Tailorable pseudocapacitors for energy storage clothes†
Liuxue Shenb,
Peng Suna,
Chuanxi Zhaoa,
Shaozao Tan*b and
Wenjie Mai*a
aSiyuan Laboratory, Guangzhou Key Laboratory of Vacuum Coating Technologies and New Energy Materials, Department of Physics, Jinan University, Guangzhou, Guangdong 510632, China. E-mail: wenjiemai@gmail.com
bDepartment of Chemistry, Jinan University, Guangzhou, Guangdong 510632, China. E-mail: tanshaozao@163.com
First published on 11th July 2016
Abstract
Recently, flexible supercapacitors as promising energy storage devices for wearable electronics have drawn great attention. Here, all-solid-state tailorable supercapacitors (TSCs) based on flexible carbon nanotube (CNT)–polymer and CNT–metal oxide composite hybrid electrodes are successfully demonstrated. The CNT–polypyrrole (PPy) based TSC devices display an excellent electrochemical performance with a volumetric capacitive of 4.4 F cm−3 at the current density of 0.04 A cm−3 (energy density of 0.39 mW h cm−3 at a power density of 9.05 mW cm−3). Strikingly, the TSCs can maintain most of the electrochemical performance and still be operational for powering small electronics like a light-emitting diode (LED) even after undergoing a random cut. When the TSCs are cut down by half of their size, the capacitance retention was ∼50%, suggesting that the tailoring process can hardly affect their performance. Moreover, the other kind of TSC devices can be fabricated by using CNT–MnO2 hybrid flexible electrodes as another example, which demonstrates the broadened potential of our design.
Introduction
Due to the increasing demand for flexible and wearable electronics (e.g. electronic paper, roll-up displays, and skin-like sensors)1,2 it is of great urgency to develop flexible energy storage devices. Recently, tailorable supercapacitors (TSCs), which exhibit the properties of supercapacitors (high power density, fast charge/discharge rate, long cycling lifetime)3–5 and the mechanical damage resistant ability of tailorable electronics,6 have attracted increasing attention. Remarkably, unlike the unchangeable shape and size of conventional capacitors and Li-ion batteries, TSCs show the merits of tailorability and conveniency. They are capable of being made into discretionary shapes and power electronics under harsh conditions, even if they are bent, twisted, cut, or punctured.The energy storage of supercapacitors (SCs) is based on two mechanisms: ion adsorption at electrode/electrolyte interfaces and fast surface redox reactions. Thus, SCs can be classified into electric double layer capacitors (EDLCs) and pseudocapacitors.7 EDLCs usually use high surface area carbon materials as electrodes, such as activated carbon (AC),8 carbon aerogels,9 carbon nanotubes (CNTs),10 and graphene.11,12 Transitional metal oxides such as ruthenium oxide (RuO2),13 manganese oxide (MnO2),14 nickel oxide (NiO)15 and conducting polymers16 like polyaniline (PANI),17,18 polypyrrole (PPy),19–21 poly(3,4-ethylenedioxythiophene) (PEDOT)22 can be applied as pseudocapacitor electrodes. Previously, many studies have focused on flexible SCs. For example, Xue et al. designed graphene–nanotube 3D architectures for wire-shaped SCs.23 Zhu et al. successfully produced flexible magnetic microtubule nanocomposite fabrics for SCs electrode applications.24 Sun et al. fabricated flexible CNT–WO3 hybrid electrodes for flexible asymmetric SCs with high performance and long cycle life.25 Zhu et al. fabricated metal nitrides solid-state asymmetric SCs for flexible electronics.26 Huang et al. fabricated self-healable and highly stretchable SCs.27 Although flexible SCs have been developed intensively, to the best of our knowledge, only one work on TSCs has been published: Xie et al. reported the shape-tailorable capability of graphene-based SCs for wearable electronics.6 However, TSCs employing pseudocapacitive materials are expected to exhibit better performance with higher capacitance.
