Nonvolatile electrical switching behavior and mechanism of functional polyimides bearing a pyrrole unit: influence of different side groups†
Zhuxin Zhou,
Lunjun Qu,
Tingting Yang,
Jinglan Wen,
Yi Zhang*,
Zhenguo Chi,
Siwei Liu,
Xudong Chen and
Jiarui Xu
PCFM Lab, GD HPPC Lab, Guangdong Engineering Technology Research Centre for High-performance Organic and Polymer Photoelectric Functional Films, State Key Laboratory of Optoelectronic Materials and Technologies, School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou 510275, China. E-mail: ceszy@mail.sysu.edu.cn; Fax: +86 20 84112222; Tel: +86 20 84112222
First published on 25th May 2016
Abstract
To better understand the structure–property relationships and the mechanisms of the electronic switching behavior of functional polyimides, a new series of diamines containing a pyrrole core with different side groups were designed and synthesized. Three novel, thermally stable and aromatic polyimides (Py6FPIs) containing the as-designed diamines were prepared. They were fabricated into memory devices sandwiched between an Al top electrode and an indium-tin oxide (ITO) bottom electrode by spin-coating. The polyimides exhibited high thermal stability with glass transition temperatures (Tg) around 300 °C as determined by DMA. The memory devices were found to show nonvolatile bistable write-once-read-many (WORM) memory characteristics with diverse switching threshold voltages and ON/OFF ratios, depending on the electron affinity of the pendent groups. The low-conductivity state and the high-conductivity state could be sustained under a constant bias or a refreshing voltage pulse of 1.0 V. The switching mechanism and the memory effects were finely demonstrated with the aid of molecular simulations and electronic absorption spectra of these Py6FPIs.
Introduction
Along with the dramatically increased capacity and drastically decreased size of semiconducting memory devices in recent decades, more and more new materials and advanced processes have been in demand. Compared with the traditional inorganic materials, organic and polymeric materials are promising candidates for the next generation of memory applications. They show notable advantages over inorganic materials, such as ease of processability, high mechanical flexibility, low fabrication cost, property modulation by molecular design through chemical synthesis, and three-dimensional (3D) multi-stacking capability for achieving high density data storage.1 Furthermore, rather than storing and retrieving data by encoding “0” (discharge) and “1” (charge) in traditional memory devices like silicon- and metal-oxide-based memory cells, memory devices based on electrically bistable resistive switching organic or polymeric materials operate by the high and low conductivity response to the applied voltage. Therefore, great efforts have been made for the development of polymer materials with excellent electrical resistive switching effects, such as vinyl polymers,2 poly(3,4-ethylenedioxy-thiophene):poly(styrene sulfonate) (PEDOT:PSS),3 poly(9,9-bis(4-diphenylaminophenyl)-2,7-fluorene) (PDPAF),4 polymer/metal hybrids5 and polymer/organic molecular blends.6Taking into account the requirements of the devices fabrication process, aromatic polyimides (PIs) are greatly superior to the polymer systems mentioned above and become promising materials for device application, due to their outstanding thermal and dimensional stability, high chemical resistance, good mechanical strength and processability.7 More importantly, the macromolecular chain of polyimide naturally possesses an electron-donor (D, diamine moiety) and electron-acceptor (A, dianhydride moiety) structure within a repeating unit, which may exhibit electrical bistability under certain external voltage. In fact, functional PIs exhibiting various memory behaviors have been extensively reported, from volatile memory properties (like Dynamic Random Access Memory (DRAM)8 and Static Random Access Memory (SRAM)8a,e,9), to nonvolatile memory properties (like FLASH memory10 and Write-Once-Read-Many (WORM) memory8a,b,11). However, researches have been mainly focused on the exploration of different diamine structures in functional polyimides with better electrical properties for data storage. The role of the donor and acceptor in these typical polymers, especially the influence of the electron affinities and the steric hindrance effect of the diamine (donor) or dianhydride (acceptor) on the electronic switching behaviors, properties and mechanism, has rarely been systematically studied and further perfected. A deeper understanding of the structure–property relationship is needed to help the design of new functional polyimides for high-performance polymer memory devices.
