Self-assembled peptide-based supramolecular hydrogel for ophthalmic drug delivery†
Renlong Lianga,
Zichao Luo‡
bc,
Guojuan Pubc,
Wei Wua,
Shuai Shia,
Jing Yubc,
Zhaoliang Zhanga,
Hao Chen*a and
Xingyi Li*a
aInstitute of Biomedical Engineering, School of Ophthalmology & Optometry and Eye Hospital, Wenzhou Medical University, 270 Xueyuan Road, Wenzhou 325027, P. R. China. E-mail: dragonhaochen@163.com; lixingyi_1984@163.com; Fax: +86 577 88833806; Tel: +86 577 88833806
bInstitute of Biomaterials and Engineering, Wenzhou Medical University, Wenzhou 325035, P. R. China
cWenzhou Institute of Biomaterials and Engineering, CNITECH, CAS, Wenzhou 325035, P. R. China
First published on 26th July 2016
Abstract
Conventional ophthalmic formulations such as eye drops normally suffer from limited therapeutic efficacy with a requirement for frequent instillation. To improve convenience and efficacy, a peptide-based supramolecular hydrogel (Nap-GFFY) was fabricated and tested for ophthalmic drug delivery. Diclofenac sodium (DIC), as a model drug, was encapsulated into the supramolecular hydrogel by simple physical mixing and then thoroughly characterized by transmission electron microscopy (TEM) and rheology. The encapsulated DIC was rapidly released from the supramolecular hydrogel over a period of 24 h study. In vitro cytotoxicity indicated that the developed Nap-GFFY hydrogel was nontoxic against different cell lines (HCEC, HLEC and L929 cells) after incubation for 24 h. Furthermore, an ocular tolerance test suggested that the developed DIC-loaded Nap-GFFY hydrogel gave rise to no eye irritation after a single instillation. More importantly, the drug concentration in the aqueous humor at 1 h after instillation of the DIC/Nap-GFFY hydrogel was significantly higher than that of commercial DIC eye drops (0.1% DIC; w/v), which indicated better corneal penetration and ophthalmic bioavailability. Overall, the developed DIC/Nap-GFFY hydrogel, as a promising ocular formulation, might have potential applications in the treatment of anterior segment disorders.
1. Introduction
Up to now, the topical instillation of eye drops has been the most important and widely accepted route of administration for treating ophthalmic disorders.1–3 Conventional topical formulations including eye drops and ointments mainly suffer from low ocular bioavailability (<5% of the total drug), owing to the defense mechanisms of the eye such as lacrimation, tear dilution and tear turnover. As a result, frequent administration of drugs is required to maintain the effective therapeutic drug concentration, which might lead to a higher risk of side effects and lower patient compliance.4–6Over the past several decades, significant efforts have been concentrated on the development of novel ocular drug delivery systems for improving in vivo bioavailability after topical instillation.4,7–9 A variety of ocular drug delivery systems including nanoparticles/nanoemulsions, hydrogels, etc., have been investigated and tested to increase corneal drug penetration and prolong pre-corneal drug residence time, yet resulting in an enhancement of ocular bioavailability.1,2,9 One attractive strategy for increasing ocular drug penetration is the use of nanoformulations (nanoparticles, nanoemulsions, micelles, etc.), which not only improve the water solubility of hydrophobic drugs, but also enable site-specific targeting in ocular drug delivery. Based on the literature data, many synthesized polymers such as poly(lactic acid) (PLA), poly(lactic acid-co-glycolic acid) (PLGA), poly(ε-caprolactone)-poly(ethylene glycol) (PCL-PEG) block copolymer and natural polymers such as cyclodextrin (CD), chitosan (CS), and hyaluronic acid (HA) have been successfully fabricated into nanoformulations for ophthalmic drug delivery.10–14 Baba et al. reported that a nanoformulation of a hydrolysable dye (∼200 nm in diameter) exhibited 50-fold higher ocular penetration than that of microsized particles.15 However, the major disadvantages of nanoformulations for ocular drug delivery are related to their instability in long-term storage and their rapid clearance from ocular tissue. Another strategy for the improvement of ocular bioavailability was to prolong the residence time of ophthalmic formulations by hydrogels.7,9,16–18 Hydrogels are hydrophilic systems composed of polymers, which form a 3D network with physical properties that render them attractive for various biomedical applications. Compared with nanoformulations, hydrogel formulations provide several advantages such as high drug-loading capacity, sustained release of drugs and prolonged pre-corneal retention time, etc.16 Over the past few years, different kinds of polymeric hydrogels (pH- and thermosensitive hydrogels) have been successfully developed for ocular drug delivery.7,16,18 There are also many commercial products based on Carbopol® hydrogel for ocular drug delivery of levofloxacin.19 Although hydrogel formulations have made some progress, they still have certain limitations such as blurred vision, eye irritation, etc.