In this work, we have successful synthesized the PPy nanoparticles via a facile chemical polymerization reaction and MnO2 nanowires through a simple hydrothermal synthesis method. Then, we obtained the flexible CNT–PPy and CNT–MnO2 hybrid electrodes by using a facile vacuum-filtering method. The as-fabricated CNT–PPy hybrid electrode reveals a high specific capacitance of 597 mF cm−2 at the current density of 4 mA cm−2 and the assembled TSCs demonstrate good electrochemical performance (energy density of 0.39 mW h cm−3 at power density of 9.05 mW cm−3). Notably, the TSCs reveal remarkable tailorability and the trimming process can hardly influence the performance of the devices. They can endure random cut from different angle or to any shape, and the TSCs are still operational to power light-emitting diode (LED). Finally, we successfully lighten LED array and driven digital watch used the tandem TSCs which demonstrates their promising application for future wearable electronics.
Experimental
Chemicals and materials
Pyrrole (AR, Aladdin), iron(III) chloride (AR, Damao Tianjin China), potassium permanganate (AR, Kaixin Henyang China), ammonium fluoride (AR, Chemical Guangzhou China), CNT (XF Nano Nanjing China), sodium dodecylbenzenesulfonate (AR, Kemiou Tianjin China), sodium sulfate (AR, Lingfeng Shanghai China), lithium chloride (AR, Kemiou Tianjin China), polyvinyl alcohol (1799, Aladdin), ethanol (AR, Chemical Guangzhou China), filter membrane (0.22 μm), and separator (40 μm in thickness, NKK TF40) were purchased from vendors.Synthesis of PPy and MnO2
2.8 mL of pyrrole (Py) was dropped into 380 mL ethanol solution (Vwater![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Vethanol = 4
Vethanol = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with magnetic stirring at 0–5 °C for 30 min. Then 20 mL 1 mol L−1 FeCl3 solution was slowly poured into the above solution and kept stirring at 0–5 °C for 10 h. After that, the suspension was collected by filtering and then washed with deionized (DI) water and pure ethanol several times. Finally, the as-prepared sample was dried overnight at 60 °C. For the synthesis procedure of MnO2, 0.316 g of KMnO4 and 0.074 g of NH4F were dissolved in 80 mL DI water under magnetic stirring at room temperature to obtain the homogeneous solution; then this mixed solution was transferred into a 100 mL Teflon-lined stainless steel autoclave. The autoclave was sealed and hydrothermally treated maintained at 150 °C for 24 h. After the autoclave was cooled down to room temperature naturally, the suspension was collected by filtering and then washed with DI water and pure ethanol several times. Finally, the as-prepared samples were dried overnight at 60 °C.
1) with magnetic stirring at 0–5 °C for 30 min. Then 20 mL 1 mol L−1 FeCl3 solution was slowly poured into the above solution and kept stirring at 0–5 °C for 10 h. After that, the suspension was collected by filtering and then washed with deionized (DI) water and pure ethanol several times. Finally, the as-prepared sample was dried overnight at 60 °C. For the synthesis procedure of MnO2, 0.316 g of KMnO4 and 0.074 g of NH4F were dissolved in 80 mL DI water under magnetic stirring at room temperature to obtain the homogeneous solution; then this mixed solution was transferred into a 100 mL Teflon-lined stainless steel autoclave. The autoclave was sealed and hydrothermally treated maintained at 150 °C for 24 h. After the autoclave was cooled down to room temperature naturally, the suspension was collected by filtering and then washed with DI water and pure ethanol several times. Finally, the as-prepared samples were dried overnight at 60 °C.
