mechanic property.36,38 This kind of polyimide aerogel is later cross-linked by some multifunctional amines called cross-linkers to fabricate covalently bonded network structures.33,34,36 These multifunctional amines impart the cross-linked polyimide aerogel a better performance, especially the much lower shrinkage. However, all of those cross-linkers are either quite expensive or not commercially available.36 The process was also implemented, by cross-linking the polyimide polymer chains via the hydrolysis and condensation reactions of some aminosilanes, including 3-aminopropyltrimethoxysilane (APTES), bis(trimethoxysilylpropyl) amine (BTMSPA) and octa(aminophenyl)-silsesquioxane (OAPS), to fabricate the silica cross-linked polyimide aerogels.33–36 Incorporation of inorganic silica also aims at improving the cross-linking extent and reducing the shrinkage.38
Theoretically speaking, high shrinkage of polyimide aerogel is attributed to the high degree of structural planarity and strong intermolecular forces.38 Generally, the major shrinkage takes place during the gelation process, which is followed by the phase separation of the polyimide chains.39 Silica structures in polyimide backbones are treated as the cross-linker, which play dual roles for reducing the shrinkage of the polyimide gels. On one hand, end-capping reaction between aminosilanes and polyimide building blocks prevents the directional growth of the individual chain (steric effect), which reduces the degree of structural planarity. On the other hand, inorganic silica tend to prevent the packing between the adjacent organic polymer chains (steric effect), which weaken the intermolecular interaction.33–36 Thus, shrinkage of the polyimide gels could be tremendously reduced by incorporating the inorganic silica into polyimide backbones during gelation process. In other word, “steric effect” (incorporation of inorganic phase) in the polyimide backbones could be an effective way to control the shrinkage. However, combination of inorganic silica phase and organic polyimide backbones by the chemical bond tend to make the preparation process complex.35 Moreover, the unmanageable hydrolysis and condensation reaction of the aminosilanes with extremely high cost act as deterrents to commercial production.33,34,36
In this study, the inorganic phase was incorporated into the polyimide backbones at a form of micro-scale hydrophobic silica aerogel powders (SAp) by a simple physical mixture. First and for the most, hydrophobic SAp were expected to “jam” the spaces between the polymer chains via the steric effect. This SAp “jammed” polyimide structures may “stretch up” the PI backbones to reduce the high shrinkage.36 Moreover, hydrophobic SAp could maintain their nano porous pore structures and hydrophobicity in the organic reaction system (SAp were not involved in the chemical reaction) and result in excellent thermal insulation and hydrophobicity.34 Certainly, the mixture of organic PI and inorganic SAp for recycling the SAp is also an advocated way for energy conservation. Last but not the least, nano porous SAp into the PI backbones were easy to be eliminated by HF solution to make a conversion into pure polyimide aerogels. Since the etch process takes place after the gelation process, the PI aerogel may retain its “stretched-up” backbones.
Experimental
Materials
Silica aerogel powders (SAp) with a diameter in the range of 20–50 um were produced by milling the silica aerogel blocks. The silica aerogel blocks were synthesized by two step sol–gel process via ambient drying. The detailed process is presented Fig. S1,† all the silica aerogel blocks were prepared with a hydrophobicity treatment, which is similar with the preparation process of the SAp products in industry. Detailed properties of the SAp and the corresponding blocks are list in Table 1.| Samples | SApa (wt%) | Density (g cm−3) | Shrinkageb (%) | Surface areac (m2 g−1) | Pore volumed (cm3 g−1) | Average pore sizee (nm) | Thermal conductivity (W m−1 K−1) | Modulus (MPa) |
|---|---|---|---|---|---|---|---|---|
| a Average of three samples.b Shrinkage was estimated via 100 × (mold diameter − sample diameter)/(mold diameter).c BET specific surface area was calculated by multipoint BET method.d Total pore volume was calculated by the single-point adsorption method.e Average pore diameter was calculated by the 4 × Vpore/σ method. — Not measured. | ||||||||
| PI/SAp-0 | 0 | 0.168 | 23.7 | 242.7 | 1.38 | 22.7 | 0.0371 | 22.7 |
| PI/SAp-20 | 20 | 0.152 | 15.6 | 375.6 | 2.27 | 24.1 | 0.0307 | 11.8 |
| P1/SAp-50 | 50 | 0.119 | 8.1 | 408.8 | 2.57 | 25.1 | 0.0289 | 6.6 |
| P1/SAp-80 | 80 | 0.092 | 7.5 | 455.2 | 2.58 | 22.7 | 0.0283 | 0.8 |
| PI/SAp-80(HF) | 0 | 0.023 | 9.1 | 288.2 | 1.03 | 14.3 | 0.0371 | — |
| PI/SAp-100(block) | 100 | 0.156 | — | 535.4 | 2.96 | 22.1 | 0.0272 | 0.6 |
| PI/SAp-100(powder) | 100 | 0.136 | — | 535.4 | 2.96 | 22.1 | 0.0321 | — |
N-Methyl-2-pyrrolidinone (NMP), acetone, 4,4′-oxydianiline (ODA), pyridine and anhydrous acetic acid were purchased from Sinopharm ChemicalReagent Co. Ltd., China. Biphenyl-3,3′,4,4′-tetracarboxylic dianydride (BPDA) were purchased from Beijing InnoChem Science & Technology Co.,Ltd., China. All reagents were used without further purification except dianhydride which needed to be dried at 125 °C for 24 h under vacuum before being used.