Supramolecular hydrogels, which are formed by the self-assembly of small molecules (MW < 2000) via non-covalent interactions (hydrogen bonding, van der Waals forces, π–π stacking, etc.) have gained considerable attention in recent years.20,21 Among supramolecular hydrogels, peptide-based supramolecular hydrogels have been developed into promising biomaterials for drug delivery and tissue engineering owing to their inherent properties such as high biocompatibility and biodegradability, minimum gelation concentration (MGC), etc.21,22 Up to now, a number of studies have been performed on peptide supramolecular hydrogels for cancer therapy and tissue engineering.20,21,23 However, as far as we know, there is little literature on peptide supramolecular hydrogels for ophthalmic drug delivery. In the present study, we attempted to fabricate a peptide supramolecular hydrogel (Nap-GFFY) for topical ocular drug delivery of diclofenac sodium (DIC; Fig. S1†). In contrast to polymeric hydrogels, the peptide supramolecular hydrogel had a low gelation concentration and was composed of nanofibers, which might be able to enhance the corneal penetration of its drug payload. Moreover, the peptide supramolecular hydrogel also exhibited tunable mechanical strength, which might be able to resist rapid clearance at the corneal surface, thus providing a more prolonged pre-corneal retention time of drugs. Therefore, it is reasonable to believe that the developed DIC-loaded Nap-GFFY hydrogel with favorable properties such as enhancing corneal penetration, prolonging pre-corneal retention time, etc., could significantly increase ocular bioavailability yet reduce the frequency of drug administration. The developed hydrogel formulation was thoroughly characterized by TEM and rheology. An in vitro release study, an in vitro cytotoxicity assay, in vivo ocular tolerance and in vivo pharmacokinetics in a rabbit model were also studied to predict its feasibility for the proposed ophthalmic application.
2. Materials and methods
2.1 Materials
Fmoc-amino acids were obtained from GL Biochem (Shanghai, China). Chemical reagents and solvents such as dimethylformamide (DMF) and dichloromethane (DCM) were from commercial sources. Diclofenac sodium (DIC) and naphthaleneacetic acid (Nap) were obtained from J&K Chemicals Ltd (Shanghai, China). Diclofenac sodium (DIC) eye drops were provided by Zhejiang Eye Hospital (Wenzhou, China). Human corneal epithelial cells (HCEC), human lens epithelial cells (HLEC) and L929 cells were bought from ATCC® (USA). All other chemicals were of analytical grade.2.2 Formation of Nap-GFFY peptide hydrogel
Nap-GFFY (Nap-Gly-Phe-Phe-Tyr) peptides were synthesized using a standard Fmoc solid-phase peptide synthesis method, as previously reported.23 In brief, 5 mg Nap-GFFY peptide and 1 equiv. Na2CO3 were suspended in 1 mL phosphate buffered solution (PBS, pH = 7.4) followed by ultrasonication for 5 min to obtain a transparent solution at 80 °C. Thereafter, a 0.5% (w/v) Nap-GFFY peptide hydrogel was formed during a cooling process within 2 min. Regarding the encapsulation of DIC, a certain amount of DIC was simply mixed with blank Nap-GFFY hydrogel to obtain DIC-loaded Nap-GFFY hydrogels.2.3 TEM observation and rheological studies
Hydrogels were placed onto a grid and stained with 0.5% (w/v) phosphotungstic acid for TEM observations (Tecnai G2 F20 system, 100 kV). Rheology experiments were carried out with a rheometer (AR-2000, TA Instruments, USA) using 40 mm cone plate. To investigate the mechanical properties, a frequency sweep from 0.1 to 100 rad s−1 was performed.2.4 In vitro release study