Fabrication of flexible CNT–PPy and CNT–MnO2 hybrid electrodes
The flexible CNT–PPy and CNT–MnO2 hybrid electrodes were prepared by a facile vacuum-filtering method. 40 mg of CNTs and the same weight of PPy or MnO2 were dispersed in 150 mL DI water and involvement 0.3 g of sodium dodecylbenzenesulfonate (SDBS) which acts as the surfactant. Subsequently, the mixture was ultrasonic treated for 30 min to form a homogeneous dispersion. Then the dispersion was vacuum-filtered and washed with DI water to remove the superfluous SDBS. Finally, the filter membrane with a layer of uniform film was dried at 60 °C for 30 min and then a hybrid film was peeled off from the filter membrane.Assembling of all-solid-state TSC devices
The TSC device was assembled using two pieces of flexible CNT–PPy hybrid electrodes with a separator (NKK TF40) and polyvinyl alcohol (PVA)/H2SO4 as gel electrolyte. The PVA/H2SO4 gel electrolyte was synthesized by 12 g of PVA were mixed with in 120 mL of 1 mol L−1 H2SO4 solution and then heated at 85 °C with vigorous stirring for 2 h. Before assembling together, the flexible CNT–PPy hybrid electrodes were immersed in the PVA/H2SO4 gel electrolyte for 5 min, and then two of them were assembled into a TSC device and maintained at room temperature overnight. Similarly, CNT–MnO2 TSC devices can be obtained using the same method and PVA/LiCl acts as the gel electrolyte (maintained at 60 °C overnight to evaporate the excess of water).Material characterization and electrochemical measurement
The morphology and structural properties of the samples were characterized by field-emission scanning electron microscopy (FE-SEM, ZEISS ULTRA 55) equipped with an energy dispersive X-ray spectrometer (EDS), transmission electron microscopy (TEM, JEOL 2100F, 200 kV), Fourier transform infrared spectrometer (FTIR EQUINO55), X-ray diffraction (XRD, Rigaku, Min-iFlex600, Cu Kα, λ = 0.15406 nm, 40 kV, Japan), and X-ray Photoelectron Spectroscopy (XPS, Thermo-VG Scientific ESCALAB 250). The electrochemical properties of the products were assessed using CHI660E electrochemical workstation, and the electrochemical impedance spectroscopy (EIS) were measured by VersaSTAT 3 (Princeton Applied Research) at frequency ranging from 100 mHz to 100 kHz with a potential amplitude of 10 mV. The cycle life was measured by using a battery test system (Neware BTS). For testing the electrochemical performance of the electrode, a three-electrode configuration was employed in 1 mol L−1 H2SO4 solution (1 mol L−1 Na2SO4 solution was used to test CNT–MnO2 hybrid electrodes) at room temperature, with Ag/AgCl electrode as the reference electrode, graphite rod as the counter electrode, and a piece of the freestanding film was used as the working electrode (the effective area about 1 cm2).Results and discussion
The schematic diagram of the fabrication process for the flexible hybrid film is shown in Fig. 1. Firstly, FeCl3 solution was slowly poured into the pyrrole ethanol solution and kept stirring at 0–5 °C for 10 h, the PPy was obtained which the suspension collected by filtering and dried overnight at 60 °C. Then, the same amount (40 mg) of CNTs and PPy were dispersed in deionized water by ultrasonication to form a homogeneous dispersion with the involvement of SDBS. Finally, the dispersion was vacuum-filtered to obtain the CNT–PPy hybrid film. | ||
| Fig. 1 Schematic illustration of the fabrication process of the flexible hybrid film and the TSC devices. | ||
As shown in Fig. 2a, the scanning electron microscopy (SEM) image of the as-synthesize PPy show that their microstructure are formed by nanoparticles. In addition, the SEM images of CNT–PPy hybrid film reveal the CNTs and PPy are well-mixed (Fig. 2b), which could remarkably enhance the electrochemical properties of CNT–PPy hybrid electrodes.