In this work, we designed and synthesized a new series of diamine molecules containing a pyrrole core with different side groups (–CH3, –CF3 and –CPh3) (Fig. 1). Novel functional aromatic PIs (Py6FPIs) containing the as-designed diamines and dianhydride of 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA) were thus prepared. The incorporation of the planar, conjugated, and electron-rich pyrrole moiety, together with the strong electron-withdrawing trifluoromethyl groups (in dianhydride) into the polyimide backbone was expected to enhance the electron donating-withdrawing property and the charge-transporting capacity along the polymer backbone for device application. We further explored the influence of the D–A interaction intensity to the device performance by finely regulating the electron affinities and the steric hindrance effect of the pendent groups, as it may have an impact on the frontier molecular orbital of the polymers. The memory devices based on the Py6FPIs exhibit nonvolatile bistable electrical switching characteristics of a WORM type memory with long retention time, varied ON/OFF ratios and diverse switching threshold voltages. Moreover, theoretical calculations for molecular orbital distribution, oscillator strength and dipole moment, combined with the absorption spectra of polyimides in thin film state and solution state, exhibiting an exact correspondence with each other, were applied to help better elucidate the switching mechanisms and the memory effects.
Results and discussion
Characterization of the diamine monomers
The three novel diamines containing pyrrole and varied pendent groups, PyCH3DA, PyCF3DA and PyCPh3DA were synthesized via the well-known Paal–Knorr reaction, followed by the reduction of the corresponding dinitro-compound (Scheme 1). The characterizations of the diamines are shown in the Experimental section, Fig. S1 and S2.† The proton resonance signals at around 5.00 ppm and the infrared absorptions at about 3350 cm−1 and 3450 cm−1, which are the characteristics signals of amino groups, indicate a dinitro-compound was successfully reduced to a diamine. 1H NMR and 13C NMR spectra, mass spectra, IR and elemental analysis clearly confirm the diamino-compounds are fully consistent with the proposed structures. Moreover, the different proton signals of the pendent benzene demonstrate that different electron affinities of the side groups do have a certain impact. For instance, as the blue rounds indicated in Fig. 2, the Hd signal of PyCF3DA (7.64–7.61 ppm) shifted to lower field as compared with that of PyCH3DA (7.06–7.04 ppm) and PyCPh3DA (7.05–6.95 ppm), referring the higher electron affinity of trifluoromethyl group (–CF3). This influence might further affect the memory device performance when the polyimides containing the corresponding side group was fabricated as the active layer. | ||
| Fig. 2 Comparison for the 1H NMR spectra of the diamines (the blue rounds indicate the Hd on the pendent phenyl rings). | ||
Characterization of the polyimides
The Py6FPIs were prepared as films by the conventional two-step polymerization as shown in Scheme 2. The reaction between the as-synthesized diamine and the commercially available dianhydride 6FDA in DMF gave a viscous precursor poly(amic acid) solution, followed by programmed thermal imidization on a glass plate. The resulting yellowish PI films were tough and flexible. The inherent viscosities of PyCH36FPI, PyCF36FPI and PyCPh36FPI in NMP are 1.19, 0.94 and 1.06 dL g−1, respectively. As shown in Fig. S3a,† the polyimides exhibited the characteristic infrared absorption bands of the imide ring at 1784 cm−1 (asymmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) and 1720 cm−1 (symmetric C
O stretching) and 1720 cm−1 (symmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching). Fig. S3b† shows the 1H NMR spectra of polyimides. The disappearance of amide bands and the absence of carboxyl absorptions in the FT-IR spectra, as well as the disappearance of the amide and carboxyl proton signals indicated a virtually complete conversion of the precursor poly(amic acid) to the polyimide. The wide-angle X-ray diffraction (WAXD) patterns (Fig. S4†) show the amorphous nature in all the as-prepared polyimide films. These properties might be attributed to the high steric hindrance effect of hexafluoroisopropyl group in the dianhydride moieties and the pendent groups in the diamines moieties, which could avoid the close packing of the macromolecular chain.
O stretching). Fig. S3b† shows the 1H NMR spectra of polyimides. The disappearance of amide bands and the absence of carboxyl absorptions in the FT-IR spectra, as well as the disappearance of the amide and carboxyl proton signals indicated a virtually complete conversion of the precursor poly(amic acid) to the polyimide. The wide-angle X-ray diffraction (WAXD) patterns (Fig. S4†) show the amorphous nature in all the as-prepared polyimide films. These properties might be attributed to the high steric hindrance effect of hexafluoroisopropyl group in the dianhydride moieties and the pendent groups in the diamines moieties, which could avoid the close packing of the macromolecular chain.