The in vitro release behavior of diclofenac sodium (DIC) from the 0.5% (w/v) Nap-GFFY hydrogels was studied in phosphate buffered solution (PBS; pH = 7.4) at 37 °C. In brief, 1 mL DIC/Nap-GFFY aqueous solution (0.1%, 0.2% and 0.3% DIC; w/v) was added to a 15 mL test tube followed by gelation at room temperature for 10 min. Subsequently, 5 mL pre-warmed PBS solution (pH = 7.4) as the release medium were added to the test tube for periodical study. At predetermined time points, a 1 mL aliquot of the release medium was withdrawn for quantitative analysis by a RP-HPLC method and the residual release medium was completely replaced with 5 mL freshly pre-warmed PBS solution for continuous study. RP-HPLC analysis was performed on a reversed-phase C18 column (4.6 × 150 mm, 5 μm, ZORBAX Eclipse XDB-C18). The mobile phase was a mixture of acetonitrile and 0.1% triethylamine/phosphate buffer solution (65/35; v/v) at a flow rate of 1.0 mL min−1 and the eluent was detected by a DAD detector at 276 nm.2.5 In vitro cytotoxicity test
To investigate the in vitro cytotoxicity of the developed Nap-GFFY hydrogel, HCEC, HLEC and L929 cells were used. In brief, the cells were seeded into 96-well plates at a density of 1 × 104 cells per well with 100 μL cell culture medium (RPMI 1640 containing 10% FBS) overnight, followed by the addition of 100 μL medium containing different concentrations of Nap-GFFY hydrogel in the range of 0–320 μg mL−1. After incubation for 24 h, cell viability was evaluated by the CCK-8 assay.2.6 Ocular tolerance test
To investigate the ocular tolerability of different formulations, a modified Draize test was performed as reported previously.24 Six male New Zealand albino rabbits (∼2.5 kg) were obtained from Wenzhou Medical University animal center and fed under pathogen-free conditions. All experimental protocols and animal care complied with the Guide for the Care and Use of Laboratory Animals (Scientific and Technological Commission of P. R. China) and were approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University. In brief, 50 μL of either 0.5% (w/v) Nap-GFFY hydrogels or 0.1% (w/v) DIC-loaded Nap-GFFY hydrogels was instilled into the lower conjunctival sac of the rabbit's right eye, while the left eye was instilled with commercial DIC eye drops (DiFei®) as a control. After instillation for 15 min, 30 min, 120 min, 24 h, 48 h and 72 h, the eyes were evaluated visually and immediately scored by a slit lamp. Furthermore, the corneal epithelial integrity and the corneal microstructure were observed by staining with fluorescein sodium and staining with hematoxylin and eosin (H&E) at 24 h after instillation.2.7 Ocular pharmacokinetics study
The ocular pharmacokinetics of 0.1% (w/v) DIC-loaded Nap-GFFY hydrogels and 0.1% (w/v) commercial DIC eye drops (DiFei®) was evaluated for a single instillation (50 μL per eye). After anesthetization with barbitone sodium, six mature male New Zealand albino rabbits (∼2.5 kg) were divided into two groups (n = 3). Each group was instilled with 50 μL of either 0.1% (w/v) DIC-loaded Nap-GFFY hydrogels or 0.1% (w/v) commercial DIC eye drops into the lower conjunctival sac of the rabbit's right eye. At predetermined time intervals (15 min, 30 min, 1 h, 2 h, 4 h, 8 h, and 24 h), samples of 20 μL aqueous humor were collected using an insulin syringe (29G) and then mixed with 80 μL mobile phase to remove the protein by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. Finally, the drug concentration in the aqueous humor as a function of time was detected by an HPLC method as described in Section 2.4.
000 rpm for 10 min. Finally, the drug concentration in the aqueous humor as a function of time was detected by an HPLC method as described in Section 2.4.