In Fig. 2c, the FTIR spectra of PPy shows a broad absorbing peak at around 3404 cm−1, which corresponds to the N–H bond stretching vibration, and the absorbing peaks at about 1700 cm−1 and 1194 cm−1, which correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and C–O bond stretching vibrations, respectively, demonstrating the pyrrole is peroxide at this polymerization process. Moreover, the two absorbing peaks at about 1553 cm−1 and 1471 cm−1 are attributed to the Py ring backbones vibration. The medium absorbing peak at about 1310 cm−1 derives from the C–N bond stretching vibration of Py ring backbones; the absorbing peak at 1045 cm−1 are attributed to C–H bond in-plane deformation of Py rings and the absorbing peaks at 925 cm−1 and 790 cm−1 both are ascribed to C–H bond out-plane deformation vibrations.28–30
O bond and C–O bond stretching vibrations, respectively, demonstrating the pyrrole is peroxide at this polymerization process. Moreover, the two absorbing peaks at about 1553 cm−1 and 1471 cm−1 are attributed to the Py ring backbones vibration. The medium absorbing peak at about 1310 cm−1 derives from the C–N bond stretching vibration of Py ring backbones; the absorbing peak at 1045 cm−1 are attributed to C–H bond in-plane deformation of Py rings and the absorbing peaks at 925 cm−1 and 790 cm−1 both are ascribed to C–H bond out-plane deformation vibrations.28–30
To further verify the electronic structure of PPy, X-ray photoelectron spectroscopy (XPS) patterns were investigated. Fig. 2d shows the XPS spectra for the C1s peak: the peaks at binding energy of 284.5 eV, 285.6 eV, 286.2 eV and 288 eV correspond to C–C bond, C–N bond, C–O bond and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, respectively. The XPS spectra for the N1s peak and the O1s peak are shown in Fig. 2e and f,31–33 and the results are in agreement with previous FTIR analysis. Then, the energy dispersive X-ray spectrometer spectrum (EDS) also reveals the existence of C, N, O and Cl elements (Fig. S2†), where the Cl signal originates from Cl ion remained in PPy within the polymerization process (Fig. S3a†). All these above characterizations prove the successful synthesis of PPy.
O bond, respectively. The XPS spectra for the N1s peak and the O1s peak are shown in Fig. 2e and f,31–33 and the results are in agreement with previous FTIR analysis. Then, the energy dispersive X-ray spectrometer spectrum (EDS) also reveals the existence of C, N, O and Cl elements (Fig. S2†), where the Cl signal originates from Cl ion remained in PPy within the polymerization process (Fig. S3a†). All these above characterizations prove the successful synthesis of PPy.
The electrochemical properties of the CNT–PPy hybrid electrodes were investigated using three-electrode cell with an Ag/AgCl electrode as reference electrode, a graphite rod as the counter electrode, and 1 M H2SO4 aqueous solution as the electrolyte. First, cyclic voltammetry (CV) curves of CNT–PPy hybrid electrodes (Fig. 3a) were measured at scan rates ranging from 5 mV s−1 to 50 mV s−1 with the voltage ranging from 0 V to 0.8 V, which suggests the typical capacitive behavior for CNT–PPy hybrid electrodes. As shown in Fig. 3b, the galvanostatic charge–discharge (GCD) test was performed at different current densities. Fig. 3c and d display the comparison of the electrochemical properties for the pure CNTs electrode and CNT–PPy hybrid electrode, which are recorded from the CV test at a scan rate of 5 mV s−1 and the GCD test at a current density of 4 mA cm−2. The area capacitance (Ca) of the electrode can be calculated by the following equations:7
 | (1.1) |
 | (1.2) |
 | (1.3) |