Thermal properties of the Py6FPIs
Thermal properties of the polyimides were investigated by DSC, DMA, TMA and TGA, and the results are summarized in Table 1 and Fig. S5.† The glass transition temperature (Tg) of all the polyimides were observed in the range of 276–287 °C by DSC, and 296–306 °C by DMA. All of the polyimides exhibited no obvious weight loss below 500 °C in nitrogen atmosphere. The decomposition temperatures (Td) at 5% and 10% weight-loss measured by TGA were recorded in the range of 515–543 °C and 537–565 °C, respectively. These remarkable thermal stability properties allowed the Py6FPIs to undergo the high temperature electronic device fabrication processes. Among the char yields of the Py6FPIs (in the range of 55–63%) obtained from the TGA curves at 900 °C, the PyCPh36FPI ranked the highest, resulted from its high aromatic contents. Besides, due to the rigidity of the polymer backbones, the polyimide films showed relatively high dimensional stabilities with the coefficient of thermal expansion (CTE) at 45–50 μm m−1 °C−1 in the recorded range of 50–250 °C. The softening temperatures (Ts) gauged by TMA were in the range of 263–279 °C, which proved the high thermal stabilities of the polyimides as well.| Polyimide | Tga (°C) | Tgb (°C) | Tsc (°C) | CTEd (μm m−1 °C−1) | Tde (°C) | Char yieldf (wt%) | |
|---|---|---|---|---|---|---|---|
| 5 wt% | 10 wt% | ||||||
| a Measured by DSC at a heating rate of 10 °C min−1 in nitrogen.b Measured by DMA at a heating rate of 5 °C min−1.c Softening temperature, measured by TMA at a heating rate of 10 °C min−1.d Calculated by TMA curves (50 to 250 °C).e Measured by TGA at a heating rate of 20 °C min−1 in nitrogen.f Residual weight percentage at 900 °C in nitrogen. | |||||||
| PyCH36FPI | 276 | 296 | 264 | 50 | 515 | 537 | 58 |
| PyCF36FPI | 287 | 306 | 279 | 49 | 522 | 543 | 55 |
| PyCPh36FPI | 277 | 298 | 263 | 45 | 543 | 565 | 63 |
Optical and electrochemical properties of the Py6FPIs
The optical properties of the polyimides were measured by UV-vis spectroscopy. The key statistics are listed in Table 2. As shown in Fig. 3a, the Py6FPIs solution exhibited a maximum absorption peak (λabsmax1) at around 328 nm with a shoulder (λabsmax2) at about 306 nm. The former peak probably corresponds to the charge-transfer transition from the pyrrole-containing diamine moieties (donor) to the dianhydride moieties (acceptor), while the latter is originated from the n–π* and local π–π* transitions of the pyrrole moieties since the λabsmax2 is well consistent with the absorption maxima of their corresponding monomers in solution (shown in Table 2 with parentheses, and in Fig. S6†). The absorption edges (λabsonset) of all the polymers were observed in the ranges of 371–377 nm. In this case, the order of λabsonset principally correlated with the nucleophilicity of the pendent group. As a strong electron acceptor group, –CF3 can reduce the nucleophilicity of the diamine moieties and weaken the CT intramolecular interaction, thus the CT absorption edge of PyCF36FPI assumed a hypsochromic shift. The λabsonset of PyCPh36FPI, yet assumed a bathochromic shift due to the electron-donating properties of triphenylmethyl group (–CPh3). For the solid thin films coated on the quartz plates, these polyimides showed a maximum absorption peak at around 332 nm with a shoulder at about 304 nm (Fig. 3b). Compared with the absorption spectra measured in the solution state, the almost unchanged λabsmax2 in the solid film state further proves that this absorption is from the local transitions of the pyrrole moieties. On the other hand, the λabsmax1 red-shifted and the absorption tails extended to a longer wavelength due to the stronger intermolecular CT interaction in the solid film state. The λabsonset of PyCPh36FPI, however, located at a shorter wavelength than that of PyCH36FPI, which is different from the order in solution state. It could be ascribed to the closed packing of PyCH36FPI molecular chain in the solid thin film state, while the bulky side group –CPh3 in PyCPh36FPI prevents the dense packing thus alleviates the intermolecular interactions.| Polyimide | In solutiona (nm) | As thin filmb (nm) | Oxidation potentialc (V) | Egd (eV) | HOMOe (eV) | LUMOf (eV) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λabsonset | λabsmax1 | λabsmax2 | λabsonset | λabsmax1 | λabsmax2 | EOXonset | EOXmax1 | EOXmax2 | ||||