2.8 Statistical analysis
Data are shown as the mean ± standard error (SE). Differences among groups were determined using one-way ANOVA analysis followed by Tukey's post hoc test (GraphPad Prism, GraphPad Software, La Jolla, CA).3. Results and discussion
3.1 Formation and characterization of hydrogels
Over the last two decades, significant efforts have been made in the development of novel ocular drug delivery systems to overcome their inherent shortcomings such as poor ocular bioavailability, frequent administration, etc. Among these ocular drug delivery systems, hydrogel formulations have gained considerable attention owing to their viscosity, rheological properties, increased pre-corneal retention time, etc.6,7,9 Pilot studies reported that a hydrogel made of Carbopol® and hydroxypropyl methylcellulose (HPMC) could greatly increase the residence time of drugs at the corneal surface, yet resulting in an enhancement of ocular bioavailability.19,25,26 In this paper, we designed and synthesized a Nap-GFFY peptide (Fig. 1A) to construct a peptide hydrogel (Nap-GFFY) for ophthalmic drug delivery. We proposed that the developed peptide hydrogel might have great potential in ocular drug delivery applications owing to several features: (1) it is a peptide, which could be considered as a biocompatible and biodegradable matrix; (2) it is an anionic hydrogel, which would give rise to no eye irritation and could increase the retention time of drugs at the corneal surface; (3) it could be able to encapsulate various hydrophilic drugs and biomacromolecules (proteins, etc.) by simple mixing. We therefore evaluated the design and investigated the self-assembly behavior of Nap-GFFY peptide in PBS (pH = 7.4). As shown in Fig. 1B, a transparent Nap-GFFY hydrogel (0.5%; w/v) could be formed during the process of heating to cooling. The minimum gelation concentration of Nap-GFFY peptide was 0.08% (w/v), which was in accordance with a previous study.23 The CD spectrum in Fig. S2† indicated that the developed Nap-GFFY hydrogel exhibited the common feature of a β-sheet structure (positive peaks at 189 nm and 227 nm and a negative peak at 204 nm).27 Regarding the loading with DIC, it was clearly observed that the encapsulation of DIC into the Nap-GFFY hydrogel did not result in a change in the hydrogel formation. The morphology of the developed DIC-loaded Nap-GFFY hydrogel was further characterized by TEM. As shown in Fig. 1C, we observed nanofibers in the DIC-loaded Nap-GFFY hydrogel. The diameter of the nanofibers was 15–30 nm and the length of the nanofibers was greater than 20 μm; these were entangled with each other to form networks for the hydrogel. Similar results were also observed by Yang et al., who demonstrated that the formation of peptide nanofibers was mainly attributed to the presence of phenylalanines (Phe), which resulted in π–π stacking between benzene rings.21,27 The mechanical properties of the Nap-GFFY hydrogel and DIC-loaded Nap-GFFY hydrogel were characterized via a model of dynamic frequency sweep. As shown in Fig. 1D, we observed that the values of G′ and G′′ of both hydrogels exhibited a weak dependence on frequency in the range of 0.1–100 rad s−1. Moreover, it was clearly observed that the encapsulation of DIC had a great effect on the mechanical strength of the Nap-GFFY hydrogel. In contrast to the values of G′ and G′′ of the Nap-GFFY hydrogel, the values of G′ and G′′ of the DIC-loaded Nap-GFFY hydrogel displayed an approximately 10-fold decrease, which might be attributed to the fact that the encapsulated DIC could interfere with π–π stacking between benzene rings, thus resulting in a decrease in the mechanical strength of the hydrogel.3.2 In vitro drug release study