The high performance all-solid-state TSCs based on flexible CNT–PPy hybrid electrodes were fabricated by a common process (see Experimental section for details). It's worth noting that the as-prepared device exhibits ultrathin and lightweight properties (0.075 cm3 in volume and 0.1 g in weight of the whole device, including the effective part of electrodes, electrolyte, and separator). CV curves measured on the as-prepared TSCs device at different scan rates over the voltage ranging from 0 V to 0.8 V and the GCD test at different current densities are shown in Fig. 4a and b, which demonstrates good electrochemical property. According to the GCD curves, the volumetric capacitance of the TSCs is calculated to be 4.4 F cm−3 at 0.04 A cm−3, which is higher than many previous reported solid-state SCs, such as H-TiO2@MnO2//H-TiO2@C asymmetric SCs38 (0.70 F cm−3 at 0.5 mA cm−2), CF/MnO2 based SCs39 (2.5 F cm−3 at 0.02 A cm−3), VOx//VN asymmetric SCs40 (1.35 F cm−3 at 0.5 mA cm−2), functionalized CNT-based asymmetric SCs41 (3 F cm−3 at 0.133 A cm−3), MnO2//Fe2O3 asymmetric SCs42 (1.5 F cm−3 at 2 mA cm−2) and VN/CNT based SCs43 (4 F cm−3 at 0.5 A cm−3). The energy density and power density of the TSCs are calculated to be 0.39 mW h cm−3 and 9.05 mW cm−3 by the following equations:7
 | (1.4) |
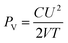 | (1.5) |
Moreover, CV curves tested at a scan rate of 5 mV s−1 show no significant change under different bending states, which reveals these TSCs' remarkable flexibility (Fig. 4c). In addition, the cycling performance of the TSCs is shown in Fig. 4d. After 3000 GCD tests, the capacitance retention remains at 70.6%, suggesting prominent cycling stability.
To demonstrate the tailorability of the flexible CNT–PPy hybrid electrode-based TSCs, a range of experiments were applied to the TSCs devices. Fig. 5a displays that the schematic of TSCs can power electronics even if they are cut. As shown in Fig. 5b, the CV curves of a wide TSCs (width of 2 cm) and its two divided parts (part 1 and part 2) show that both segments own almost half the capacitance of the original CNT–PPy based TSCs. It is obvious that the tailoring process can hardly affect the performance of the TSCs. Furthermore, three tandem TSCs were connected to a closed circuit with a red LED. It can be seen that the red LED was lighten after charging at 2.4 V for 30 seconds.
Subsequently, during the LED was still lighting, the tandem TSCs were cut by a pair of scissors (Fig. 5c and the movie-S1†). The LED keeps alight during and after the tailored process with little brightness change, suggesting the excellent stability and tailorable characteristics of the TSC. In addition, we further designed and performed a series of experiments to prove the potential applications of the all-solid-state TSC device. As displayed in movie-S2,† a digital watch was driven by three tandem TSCs, meanwhile, four tandem TSCs successfully lightened the green LEDs array (32 green LEDs) and the blue LEDs array (53 blue LEDs). Based on the above demonstrations, it can be proved that the TSC devices present a bright prospect for applying in wearable electronics.
Finally, in order to demonstrate that the design of TSCs is not limited in polymer based electrodes but applicable in metal oxide based electrodes, we further fabricated CNT–MnO2 hybrid electrode and explore the performance of CNT–MnO2 hybrid film using a similar technique (Fig. 1). The SEM image of the as-grown samples shows the typical morphology of MnO2 NWs (Fig. 6a), and the transmission electron microscopy (TEM) image (Fig. 6b) further illustrates the smooth surface of MnO2 NWs with an average diameter of ∼40 nm. As shown in Fig. 6c, the high-resolution TEM (HRTEM) image from the circled area clearly reveals that the as-prepared MnO2 NWs possess high quality. The fringe spacing of 0.487 nm corresponds to the interplanar spacing of (200) plane. The corresponding selected area electron diffraction (SAED) pattern could be indexed and results indicate the obtained structure to α-MnO2 phase, with the growth direction along [100] (Fig. 6d). To further confirm the crystallinity and composition of the obtained MnO2 NWs, X-ray diffraction (XRD) and XPS techniques were used (see Fig. S5 and S6†),44 the analysis result prove the successful synthesis of MnO2. In Fig. 6e, the SEM image of hybrid film shows the CNTs and MnO2 NWs are closely intertwined, which contributes to its flexibility and electrochemical performance.