Characterization
The bulk density of the samples were calculated from weight and volume of the cylindrical samples. Shrinkage was estimated via 100 × (mold diameter − sample diameter)/(mold diameter). The morphology of the aerogels were observed by scanning electron microscopy (SEM, Philips-XL30FEG). Samples were fractured at room temperature and sputter coated with gold before the SEM observation. The specific surface area and pore size distribution of the aerogels were obtained from nitrogen adsorption–desorption isotherms at 77 K, analyzed by using a Quantachrome Autosorb-1 analyzer. All samples were out-gassed by heating at 80 °C for 8 h under a vacuum before collecting adsorption and desorption isotherm data by using nitrogen as the adsorbent at 77 K. The surface area of the sample was calculated by the Brunauer–Emmett–Teller (BET) method, and pore size distribution for the mico-pores and meso-pores was determined by modeling using the BJH theory. TG-DSC analysis was conducted from room temperature to 900 °C with a heating rate of 10 °C min−1 under nitrogen atmosphere on a SDT Q600 instrument (TA, America). Pore size distribution from the desorption curves obtained by the mercury intrusion method was mainly used to determine the macro pores in the samples. Thermal conductivity was measured on TPS 2500S thermal constants analyser (Hot Disk, Sweden) at room temperature and mean value was determined by three times measurements. The compression test was taken on a CMT 5105 universal materials testing machine under room conditions with a constant compression speed at 10% of the sample length per minute. Samples were polished smoothly using sandpapers to make sure that the top and bottom surfaces were parallel before installation between the two compression plates of the testing machine. The elastic modulus was taken as the slope of the initial linear portion in the obtained stress–strain curve of the compression. Contact angles (CA) were measured using a JC2000A optical contact-angle software.Preparation of silica-aerogel-powders “jammed” polyimide aerogels
Polyamide acid (PAA) oligomers, the precursor of the polyimide, were derived from the dianhydride (BPDA) and diamines (ODA) with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Firstly, BPDA (2.556 g) was slowly added to a stirred solution of ODA (1.672 g) in NMP, and the mixture was stirred for nearly 30 minutes until all the solids were dissolved to form the PAA solution. Then the silica aerogel powders with a mass fraction of 0%, 20%, 50%, and 80% were slowly added into the PAA solution. The mixture was vigorously stirred for 1 h, after which acetic anhydride (6.57 ml) and pyridine (5.62 ml) were added. The mixture solution was continually and vigorously stirred for 3 minutes, after which it was poured into a polytetrafluoroethylene mold. The gel was removed from the mold after gelation, and soaked in fresh NMP to remove the acetic anhydride and pyridine. The solvent within the obtained gel was exchanged gradually over 24 h intervals as follows: 75/25 vol% NMP/acetone, 50/50 vol% NMP/acetone and 25/75 vol% NMP/acetone, before being exchanged with 100% acetone three times and then dried by supercritical CO2 extraction. The detailed process is illustrated in Scheme 1.
1. Firstly, BPDA (2.556 g) was slowly added to a stirred solution of ODA (1.672 g) in NMP, and the mixture was stirred for nearly 30 minutes until all the solids were dissolved to form the PAA solution. Then the silica aerogel powders with a mass fraction of 0%, 20%, 50%, and 80% were slowly added into the PAA solution. The mixture was vigorously stirred for 1 h, after which acetic anhydride (6.57 ml) and pyridine (5.62 ml) were added. The mixture solution was continually and vigorously stirred for 3 minutes, after which it was poured into a polytetrafluoroethylene mold. The gel was removed from the mold after gelation, and soaked in fresh NMP to remove the acetic anhydride and pyridine. The solvent within the obtained gel was exchanged gradually over 24 h intervals as follows: 75/25 vol% NMP/acetone, 50/50 vol% NMP/acetone and 25/75 vol% NMP/acetone, before being exchanged with 100% acetone three times and then dried by supercritical CO2 extraction. The detailed process is illustrated in Scheme 1.
 | ||
| Scheme 1 Detailed reaction mechanism of the precursor polyamide acid (PAA) and the entire procedure for the preparation of PI/SAp composite aerogels. | ||
Conversion into polyimide aerogel ultra-low density
The composite gels with 80% SAp (PI/SAp-80) after the solvent exchange were socked in HF solution (10%) for 24 h to eliminate the SAp. The obtained polyimide gels were carefully fished out and rinsed with acetone 3 times, and then were dried by supercritical CO2 extraction to produce the ultra-light polyimide aerogel PI/SAp-80 (HF).Results and discussion
The detailed properties of the silica-aerogel-powders “jammed” polyimide aerogels (PI/SAp) are list in Table 1. Typical samples processed with different content of SAp discussed below are shown in Fig. 1. The mass fraction of SAp (ratio of SAp in the total mass of all the solid raw material including dianhydride, diamines, and SAp) is varied from 0% to 100% to fabricate the samples PI/SAp-0 (the pure polyimide aerogel without SAp), PI/SAp-20, PI/SAp-50, PI/SAp-80, and PI/SAp-100 (the pure silica aerogel blocks or powders without PI).From Fig. 1, we can see that the samples turn light yellow from dark yellow following the increased mass fraction of SAp in polyimide backbones. Generally speaking, polyimide backbones are orange or yellow, and the SAp are white (Fig. 1f).29–36 Thus, light yellow of the sample is derived from the mixture of yellow polyimide and white SAp. The color uniformity of all the samples, to a large extent, indicates the homogeneous mixture of SAp.