The in vitro release of DIC from a 0.5% Nap-GFFY hydrogel was investigated in PBS (pH = 7.4) at 37 °C. As shown in Fig. 2, it was clearly observed that DIC was quickly released from the Nap-GFFY hydrogel in a period of 24 h study. The drug release rate from the hydrogel was dependent on the initial drug loading. The release of DIC from the hydrogel could be accelerated by using a higher DIC concentration (release rates: 0.3% DIC > 0.2% DIC > 0.1% DIC). In general, the drug release from the hydrogel was mainly controlled by the degradation rate of the hydrogel and the diffusion of the drug from the hydrogel.28 In contrast to previously reported polymeric hydrogels, the developed Nap-GFFY hydrogel exhibited a relatively low mechanical strength.29,30 From the result of an in vitro degradation test (Fig. S3†), it was clearly observed that all hydrogel samples exhibited obvious degradation after study for 96 h (30–50% degradation), suggesting that erosion of the hydrogels occurred. However, DIC was almost completely released from the hydrogel after 24 h study, indicating that the release of the drug from the hydrogel was mainly modulated by the manner of diffusion rather than degradation of the hydrogel.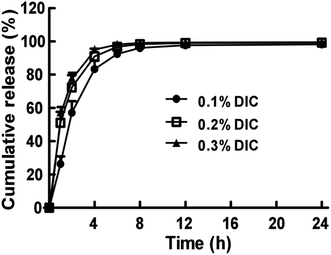 | ||
| Fig. 2 Cumulative drug release profile from DIC-loaded Nap-GFFY hydrogels (0.1%, 0.2% and 0.3% DIC, w/v) at 37 °C in PBS (pH = 7.4). The bars shown are the mean ± SE (n = 3). | ||
3.3 In vitro cytotoxicity test
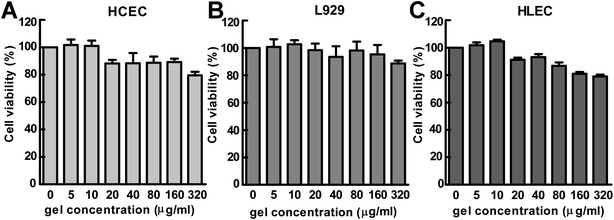
Because the developed Nap-GFFY hydrogel might have potential applications in the field of ocular drug delivery, the biocompatibility of the hydrogel should be carefully investigated before its further in vivo application. Therefore, we used a CCK-8 assay to test the cytotoxicity of the Nap-GFFY hydrogel against L-929 mouse fibroblasts (L929), human corneal epithelial cells (HCEC) and human lens epithelial cells (HLEC). As shown in Fig. 3, more than 80% of cells survived after incubation for 24 h with 5–160 μg mL−1 Nap-GFFY hydrogel, which suggested that the developed Nap-GFFY hydrogel had great biocompatibility. The great biocompatibility of the hydrogel enabled its further development as a formulation for ocular drug delivery.3.4 Ocular tolerance test
For a novel formulation to be proposed as an ophthalmic drug delivery system, it is very important to evaluate not only its biopharmaceutical properties but also its ocular tolerability. Therefore, the potential for ocular irritation of the Nap-GFFY hydrogel and DIC-loaded Nap-GFFY hydrogel was evaluated by using a Draize test in rabbits. As shown in Fig. 4, it was clearly observed that there were no signs such as corneal opacity, conjunctival redness, etc., within 72 h of the instillation of various formulations. Moreover, the corneal epithelium of rabbits instilled with various formulations retained an intact structure at 24 h, as indicated by staining with sodium fluorescein and H&E (Fig. 4). Taking into consideration the fact that rabbit eyes are more susceptible to extraneous substances than human eyes, it is therefore reasonable to believe that the developed Nap-GFFY hydrogel and DIC-loaded Nap-GFFY hydrogel possessed excellent ocular tolerability that was suitable for ocular drug delivery applications.3.5 In vivo pharmacokinetics study
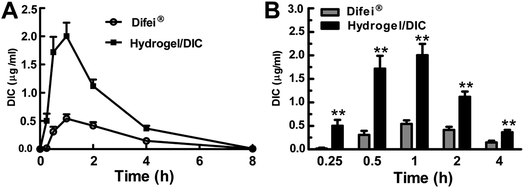