In order to address the effect of the presence of MnO2 on the electrode materials, Fig. 6f and g give the comparison of the electrochemical properties for the pure CNTs electrode and CNT–MnO2 hybrid electrode. Both CV tests were performed in 1 mol L−1 aqueous Na2SO4 electrolyte with a three-electrode configuration at a scan rate of 5 mV s−1 and GCD tests were recorded at a current density of 2 mA cm−2. It can be seen that the introduction of hybrid electrode could greatly enhance the areal capacitance up to 135 mF cm−2 at 2 mA cm−2 increases by 3 times compared to the pure CNTs electrode (Fig. S7†).
In addition, we also used the CNT–MnO2 hybrid electrodes to fabricate all-solid-state TSCs. CV curves measured on this as-prepared TSCs device at different scan rates from 5 to 50 mV s−1 exhibits the typical pseudocapacitive characteristic (Fig. 6h). GCD tests were performed with different current densities, over the voltage from 0 V to 0.8 V (Fig. 6i), demonstrating a good electrochemical performance of the device. The volumetric capacitance of the TSCs is calculated to be 2.6 F cm−3 at 0.02 A cm−3 and the TSCs based on CNT–MnO2 hybrid electrodes display prominent cycling stability of 88.8% capacitance retention after 3000 cycles (Fig. 6j).
Furthermore, the tailorability of the CNT–MnO2 hybrid electrode-based TSCs have been proved. As shown in Fig. 6k, when a piece of TSC was cut down by half of its size, the capacitance retention was ∼50%, which is well proportional to the area of the TGS. The CV curves of two divided parts show that both segments own almost half the capacitance of the original CNT–MnO2 based TSCs.
Conclusions
In this work, we successful fabricated tailorable all-solid-state supercapacitors based on the flexible CNT–PPy and CNT–MnO2 hybrid electrodes, separately. The CNT–PPy hybrid electrode displays high area capacitance of 597 mF cm−2 at 4 mA cm−2 compared with pure CNTs electrode, almost 11 times enhancement. Likewise, the CNT–MnO2 hybrid electrodes with a roughly 3 fold increase were successful synthesized as well. In addition, the TSCs device based on CNT–PPy hybrid electrode exhibited rather good electrochemical performance (energy density of 0.39 mW h cm−3 at power density of 9.05 mW cm−3) and prominent cycling stability while the TSCs device based on CNT–MnO2 hybrid electrode also reveals pretty good performance and long cycling life (88.9% retention after 3000 cycles). Furthermore, these TSCs showed remarkable flexibility and tailorability because the process of bending and tailoring can hardly affect the performance of the devices. They can be separated into two parts while each segment is still operational and has ∼50% of the original capacitance, which is a significant step forward for practical energy storage garment. Eventually, we successfully lightened LED array and drove a digital watch using the tandem TSCs, which demonstrates their promising application for wearable electronics.Acknowledgements
We are grateful for the financial supports from the National Natural Science Foundation of China (Grants 51172099 and 21376104) and the Natural Science Foundation of Guangdong Province, China (Grants 2014A030306010 and 2014A030310302).References
- B. D. Gates, Science, 2009, 323, 1566–1567 CrossRef CAS PubMed.
- J. J. Boland, Nat. Mater., 2010, 9, 790–792 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- Y. Gogotsi and P. Simon, Science, 2011, 334, 917–918 CrossRef CAS PubMed.
- M. Beidaghi and Y. Gogotsi, Energy Environ. Sci., 2014, 7, 867–884 CAS.
- B. Xie, C. Yang, Z. Zhang, P. Zou, Z. Lin, G. Shi, Q. Yang, F. Kang and C. P. Wong, ACS Nano, 2015, 9, 5636–5645 CrossRef CAS PubMed.
- P. Yang and W. Mai, Nano Energy, 2014, 8, 274–290 CrossRef CAS.
