Effects of chitin nanowhiskers on the thermal, barrier, mechanical, and rheological properties of polypropylene nanocomposites
Abstract
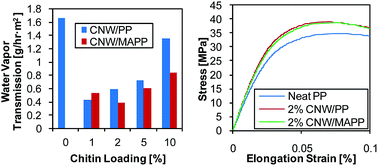
Chitin nanowhisker (CNW)/polypropylene (PP) composites with various CNW loadings fabricated through melt blending were characterized for their morphology, thermal properties, water barrier properties, mechanical properties, and rheological properties. The effects of CNWs and compatibilizer on these properties were investigated. Morphological studies of the composites revealed good dispersion of CNWs within the polymer matrix, which translated into improvements in elastic modulus, ultimate tensile strength, and water barrier properties. These results were further supported by thermal and rheological properties showing increase in crystallinity and extensional viscosity of the composites respectively. Moreover, the addition of a compatibilizer suggested reinforcement and enhanced interaction between the CNW and PP as a significant improvement in water barrier properties was observed. Based on the results, the multiple properties that were explored in this study can be tailored with the addition of CNWs, which is a biodegradable filler, for various sustainable packaging and industrial applications.

 Please wait while we load your content...
Please wait while we load your content...