As expected, mass fraction of the SAp has a great effect on the shrinkage and bulk density. As show in Fig. 2, the pure PI aerogel PI/SAp-0 has a shrinkage of about 23.7%. After mass fraction of SAp increases from 20–80% in the PI/SAp-20, PI/SAp-50, and PI/SAp-80, shrinkage of the samples drastically decreases from 15.6% to 7.5%. Since the different shrinkages generally come from the combination of solvent interactions, chain rigidity and chain packing, there is no need to take a consideration on the solvent interactions in the two systems with the same solvent NMP.36 The major shrinkage of the samples was observed during the gelation process, which is followed by the phase separation of the polyimide chains.39 For the linear polyimide aerogel (dianhydride and diamines with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), chains packing during the phase separation process is an key factor for the high shrinkage.36,39 However, introduction of micro-scale SAp effectively prevents the polyimide chains packing together during the gelation process, because the inorganic silica phase tend to “jam” the spaces between the polymer chains (steric effect). Since the major shrinkage during the gelation process has been controlled by the “jammed” effect, shrinkage of the obtained PI/SAp composite aerogels decreases as low as 7.5% with the increased content of SAp. In comparison with the expensive cross-linkers used for decreasing the shrinkage by a chemical reaction, this simple physical mixtures seems to be the superior.33–36
1), chains packing during the phase separation process is an key factor for the high shrinkage.36,39 However, introduction of micro-scale SAp effectively prevents the polyimide chains packing together during the gelation process, because the inorganic silica phase tend to “jam” the spaces between the polymer chains (steric effect). Since the major shrinkage during the gelation process has been controlled by the “jammed” effect, shrinkage of the obtained PI/SAp composite aerogels decreases as low as 7.5% with the increased content of SAp. In comparison with the expensive cross-linkers used for decreasing the shrinkage by a chemical reaction, this simple physical mixtures seems to be the superior.33–36
With a same trend, density of SAp “jammed” polyimide aerogels (show in Fig. 2) decrease from 0.168 g cm−3 to 0.092 g cm−3 with the increased SAp. As we can see from Table 1, bulk density of the pure silica aerogel block corresponding to the SAp-100 is 0.156 g cm−3 and pure PI aerogel PI/SAp-0 is 0.168 g cm−3. However, density of the SAp “jammed” polyimide aerogels (PI/SAp-20, 50, and 80) are ranging from 0.092 g cm−3 to 0.152 g cm−3, which is much lower than that of the two pure aerogels. Unlike the previously reported polyimide-silica hybrid aerogels produced by the chemical reaction,29–36 SAp “jammed” polyimide aerogels (PI/SAp-20, 50, and 80) are all produced by just a physical mixture. Thus, mixture of the micro-scale SAp tend to form some interfaces, include the gap among SAp, and the boundary between SAp and PI backbones. These interfaces, to a large extent, increase the total volume of the samples and result in a low density.
Microstructure of all the samples was evaluated in terms of their pore size distribution and nano morphology of their frameworks. As show in the SEM images in Fig. 3 and S3,† PI/SAp-0 is nano fibrous networks, which is formed by the intertwining of the polyimide chains with a diameter of about 30 nm. Introduction of particulate PI/SAp-100 (Fig. 3e) means the combination of the organic phase and inorganic phase. Since there is no chemical reaction between them, combination of the two phase is just a physical mixture. Polyimide backbones play a role like adhesives, which ‘glue’ the SAp together. As show in Fig. 3b–d, we can see that the particulate SAp in PI backbones have a preserved pore structure. From Fig. 3b, we can see the boundary between fibrous PI and particulate SAp in the sample PI/SAp-20, while the boundary turned undistinguishable with the increased SAp in PI/SAp-50 and PI-SAp80. Since 50% mass fraction of light weight SAp means a high volume fraction, the major space in the SAp “jammed” polyimide aerogels is mostly occupied by SAp. Namely, the major phase of the microstructure in the sample PI/SAp-50 and PI/SAp-80 is particulate SAp.
As show in Fig. 4, all the samples have the same H1 hysteresis loop in their nitrogen absorption–desorption isotherms. The PI/SAp-100 are particulate and consist of joint particles, while PI/SAp-0 are nano fibrous and tend to fabricate the cylindrical pore between two long straight chains. Therefore, both of the two nano structure could exhibit the type H1 loop in their in their nitrogen absorption–desorption isotherms.36,40 The increasing of the volume adsorbed at relative pressure 0.9 and the narrow desorption hysteresis loop indicates the meso (2–50 nm) and macro porosity (over 50 nm).36 Indeed, BJH desorption plots (show as insets in Fig. 4) give broad pore-size distributions from micro porous to macro porous for the samples. PI/SAp-100 is narrow and monomodal and has distribution peak at around 13 nm. While PI/SAp-0 show relatively broader isolated peak at about 30 nm. However, after the combination of the PI and SAp, there are two distribution peaks at 15 nm and 30 nm appearing in the SAp “jammed” polyimide aerogels (PI/SAp-20 and PI/SAp-50). From Fig. 4, we can see that the relative intensity of the peak at 30 nm declines with the increased SAp, and is merged by the strong peak at 15 nm in the sample PI/SAp-80. Since there are only two phase PI and SAp in the SAp “jammed” polyimide aerogels, it is reasonable that the peaks at 15 nm and 30 nm appearing in the samples of PI/SAp-20 and PI/SAp-50 respectively represents the SAp and PI.