- L. Wei, N. Nitta and G. Yushin, ACS Nano, 2013, 7, 6498–6506 CrossRef CAS PubMed.
- E. Gallegos-Suárez, A. F. Pérez-Cadenas, F. J. Maldonado-Hódar and F. Carrasco-Marín, Chem. Eng. J., 2012, 181–182, 851–855 CrossRef.
- C. Portet, G. Yushin and Y. Gogotsi, Carbon, 2007, 45, 2511–2518 CrossRef CAS.
- M. F. El-Kady, V. Strong, S. Dubin and R. B. Kaner, Science, 2012, 335, 1326–1330 CrossRef CAS PubMed.
- H. Yi, H. Wang, Y. Jing, T. Peng, Y. Wang, J. Guo, Q. He, Z. Guo and X. Wang, J. Mater. Chem. A, 2015, 3, 19545–19555 CAS.
- R. Warren, F. Sammoura, F. Tounsi, M. Sanghadasa and L. Lin, J. Mater. Chem. A, 2015, 3, 15568–15575 CAS.
- Z. Yu and J. Thomas, Adv. Mater., 2014, 26, 4279–4285 CrossRef CAS PubMed.
- M. Yu, W. Wang, C. Li, T. Zhai, X. Lu and Y. Tong, NPG Asia Mater., 2014, 6, e129 CrossRef CAS.
- C. Yang, H. Wei, L. Guan, J. Guo, Y. Wang, X. Yan, X. Zhang, S. Wei and Z. Guo, J. Mater. Chem. A, 2015, 3, 14929–14941 CAS.
- L. Wang, X. Feng, L. Ren, Q. Piao, J. Zhong, Y. Wang, H. Li, Y. Chen and B. Wang, J. Am. Chem. Soc., 2015, 137, 4920–4923 CrossRef CAS PubMed.
- Y. Wang, H. Wei, J. Wang, J. Liu, J. Guo, X. Zhang, B. L. Weeks, T. D. Shen, S. Wei and Z. Guo, J. Mater. Chem. A, 2015, 3, 20778–20790 CAS.
- Y. Huang, H. Li, Z. Wang, M. Zhu, Z. Pei, Q. Xue, Y. Huang and C. Zhi, Nano Energy, 2016, 22, 422–438 CrossRef CAS.
- H. Wei, C. He, J. Liu, H. Gu, Y. Wang, X. Yan, J. Guo, D. Ding, N. Z. Shen, X. Wang, S. Wei and Z. Guo, Polymer, 2015, 67, 192–199 CrossRef CAS.
- Y. Huang, J. Tao, W. Meng, M. Zhu, Y. Huang, Y. Fu, Y. Gao and C. Zhi, Nano Energy, 2015, 11, 518–525 CrossRef CAS.
- Y. Zeng, Y. Han, Y. Zhao, Y. Zeng, M. Yu, Y. Liu, H. Tang, Y. Tong and X. Lu, Adv. Energy Mater., 2015, 5, 1402176 CrossRef.
- Y. Xue, Y. Ding, J. Niu, Z. Xia, A. Roy, H. Chen, J. Qu, Z. L. Wang and L. Dai, Sci. Adv., 2015, 1, e1400198 Search PubMed.
- J. Zhu, M. Chen, H. Wei, N. Yerra, N. Haldolaarachchige, Z. Luo, D. P. Young, T. C. Ho, S. Wei and Z. Guo, Nano Energy, 2014, 6, 180–192 CrossRef CAS.
- P. Sun, Z. Deng, P. Yang, X. Yu, Y. Chen, Z. Liang, H. Meng, W. Xie, S. Tan and W. Mai, J. Mater. Chem. A, 2015, 3, 12076–12080 CAS.
- C. Zhu, P. Yang, D. Chao, X. Wang, X. Zhang, S. Chen, B. K. Tay, H. Huang, H. Zhang, W. Mai and H. J. Fan, Adv. Mater., 2015, 27, 4566–4571 CrossRef CAS PubMed.