Curves of the drug concentration in the aqueous humor versus time after the instillation of 50 μL 0.1% (w/v) commercial DIC eye drops (DiFei®) and 0.1% (w/v) DIC-loaded Nap-GFFY hydrogels are shown in Fig. 5. From Fig. 5, it was clearly observed that the drug concentration in the aqueous humor quickly reached the maximum drug concentration (Cmax) at 1 h after instillation of both formulations. In comparison with the Cmax of DiFei® (Cmax = 0.54 ± 0.21 μg mL−1), the Cmax of the DIC-loaded Nap-GFFY hydrogel (Cmax = 2.00 ± 0.67 μg mL−1) exhibited an approximately 4-fold increase, which might be attributed to the fact that nanofibers of hydrogels could promote the corneal penetration of drugs. Similar results were also reported by previous studies, in which various polymeric nanoformulations (nanoparticles, nanospheres, liposomes, etc.) or nano-prodrugs could significantly enhance the corneal penetration of drugs after ocular instillation owing to its small size effect.10,13 Furthermore, the areas under the curve (AUC0–8 h) for DiFei® and the DIC-loaded Nap-GFFY hydrogel were 1.58 ± 0.75 μg mL−1 h−1 and 4.95 ± 1.61 μg mL−1 h−1, respectively (Table 1). The fact that the ophthalmic bioavailability of the DIC-loaded Nap-GFFY hydrogel was higher than that of DiFei® might be related to its ability to increase the pre-corneal retention time and corneal penetration of drugs. Overall, our developed DIC-loaded Nap-GFFY hydrogel could greatly improve ophthalmic bioavailability and might have potential applications in the treatment of anterior segment disorders.| Key parameter | Diclofenac sodium-loaded Nap-GFFY hydrogel | Diclofenac sodium eye drops |
|---|---|---|
| Volume of instillation | 50 μL | 50 μL |
| AUC0–8 h (μg mL−1 h−1) | 4.95 ± 1.61 | 1.58 ± 0.75 |
| Cmax (μg mL−1) | 2.00 ± 0.67 | 0.54 ± 0.21 |
| Tmax (h) | 1 | 1 |
4. Conclusion
In the present study, a peptide hydrogel (Nap-GFFY) was successfully constructed for the ophthalmic drug delivery of DIC. The encapsulation of DIC into the Nap-GFFY hydrogel resulted in a decrease in the mechanical strength of the hydrogel. A TEM observation showed that the developed DIC-loaded Nap-GFFY hydrogel had a nanofibrous structure. An in vitro release study indicated that DIC was quickly released from the Nap-GFFY hydrogel within 24 h. By increasing the initial loading of DIC, the release rate increased accordingly. An in vitro cytotoxicity test showed that the developed hydrogel exhibited great biocompatibility. An ocular tolerance test indicated that the developed hydrogel possessed excellent ocular tolerability. More importantly, the developed DIC-loaded Nap-GFFY hydrogel could greatly improve ophthalmic bioavailability after a single instillation in comparison with commercial DIC eye drops (DiFei®). In conclusion, the developed Nap-GFFY hydrogel formulation is a promising candidate for ophthalmic drug delivery.Acknowledgements
This study was supported by the National Natural Science Foundation of China (51303136 and 51403159), the Key Program for International S&T Cooperation Projects of China (2015DFA50310), National Science and Technology Major Project (2014ZX09303301) and Science and Technology Bureau of Wenzhou city (Y20140703 and Y20140141).References
- J. M. Kaiser, H. Imai, J. K. Haakenson, R. M. Brucklacher, T. E. Fox, S. S. Shanmugavelandy, K. A. Unrath, M. M. Pedersen, P. Dai and W. M. Freeman, J. Nanomed. Nanotechnol., 2013, 9, 130–140 CrossRef CAS PubMed.
- S. Liu, L. Jones and F. X. Gu, Macromol. Biosci., 2012, 12, 608–620 CrossRef CAS PubMed.
- X. Yuan, D. C. Marcano, C. S. Shin, X. Hua, L. C. Isenhart, S. C. Pflugfelder and G. Acharya, ACS Nano, 2015, 9, 1749–1758 CrossRef CAS PubMed.
- L. Gan, J. Wang, M. Jiang, H. Bartlett, D. Ouyang, F. Eperjesi, J. Liu and Y. Gan, Drug Discovery Today, 2013, 18, 290–297 CrossRef CAS PubMed.
- D. Achouri, K. Alhanout, P. Piccerelle and V. Andrieu, Drug Dev. Ind. Pharm., 2013, 39, 1599–1617 CrossRef CAS PubMed.
- T. R. R. Singh and D. Jones, J. Pharm. Pharmacol., 2014, 66, 487–489 CrossRef CAS.
- H. Almeida, M. H. Amaral, P. Lobao and J. M. S. Lobo, Drug Discovery Today, 2013, 19, 400–412 CrossRef PubMed.
- R. M. Dutescu, C. Panfil, O. M. Merkel and N. Schrage, Eur. J. Pharm. Biopharm., 2014, 88, 123–128 CrossRef CAS PubMed.