 | ||
| Fig. 4 Nitrogen absorption–desorption isotherms of all the obtained samples. Insets show pore-size distribution derived from BJH desorption plots and data are summarized in Table 1 (a) is the pure PI aerogel (PI/SAp-0); (f) is the silica aerogel powders (PI/SAp-100); (b–d) are the PI/SAp composite aerogels PI/SAp-20, PI/SAp-50, and PI/SAp-80; (e) is the ultralight polyimide aerogel PI/SAp-80(HF) produced by the elimination of SAp in the sample PI/SAp-80. | ||
This evidence provides a further confirmation that combination of the PI and SAp is just a physical mixture, and the “jammed” effect on the PI backbones should not be related the chemical reaction in the organic system. In other word, incorporation of SAp certainly not affect the nano-scale structures of PI. Meanwhile, slightly shifting of distribution peak for the SAp strongly indicates that the particulate SAp in PI backbones have a preserved pore structure (SEM images in Fig. 3b–d). In addition, merge of the peak for the PI in the PI/SAp-80 indicates that major phase of the microstructure in the sample PI/SAp-80 has been occupied by particulate SAp, which is also consistent with the conclusions derived from the nano morphology.
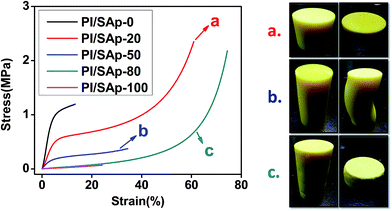
The Young's modulus of the samples is evaluated as the initial linear slope of the stress–strain curves and the specific Young's modulus (E/ρ) is evaluated as the specific value between compression modulus and the bulk density. Fig. 5 shows the stress–strain curves and the image of the SAp “jammed” polyimide aerogels before and after compression test. In nature, pure polyimide aerogels have excellent mechanical strength34 while pure silica aerogel are fragile.13,14 Indeed, pure polyimide aerogel remains the excellent strength, which has the highest slope value in the stress–strain curves. It may be expected that the SAp “jammed” polyimide aerogels PI/SAp-50 and PI/SA-80 would show a performance like the silica aerogel, because the major phase of the two samples is SAp. However, unlike the fragile silica aerogel block which shatters into many tiny fragments after compression, all the SAp “jammed” polyimide aerogels remains in a single piece and turns into a dense solid after application of a large compressive load. As we can see from Fig. 5, the silica aerogel block fails at a low strain about 25%, while the SAp “jammed” polyimide aerogels have long continues curves and didn't break at strain over 60%. All of the abovementioned indicates that the micro-scale SAp are strongly glued by the polyimide backbones to reform an integrated composite block. As show in Table 2, modulus of the pure polyimide aerogel PI/SAp-0 was 22.7 MPa, while silica aerogel block has a modulus of about 0.6 MPa. After the combination of PI and SAp, strength of the samples decrease from 11.8–0.8 MPa with the increased SAp. Previously reported chemically bonded polyimide-silica hybrid aerogels with same density have compressive modulus ranging from 1.7–12 MPa, which is similar with the SAp “jammed” polyimide aerogels produced by the physical mixture. To sum up, it is an effective way to reform the integrated blocks with enhanced strength by just mixing PI and SAp.
| Samples | SAp (wt%) | Bulk density (g cm−3) | Modulus (MPa) | Specific modulus (J g−1) | Sound speed (m s−1) | Ultimate strain (MPa) |
|---|---|---|---|---|---|---|
| PI/SAp-0 | 0 | 0.168 | 22.7 | 135.2 | 368 | Over 80% |
| PI/SAp-20 | 20 | 0.152 | 11.8 | 77.4 | 278 | Over 80% |
| PI/SAp-50 | 50 | 0.119 | 6.6 | 55.8 | 236 | Over 80% |
| PI/SAp-80 | 80 | 0.092 | 0.8 | 8.5 | 92 | Over 70% |
| PI/SAp-100 | 100 | 0.156 | 0.6 | 4.5 | 67 | About 25% |
With a same trend, specific modulus of the samples decrease from 77.4–8.5 J g−1 after the incorporation of SAp in polyimide backbones. As seen in Table 2, the low values of the specific modulus translate into speed of sound waves (278 m s−1 to 92 m s−1 calculated according to eqn (1)), rendering those materials suitable for acoustic insulation.41,42 Moreover, the controllable sound speed with board range from 67 m s−1 to 368 m s−1 for the aerogel materials derived from the combination of organic and inorganic phases (such as PI and SAp) indicates a simple and practicable method to design and control the sound speed.43
 | (1) |
Thermal conductivity of the SAp was measured by imbedding the test probe into the powders which was padded into small cylindrical carton. Meanwhile, the appropriate compression was acted on the cartons for the densification of the SAp, because air resided in the gap between the SAp is a key factors for the test result. As show in Fig. 6, thermal conductivity of the silica powders is 0.032 W m−1 K−1, which is higher than that of the corresponding blocks (0.027 W m−1 K−1). The pure polyimide aerogel PI/SAp-0 has a thermal conductivity of about (0.037 W m−1 K−1). However, after combining the SAp with the PI backbones, thermal conductivity of the SAp “jammed” polyimide aerogels decreases in the range of 0.031–0.028 W m−1 K−1.