- Y. Huang, M. Zhong, Y. Huang, M. Zhu, Z. Pei, Z. Wang, Q. Xue, X. Xie and C. Zhi, Nat. Commun., 2015, 6, 10310 CrossRef CAS PubMed.
- C. Chen, X. Fu, T. Ma, W. Fan, Z. Wang and S. Miao, J. Appl. Polym. Sci., 2014, 131, 40814 Search PubMed.
- Y.-T. Weng, H.-A. Pan, N.-L. Wu and G. Z. Chen, J. Power Sources, 2015, 274, 1118–1125 CrossRef CAS.
- Y. Liu, J. Zhou, J. Tang and W. Tang, Chem. Mater., 2015, 27, 7034–7041 CrossRef CAS.
- J. S. Lee, D. H. Shin and J. Jang, Energy Environ. Sci., 2015, 8, 3030–3039 CAS.
- W. Tian, X. Mao, P. Brown, G. C. Rutledge and T. A. Hatton, Adv. Funct. Mater., 2015, 25, 4803–4813 CrossRef CAS.
- S. Zheng, H. Ju and X. Lu, Adv. Energy Mater., 2015, 5, 1500871 CrossRef.
- Y. Chen, L. Du, P. Yang, P. Sun, X. Yu and W. Mai, J. Power Sources, 2015, 287, 68–74 CrossRef CAS.
- H. Wei, Y. Wang, J. Guo, X. Yan, R. O'Connor, X. Zhang, N. Z. Shen, B. L. Weeks, X. Huang, S. Wei and Z. Guo, ChemElectroChem, 2015, 2, 119–126 CrossRef CAS.
- L. Y. Yuan, B. Yao, B. Hu, K. F. Huo, W. Chen and J. Zhou, Energy Environ. Sci., 2013, 6, 470–476 CAS.
- G. Nystrom, A. Razaq, M. Stromme, L. Nyholm and A. Mihranyan, Nano Lett., 2009, 9, 3635–3639 CrossRef PubMed.
- X. Lu, M. Yu, G. Wang, T. Zhai, S. Xie, Y. Ling, Y. Tong and Y. Li, Adv. Mater., 2013, 25, 267–272 CrossRef CAS PubMed.
- X. Xiao, T. Li, P. Yang, Y. Gao, H. Jin, W. Ni, W. Zhan, X. Zhang, Y. Cao, J. Zhong, L. Gong, W.-C. Yen, W. Mai, J. Chen, K. Huo, Y.-L. Chueh, Z. L. Wang and J. Zhou, ACS Nano, 2012, 6, 9200–9206 CrossRef CAS PubMed.
- X. Lu, M. Yu, T. Zhai, G. Wang, S. Xie, T. Liu, C. Liang, Y. Tong and Y. Li, Nano Lett., 2013, 13, 2628–2633 CrossRef CAS PubMed.
- X. Xiao, T. Li, Z. Peng, H. Jin, Q. Zhong, Q. Hu, B. Yao, Q. Luo, C. Zhang, L. Gong, J. Chen, Y. Gogotsi and J. Zhou, Nano Energy, 2014, 6, 1–9 CrossRef CAS.
- P. Yang, Y. Ding, Z. Lin, Z. Chen, Y. Li, P. Qiang, M. Ebrahimi, W. Mai, C. P. Wong and Z. L. Wang, Nano Lett., 2014, 14, 731–736 CrossRef CAS PubMed.
- X. Xiao, X. Peng, H. Jin, T. Li, C. Zhang, B. Gao, B. Hu, K. Huo and J. Zhou, Adv. Mater., 2013, 25, 5091–5097 CrossRef CAS PubMed.
- L. Du, P. Yang, X. Yu, P. Liu, J. Song and W. Mai, J. Mater. Chem. A, 2014, 2, 17561–17567 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11733c |
| This journal is © The Royal Society of Chemistry 2016 |