| a Measured in dilute solution in DMF (∼2 × 10−5 M).b Spin-coated thin film on quartz plate.c Obtained from cyclic voltammograms versus Ag/AgCl in CH3CN.d Energy gap = 1240/λabsonset of the polyimide thin film.e The HOMO energy levels relative to ferrocene (−4.80 eV) were calculated from Eonset.f LUMO = HOMO + Eg.g Data in parentheses are the maximum UV-vis absorption wavelength measured in THF solution (10−5 M). | ||||||||||||
| PyCH36FPI | 374 | 329 | 307 (307)g | 395 | 334 | 305 | 0.90 | 1.11 | 1.53 | 3.14 | –5.32 | –2.18 |
| PyCF36FPI | 371 | 325 | 305 (300) | 385 | 328 | 303 | 1.00 | 1.17 | 1.59 | 3.22 | –5.42 | –2.20 |
| PyCPh36FPI | 377 | 331 | 307 (305) | 392 | 335 | 304 | 0.97 | 1.15 | 1.55 | 3.16 | –5.39 | –2.23 |
 | ||
| Fig. 3 Normalized UV-vis absorption spectra of Py6FPIs: (a) in DMF (∼2 × 10−5 M); (b) PI films spin-coated on quartz plates. | ||
Fig. 4 depicts the cyclic voltammograms (CV) of the polyimide cast films on the ITO-coated glass slides, which were used as working electrodes in CH3CN containing 0.1 M solution of TBAP as supporting electrolyte and saturated Ag/AgCl as reference electrode. The related electrochemical properties are also summarized in Table 2. These PIs showed two oxidation peaks in the range of 1.11–1.17 V and 1.53–1.59 V, respectively. The onset oxidation potentials for PyCH36FPI, PyCF36FPI and PyCPh36FPI were 0.90, 1.00 and 0.97 V respectively. Note that the oxidation potential of ferrocene (external standard) was 0.38 V versus Ag/AgCl measured by CV from a polymer-free ITO-coated glass slide. Thus the estimated highest occupied molecular orbital (HOMO) energy levels were −5.32, −5.42 and −5.39 eV, respectively, based on the reference energy level of ferrocene (4.8 eV below vacuum level). Also note that the band gap estimated from the absorption edge of polymer thin films were 3.14, 3.22 and 3.16 eV, respectively. The lowest unoccupied molecular orbital (LUMO) energy levels were −2.18, −2.20 and −2.23 eV, respectively. These results indicate that the variation of the pendent groups with different electron affinities have a certain impact on the HOMO and LUMO energy levels. Hence, the hole-injecting and electron-injecting properties of the resulting memory devices, more or less, may be differed.
Electrical switching effects and memory performances of the ITO/Py6FPI/Al devices
The memory behavior of Py6FPIs is demonstrated by the I–V characteristics of ITO/Py6FPI/Al device. Fig. 5 shows the I–V curves of the ITO/Py6FPIs/Al devices under an initially positive applied voltage. The I–V curves of ITO/PyCH36FPI/Al device (Fig. 5a) are used as an example in illustrating the memory effects. In the first sweep under the positive voltage from 0 to 4.1 V, the device was in the low conductivity state, which was assigned as OFF state or “0” signal in data storage. However, an abrupt increase in current from 10−10 to 10−5 A occurred at the continuous sweeping voltage around 4.1–4.2 V, which was defined as the switching threshold voltage, indicating the device transferred from a low conductivity state to a high conductivity state (as-signed as ON state or “1” signal in data storage). These distinct conductivity states allow the reading process at a relatively low voltage (e.g., 1.0 V) for ON signal or OFF signal without interference. During the continuous positive sweeping to 5.0 V, the device remained in the ON state with an ON/OFF ratio over 105. This electronic transition from the OFF state to the ON state corresponds to the “writing” process in a digital memory cell. The device kept in ON state during the subsequent reverse sweep (the 2nd sweep) from 5.0 V to 0.0 V. Even after the device was power-off for about 3 min, the ON state could be preserved in the following negative sweeps from 0.0 to −5.0 V (the 3rd sweep) and −5.0 to 0.0 V (the 4th sweep), as well as the positive sweeps from 0.0 to 5.0 V (the 5th sweep) and 5.0 to 0.0 V (the 6th sweep), without relaxation to the OFF state (defined as the “erasing” process). For the other two memory devices applying PyCF36FPI and PyCPh36FPI as the active layer, similar switching properties were observed. The I–V characteristics demonstrate that the ITO/Py6FPI/Al devices exhibited nonvolatile WORM type memory effect.To make further investigations on the performance of the WORM type memory effects, the ON/OFF current ratio, the retention time of the device and the read cycles were evaluated. Fig. 6 shows that the pyrrole-containing polyimide devices had varied ON/OFF current ratios from 103 to 5 × 105 depending on the pendent groups.