Theoretically, the total thermal conductivity of porous materials is the sum of the convective thermal conductivity kc, the radiative thermal conductivity kr, the solid thermal conductivity ks, and the gaseous thermal conductivity kg.36,44–46
| ktotal = kc + kr + ks + kg | (2) |
Since the pore size of the aerogel is in nano-scale, the convective heat transfer between gas and solid phases can be neglected. Meanwhile, radiative thermal conductivity at ambient temperature gives little contribution to the total thermal conductivity.36,44 Therefore, the main contribution to the aerogel at ambient temperature is derived from ks, and kg.
| kambient = ks + kg | (3) |
Solid thermal conductivity can be written as follows:36,44
 | (4) |
The gaseous thermal conductivity of porous materials is frequently expressed as:36,44
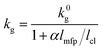 | (5) |
Average pore size of the aforementioned samples (see in Table 1) are all located at about 22–25 nm, which is smaller than the mean free path (about 70 nm) of a gas molecule. This makes the air molecules in the pores difficult to collide with each other, which leads to low gas thermal conductivity.36,44 Since thermal conductivity of the SAp was evaluated by just imbedding the test probe into the powders under a simple compression, air resided in the gap (generally the macro pores) between the micro-scale SAp increases the gas thermal conductivity. Thus, SAp show a much higher thermal conductivity than that of the corresponding blocks with the same density.36,46 Combination of SAp and PI means that the gap between the micro-scale silica powders, to some extent, should be occupied by the PI backbones. In other words, gas thermal conductivity of the impacted SAp in cylindrical carton is much higher than that of the PI-glued SAp. Moreover, decrease in thermal conductivity from 0.031–0.028 W m−1 K−1 following the increased SAp for the SAp “jammed” polyimide aerogels should be attributed to the decrease in bulk density. From eqn (5), we can see that when bulk density of the sample decreases, solid thermal conductivity decrease. It is notable that density of the SAp “jammed” polyimide aerogels are all lower than that of pure silica aerogel blocks, while total thermal conductivity is higher. As seen in eqn (3), the main contribution to the total thermal conductivity of porous materials at ambient temperature is derived from gas and solid thermal conductivity. The lower bulk density means the lower solid thermal conductivity in SAp “jammed” polyimide aerogels comparing with the pure silica aerogel blocks. Thus, the higher total thermal conductivity indicate their higher gas thermal conductivity, which may result from the macro pores formed between the interfaces of the SAp “jammed” polyimide aerogels.
Fig. 7 show the hydrophobicity of the samples, and insets are the photographs for the contact angles. Water contact angle of the pure polyimide aerogel PI/SAp-0 is about 96°, because the imide rings in the polyimide backbones are hydrophilic.34 Incorporation of super-hydrophobic SAp with water contact angle of about 155° tremendously enhance the hydrophobicity of the composite aerogels, which have water contact angle in the range of 130–152°. Fig. S2† show the samples under the water contact angle test. Water droplets on PI/SAp-0 were easy to spread out and quickly absorbed into the sample, which result in some scallops on the surface of the sample. While PI/SAp-20, PI/SAp-50, and PI/SAp-80 show a strong repellency when the water droplets try to get close to them. The small water droplets even can't stay on the surface of the SAp “jammed” polyimide aerogels until increasing their volume.
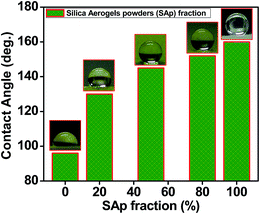 | ||
| Fig. 7 Water contact angle of the pure polyimide aerogel (PI/SAp-0), PI/SAp composite aerogels (PI/SAp-20, PI/SAp-50, and PI/SAp-80), and pure silica aerogel (PI/SAp-100). | ||
In nature, the process of water absorption on the aerogels take place by wetting and capillary action.47,48,50 Generally, the contact angle provides an inverse measure of wettability and the capillary force is often depending on the porous structure of the samples. Thus, the poor water resistance of the traditional polyimide aerogels is mainly attributed to their hygroscopic imide rings and nano porous structures.48–50 Despite their outstanding properties and great potential for application, poor water resistance of the polyimide aerogels is still a big challenge. Incorporation of super-hydrophobic SAp is a convenient and effective rote to enhance the hydrophobicity of polyimide aerogels.50 Overall, the excellent hydrophobicity indicates the superiority of the SAp “jammed” polyimide aerogels for the application in harsh environment.
Thermal stability of the samples was tested by TGA-DSC analysis in N2 without drying the samples before measurement. From the TGA curves in Fig. 8, we can see that all the samples have a weight loss at 100 °C, which represents the absorbed water.36 Decreasing of the absorbed water content from 2.87% to 0.85% (as list in Table 3) with the increased SAp, to some extent, provide a confirmation that incorporation of super-hydrophobic SAp is an effective way to enhance the poor water resistance.
 | ||
| Fig. 8 Thermogravimetric analysis (TGA) curves in N2 of the pure polyimide aerogel (PI/SAp-0), PI/SAp composite aerogels (PI/SAp-20, PI/SAp-50, and PI/SAp-80), and pure silica aerogel (PI/SAp-100). | ||
| Samples | SAp (wt%) | Tinitiala (°C) | T10b (°C) | Tmaxc (°C) | Weight residue at 900 °C (%) | Weight loss at 100 °C (%) |
|---|---|---|---|---|---|---|
| a Initial decomposition temperature.b Temperature of 10% weight loss.c The maximum decomposition temperature. | ||||||
| PI/SAp-0 | 0 | 569 | 583 | 591 | 64.73 | 2.87 |
| PI/SAp-20 | 20 | 562 | 583 | 604 | 65.72 | 2.05 |
| PI/SAp-50 | 50 | 550 | 587 | 607 | 73.15 | 1.73 |
| PI/SAp-80 | 80 | 525 | 590 | 607 | 79.26 | 1.35 |
| PI/SAp-100 | 100 | 400 | 900 | 535 | 90.26 | 0.85 |
The initial decomposition temperature, temperature of 10% weight loss, maximum decomposition, and weight residue at 900 °C were list in Table 3. Residual mass of the samples after the pyrolysis is about 64.7%, 65.7%, 73.2%, and 79.3%, which is corresponding to the sample PI/SAp-0, PI/SAp-20, PI/SAp-50, and PI/SAp-80. This increased tendency is attributed to the increased SAp in PI backbones, because PI/SAp-100 has a residual mass of about 90.3% after the pyrolysis. This residual mass is much higher than that of pure polyimide aerogel. Thus, higher content of SAp means higher residual mass after the pyrolysis.