- C. Lu, P. Zahedi, A. Forman and C. Allen, J. Pharm. Sci., 2014, 103, 216–226 CrossRef CAS PubMed.
- T. Stukenkemper, A. Dose, M. Caballo Gonzalez, A. J. J. Groenen, S. Hehir, V. Andres-Duerrero, R. Herrero Vanrell and N. R. Cameron, Macromol. Biosci., 2015, 15, 138–145 CrossRef CAS PubMed.
- J. F. Fangueiro, T. Andreani, M. A. Egea, M. L. Garcia, S. B. Souto, A. M. Silva and E. B. Souto, Int. J. Pharm., 2014, 461, 64–73 CrossRef CAS PubMed.
- N. Mohammed, N. S. Rejinold, S. Mangalathillam, R. Biswas, S. V. Nair and R. Jayakumar, J. Biomed. Nanotechnol., 2013, 9, 1521–1531 CrossRef CAS PubMed.
- S. Shi, Z. Zhang, Z. Luo, J. Yu, R. Liang, X. Li and H. Chen, Sci. Rep., 2015, 5, e11337 CrossRef PubMed.
- X. Li, L. Li, Z. Zhang and H. Chen, Curr. Drug Metab., 2013, 14, 857–862 CrossRef CAS PubMed.
- K. Baba, Y. Tanaka, A. Kubota, H. Kasai, S. Yokokura, H. Nakanishi and K. Nishida, J. Controlled Release, 2011, 153, 278–287 CrossRef CAS PubMed.
- Y. Gao, Y. Sun, F. Ren and S. Gao, Drug Dev. Ind. Pharm., 2010, 36, 1131–1138 CrossRef CAS PubMed.
- A. Ribeiro, F. Veiga, D. Santos, J. J. Torres-Labandeira, A. Concheiro and C. Alvarez-Lorenzo, Biomacromolecules, 2011, 12, 701–709 CrossRef CAS PubMed.
- S. S. Anumolu, A. S. DeSantis, A. R. Menjoge, R. A. Hahn, J. A. Beloni, M. K. Gordon and P. J. Sinko, Biomaterials, 2010, 31, 964–974 CrossRef CAS PubMed.
- K. M. Hosny, AAPS PharmSciTech, 2010, 11, 241–246 CrossRef CAS PubMed.
- H. Wang and Z. Yang, Soft Matter, 2012, 8, 2344–2347 RSC.
- H. Wang and Z. Yang, Nanoscale, 2012, 4, 5259–5267 RSC.
- M. Ikeda, T. Tanida, T. Yoshii and I. Hamachi, Adv. Mater., 2011, 23, 2819–2822 CrossRef CAS PubMed.
- C. Ou, J. Zhang, X. Zhang, Z. Yang and M. Chen, Chem. Commun., 2013, 49, 1853–1855 RSC.
- X. Li, Z. Zhang and H. Chen, Int. J. Pharm., 2013, 448, 96–100 CrossRef CAS PubMed.
- Z. Liu, J. Li, S. Nie, H. Liu, P. Ding and W. Pan, Int. J. Pharm., 2006, 315, 12–17 CrossRef CAS PubMed.
- A. Ludwig, Adv. Drug Delivery Rev., 2005, 57, 1595–1639 CrossRef CAS PubMed.
- N. J. Greenfield, Nat. Protoc., 2006, 1, 2876–2890 CrossRef CAS PubMed.
- C. He, S. W. Kim and D. S. Lee, J. Controlled Release, 2008, 127, 189–207 CrossRef CAS PubMed.
- Y. Huang, H. Yu and C. Xiao, Carbohydr. Polym., 2007, 69, 774–783 CrossRef CAS.
- M. Changez, K. Burugapalli, V. Koul and V. Choudhary, Biomaterials, 2003, 24, 527–536 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Chemical structure of DIC; circular dichroism (CD) spectrum of Nap-GFFY supramolecular hydrogel; in vitro degradation test of DIC/Nap-GFFY supramolecular hydrogels. See DOI: 10.1039/c6ra11691d |
| ‡ Luo ZC carried out equal work with Liang RL and was co-first author of this paper. |
| This journal is © The Royal Society of Chemistry 2016 |