As it can be seen from Table 3, the decomposition temperature of the pure polyimide aerogel PI/SAp-0 starts around 570 °C. The PI/SAp-100 has an onset of decomposition temperature at about 400 °C, which is attributed to the thermal decomposition of the methyl groups.54 It means the super-hydrophobic composite aerogels should maintain their self-cleaning ability well under this temperature. It may be expected that incorporation of inorganic silica phase can, to some extent, maintain or enhance the thermal stability of the organic polyimide polymer.55 However, the initial decomposition temperature of the unbounded hybrids decrease with the incorporation of the SAp from 560 °C to 525 °C (as list in Table 3, and show Fig. 8). On one hand, combination of the two phase PI and SAp is just a physical mixture, there was no chemical bonds between the silica networks and polyimide backbones. As previously reported, the bonded composite of polyimide and silica has a higher decomposition temperature than the un-bonded one.37 On the other hand, introduction of micro-scale SAp effectively prevent the strong interaction between chains (steric effect), which is the key factor for the high thermal stability of polyimide backbones.56 Both of the two hands lead to a decrease in the decomposition temperature after the incorporation of SAp in PI backbones. In addition, slight weight loss of the PI/SAp-80 occurred at around 200 °C may be attributed to the residual solvent NMP.31
Ultra-light polyimide aerogel PI/SAp-80(HF) was obtained by eliminating the SAp by HF solution from the corresponding gels of the sample PI/SAp-80. As we can see from Table 1, bulk density of the aerogel PI/SAp-80(HF) with pure polyimide backbones is only 0.02 g cm−3, which is much lower than all the previously reported polyimide aerogels.33–36,51–53 Ultra-low density of the sample PI/SAp-80(HF) should be attributed to the decreased weight and the maintained volume. On one hand, elimination of the 80% SAp greatly reduces the mass of the sample, and what remains is just the polyimide backbones. On the other hand, the more important factor is that volume of the eroded sample PI/SAp-80(HF) almost keeps unchanged. As show in Table 1, shrinkage of the PI/SAp-80(HF) slight increase from 7.5% to 9.1% after the elimination of the SAp in the gels of sample PI/SAp-80, because shrinkage of the polyimide backbones generally took place during the gelation process. As mentioned above, the steric effect result in a SAp “jammed” PI backbones during the gelation process. And PI aerogels can retain its “stretched-up” backbones after the drying, because the HF solution can't destroy the PI backbones. Therefore, inorganic SAp can be treated as the shrinkage inhibitor and the pore former to control the microstructure of the polyimide aerogels.
As seen in Fig. 4d–f, decrease in the area of hysteresis loop and the absorbed volume for the PI/SAp-80(HF) indicates that the samples have a decrease in the number of meso-size pore in comparison with the PI/SAp-80. Since the SAp are micro-scale, the residual polyimide backbones after etch is almost constructed by the macro porous frameworks. Pore size distribution from the desorption curves obtained by the mercury intrusion method and the microstructure in SEM provide a confirmation for the increase in macro pores. From Fig. 9 we can see that microstructure of the PI/SAp-80 is the combination of nano fibrous polyimide chains and particulate SAp. After the elimination of SAp, what remains is just the nano porous polyimide polymer networks with obvious open macro pores. Moreover, pore size distribution of the sample before and after the corrosion also indicate a dramatic increase in the number of macro pores. Those provide a further confirmation for the aforementioned interfaces and gapes exist in the SAp “jammed” polyimide aerogels. In addition, increase in total thermal conductivity after corrosion from 0.028 W m−1 K−1 to 0.037 W m−1 K−1 (as list in Table 1), to a great extent, can be attributed to the increased number of macro pores. Although solid thermal conductivity of the ultra-light sample PI/SAp-80(HF) decrease, the key factor is the dramatic increased gas thermal conductivity resulted from the abundant macro pores.36,44
Conclusion
Combination of organic polyimide (PI) and inorganic silica aerogel powders (SAp) were used to fabricate the PI/SAp composite aerogels. On one hand, hydrophobic SAp “jammed” polyimide mixture greatly reduces the shrinkage as low as 7.5% and enhance the hydrophobicity (water contact angle up to 152°), which are the two major challenges of the traditional polyimide aerogels. In addition, the composite aerogels with 80% SAp was transformed into the pure polyimide by just eliminating of the SAp. The obtained polyimide aerogel has ultra-low density of about 0.02 g cm−3, which is much lower than all the previously reported polyimide aerogels. On the other hand, SAp were “glued” together with preserved porous structures by the polyimide backbones. And the PI/SAp composite aerogels exhibit stronger mechanic strength than the pure silica aerogel blocks with a same density. Overall, the SAp “jammed” polyimide aerogels have excellent thermal insulation (thermal conductivity is low to 0.028 W m−1 K−1) and a high thermal stability with an onset decomposition temperature at about 550 °C in N2.Acknowledgements
We are thankful for financial support from Bayer-Tongji Eco-Construction & Material Academy. National High Technology R&D Program of China (2013AA031801), National Natural Science Foundation of China (51172163), Science and Technology Innovation Fund of Shanghai Aerospace, China (SAST201321).References
- S. S. Kistler, Nature, 1931, 127, 741 CrossRef CAS.
- N. Hüsing and U. Schubert, Angew. Chem., Int. Ed., 1998, 37, 22–45 CrossRef.
- A. Du, B. Zhou, Z. Zhang and J. Shen, Materials, 2013, 6, 941–968 CrossRef PubMed.
- M. A. B. Meador, E. McMillon, A. Sandberg, E. Barrios, N. G. Wilmoth, C. H. Mueller and F. A. Miranda, ACS Appl. Mater. Interfaces, 2014, 6, 6062–6068 Search PubMed.
- A. P. Katsoulidis, J. Q. He and M. G. Kanatzidis, Chem. Mater., 2012, 24, 1937–1943 CrossRef CAS.
- A. Du, B. Zhou, W. Xu, Q. Yu, Y. Shen, Z. Zhang, J. Shen and G. Wu, Langmuir, 2013, 29, 11208–11216 CrossRef CAS PubMed.
- H. Sun, Z. Xu and C. Gao, Adv. Mater., 2013, 25, 2554–2560 CrossRef CAS PubMed.
- E. R. Bardy, J. C. Mollendorf and D. R. J. Pendergast, Heat Transfer, 2007, 129, 232–235 CrossRef CAS.
- G. H. Herrmann, H. Bednarek, H. Reichert, R. Stephans and R. Strahle, H & V Engineer, 1995, 68, 8–11 Search PubMed.
- K. Kanamori, M. Aizawa, K. Nakanishi and T. Hanada, Adv Mater., 2007, 19, 1589–1593 CrossRef CAS.
- G. Zhang, A. Dass, A. M. Rawashdeh, J. Thomas, J. A. Counsil, C. Sotiriou-Leventis, E. F. Fabrizio, F. Ilhan, P. Vassilaras, D. A. Scheiman, L. McCorkle, A. Palczer, J. C. Johnston, M. A. B. Meador and N. Leventis, J. Non-Cryst. Solids, 2004, 350, 152–164 CrossRef CAS.
- J. P. Randall, M. A. B. Meador and S. C. Jana, J. Mater. Chem. A., 2013, 1, 6642–6652 RSC.
- Z. S. Deng, J. Wang, A. M. Wu, J. Shen and B. Zhou, J. Non-Cryst. Solids, 1998, 225, 101–104 CrossRef CAS.
- K. E. Parmenter and F. Milstein, J. Non-Cryst. Solids, 1998, 223, 179–189 CrossRef CAS.
- C. Jin, Aerogels Handbook, Advances in Sol-Gel Derived Materials and Technologies, 2011, DOI:10.1007/978-1-4419-7589-8–40.
- J. Chandradass, S. Kang and D. Bae, J. Non-Cryst. Solids, 2008, 354, 4115–4119 CrossRef CAS.
- Z. T. Mazraeh-shahi, A. M. Shoushtari, A. R. Bahramian and M. Abdouss, Fibers Polym., 2014, 15, 2154–2159 CrossRef CAS.
- Z. Zhang, J. Shen, X. Ni, G. Wu, B. Zhou, M. Yang, X. Gu, M. Qian and Y. Wu, J. Macromol. Sci., Part A: Pure Appl.Chem., 2006, 43, 1663–1667 CrossRef CAS.
- X. Yang, Y. Sun, D. Shi and J. Liu, Mater. Sci. Eng., A, 2011, 528, 4830–4836 CrossRef.
- M. A. B. Meador, C. M. Scherzer, B. N. Nguyen, D. Quade and S. L. Vivod, ACS Appl. Mater. Interfaces, 2010, 2, 2162–2168 Search PubMed.
- M. A. B. Meador, A. S. Weber, A. Hindi, M. Naumenko, L. McCorkle, D. Quade, S. L. Vivod, G. L. Gould, S. White and K. Deshpande, ACS Appl. Mater. Interfaces, 2009, 1, 894–906 Search PubMed.
- B. N. Nguyen, M. A. B. Meador, M. E. Tousley, B. Shonkwiler, L. McCorkle, D. A. Scheiman and A. Palczer, ACS Appl. Mater. Interfaces, 2009, 1, 621–630 Search PubMed.
- N. Leventis, C. Sotiriou-Leventis, G. Zhang and A. M. M. Rawashdeh, Nano Lett., 2002, 2, 957–960 CrossRef CAS.
- M. A. B. Meador, L. A. Capadona, L. McCorkle, D. S. Papadopoulos and N. Leventis, Chem. Mater., 2007, 19, 2247–2260 CrossRef CAS.
- B. N. Nguyen, M. A. B. Meador, A. Medoro, V. Arendt, J. Randall, L. McCorkle and B. Shonkwiler, ACS Appl. Mater. Interfaces, 2010, 2, 1430–1443 Search PubMed.
- D. J. Boday, R. J. Stover, B. Muriithi, M. W. Keller, J. T. Wertz, K. A. D. Obrey and D. A. Loy, ACS Appl. Mater. Interfaces, 2009, 1, 1364–1369 Search PubMed.
- D. P. Mohite, Z. J. Larimore, H. Lu, J. T. Mang, C. Sotiriou-Leventis and N. Leventis, Chem. Mater., 2012, 24, 3434–3448 CrossRef CAS.
- Y. Duan, S. C. Jana, B. Lama and M. P. Espe, Langmuir, 2013, 29, 6156–6165 CrossRef CAS PubMed.
- W. Dong, W. Rhine and S. White, Mater. Res. Soc. Symp. Proc., 2011, 1306, DOI:10.1557/opl.2011.380.
- W. Wu, K. Wang and M. S. Zhan, Ind. Eng. Chem. Res., 2012, 51, 12821–12826 CrossRef CAS.
- J. Guo, B. N. Nguyen, L. Li, M. A. B. Meador, D. A. Scheiman and M. Cakmak, J. Mater. Chem. A., 2013, 1, 7211–7221 RSC.
- P. Yan, B. Zhou and A. Du, RSC Adv., 2014, 4, 58252–58259 RSC.
- H. Guo, M. A. B. Meador, L. McCorkle, D. J. Quade, J. Guo, B. Hamilton, M. Cakmak and G. Sprowl, ACS Appl. Mater. Interfaces, 2011, 3, 546–552 Search PubMed.
- H. Guo, M. A. B. Meador, L. McCorkle, D. J. Quade, J. Guo, B. Hamilton and M. Cakmak, ACS Appl. Mater. Interfaces, 2012, 4, 5422–5429 Search PubMed.
- X. Pei, W. Zhai and W. Zheng, Langmuir, 2014, 30, 13375–13383 CrossRef CAS PubMed.
- S. Wu, A. Du, S. Huang, W. Sun, G. Zu, Y. Xiang, C. Li and B. Zhou, RSC Adv., 2016, 6, 22868–22877 RSC.
- J. Lin, Y. Liu, W. Yang, Z. Xie, P. Zhang, X. Li, H. Lin and J. Lei, Polym. Res., 2014, 21, 531–539 CrossRef.
- M. A. B. Meador, E. Malow, Z. He and L. McCorkle, Polym. Prepr., 2011, 52, 21–22 Search PubMed.
- K. Wakabayashi, S. Kohama, S. Yamazaki and K. Kimura, Polymer, 2007, 48, 458–466 CrossRef CAS.
- M. Kruk and M. aroniec, Chem. Mater., 2001, 13, 3169–3183 CrossRef CAS.
- S. Putselyk, G. Eska, S. Abe and K. Matsumoto, Phys. B, 2003, 329, 309–310 CrossRef.
- N. Leventis, C. Chidambareswarapattar, D. P. Mohite, Z. J. Larimore, H. Lu and C. Leventis, J. Mater. Chem., 2011, 21, 11981–11986 RSC.
- S. Motahari, H. Javadi and A. Motahari, J. Mater. Civ. Eng., 2015, 27, 04014237 CrossRef.
- G. Zu, J. Shen, W. Wang, L. Zou, Y. Lian, Z. Zhang, B. Liu and F. Zhang, Chem. Mater., 2014, 26, 5761–5772 CrossRef CAS.
- O. J. Lee, K. H. Lee, T. J. Yim, S. Y. Kim and K. P. Yoo, J. Non-Cryst. Solids, 2002, 298, 287–292 CrossRef CAS.
- G. H. Tang, C. Bi, Y. Zhao and W. Q. Tao, Energy, 2015, 90, 701–721 CrossRef CAS.
- V. Cnudde, M. Dierick, J. Vlassenbroeck, B. Masschaele, E. Lehmann, P. Jacobs and L. V. Hoorebeke, Nucl. Instrum. Methods Phys. Res., Sect. B, 2008, 266, 155–163 CrossRef CAS.
- S. Yun, H. Luo and Y. Gao, RSC Adv., 2014, 4, 4535–4542 RSC.
- H. J. Wang, A. Kleinhammes, T. P. McNicholas, J. Liu and Y. Wu, J. Phys. Chem. C, 2014, 118, 8474–8480 CrossRef CAS.
- S. Wu, A. Du, S. Huang, W. Sun, Y. Xiang and B. Zhou, Mater. Lett., 2016, 176, 118–121 CrossRef CAS.
- M. A. B. Meador, C. R. Alemán, K. Hanson, N. Ramirez, S. L. Vivod, N. Wilmoth and L. McCorkle, ACS Appl. Mater. Interfaces, 2015, 7, 1240–1249 Search PubMed.
- K. Kawagishi, H. Saito, H. Furukawa and K. Horie, Macromol. Rapid Commun., 2007, 28, 96–100 CrossRef CAS.
- M. A. B. Meador, E. J. Malow, R. Silva, S. Wright, D. Quade, S. L. Vivod, H. Guo, J. Guo and M. Cakmak, ACS Appl. Mater. Interfaces, 2012, 4, 536–544 Search PubMed.
- H. Budunoglu, A. Yildirim, M. O. Guler and M. Bayindir, ACS Appl. Mater. Interfaces, 2011, 3, 539–545 Search PubMed.
- G. Ragosta and P. Musto, eXPRESS Polym. Lett., 2009, 3, 413–428 CrossRef CAS.
- L. Liu, X. Zheng, Z. Sun and Y. Wang, Liq. Cryst., 2015, 42, 1382–1390 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: The detailed process for the synthesis of the hydrophobic silica aerogel is provided to state that the silica aerogel powders used in this work is similar with the scraped ones from the industrial production. Graphs for the water contact angle tests and the detailed water absorbed value in TGA curves are provided to confirm the enhanced moisture resistance. SEM image with two different magnifications are provided to exhibit the microstructures of the samples in more detail. TG-DCS curves are provided to indicate the high thermal stability of the samples. See DOI: 10.1039/c6ra11801a |
| ‡ These authors contributed equally to this work and should be considered co-first authors. |
| This journal is © The Royal Society of Chemistry 2016 |