The relatively low ON/OFF current ratio for PyCPh36FPI probably results from the stronger π–π interaction between molecular chains, considering three crowded benzene rings in the triphenylmethyl group. Yet, they all exhibited high stabilities during the measurement of the retention times for the ON and OFF states, as shown in Fig. 7. By applying a constant reading voltage of 1.0 V for the ON state and the OFF state, no obvious degradation in current value was observed for an hour or even longer. In addition, both the ON state and the OFF state retained stable at read pulses of 1.0 V (each pulse lasts 0.5 ms) without degradation (Fig. 8). These results suggest that the memory devices possessed good reproducibility and stability.
 | ||
| Fig. 7 The retention times for the ON and OFF states of the ITO/Py6FPI/Al devices using (a) PyCH36FPI, (b) PyCF36FPI or (c) PyCPh36FPI as active layer under a constant bias of 1.0 V. | ||
Switching mechanism
To better illustrate the transition process from the ON state to the OFF state, the I–V curves in both states are analyzed in detail using theoretical models. Under a low positive voltage (e.g., 0.5–2.5 V, Fig. 9a), the I–V curves for PyCH36FPI of the ON state follows the ohmic conduction model14 described by eqn (1).| J = qnμV/d | (1) |
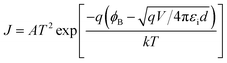 | (2) |
 | ||
| Fig. 9 Experimental (dots) and fitted (solid line, for Schottky emission model) I–V curves for the (a) ON state and (b) OFF state of the ITO/PyCH36FPI/Al devices. | ||
This may help better elucidate the variations in switching threshold voltages for Py6FPIs with deferent side groups. As mentioned above, the strongly electron-withdrawing trifluoromethyl groups significantly lower the HOMO value, and increase the energy barrier between the ITO electrode and the HOMO of PyCF36FPI. Thus, under an insufficient applied voltage, electrons are difficult to transit to the HOMO of PyCF36FPI. While, a lower applied voltage can let the electrons hop up to the HOMO of PyCH36FPI and PyCPh36FPI. As a matter of fact, the order of barrier values relative to ITO (work function – 4.7 eV), PyCH36FPI (0.62 eV) < PyCPh36FPI (0.69 eV) < PyCF36FPI (0.72 eV), matched the order of switching threshold voltages PyCH36FPI (4.15 V) < PyCPh36FPI (4.20 V) < PyCF36FPI (7.55 V).
On the other hand, the nonvolatile WORM type memory effects could be explained by the electric field induced “conformation-coupled charge transfer”.11a When the exited states are formed under an electric field, the conformation-coupled charge transfer process may occur, leading to a twisted molecular chain. Thus, a large potential energy barrier for the dissociation of CT complexes arises due to the steric hindrance for the return of the electrons, which eventually stabilized the conductive CT state and retained the high conductivity states. They offer continuous open channels for charge carriers to move in both directions. Under these circumstances, no matter which direction of external electric field is applied, the memory device will maintain at the ON state instead of returning to the low conductivity OFF state, exhibiting nonvolatile WORM type memory behaviors.
In this view, the more stable the CT complexes are, the more possibility for the polyimide to possess nonvolatile WORM properties. Under an applied field, the dipole moment of exciton changes and leads to a splitting of the original exciton state.16 The evaluation of the dipole moment helps to understand the memory characteristics. The theoretical dipole moments for the BU of PyCH36FPI, PyCF36FPI and PyCPh36FPI are 3.70, 2.32 and 3.82 debye, respectively. Herein, the reported triphenylamine-containing TP6F-PI,8g whose BU dipole moment was calculated as 2.07 debye in the study, is taken for comparison. Since TP6F-PI possesses a lower dipole moment which leads to a more unstable CT complex, it may have less possibility for a WORM type memory than the reported polyimides herein. In fact, the device based on TP6F-PI exhibited volatile DRAM behavior, while our devices all exhibited nonvolatile WORM behavior. Therefore, a WORM type memory can be gained by introducing more nucleophilicity groups such as pyrrole to the donor moieties of a polyimide chain.
In order to better understand the electrical switching behaviors of the memory devices, molecular simulations were used to demonstrate the electronic properties. The calculations of the frontier orbitals and the dipole moment of the basic unit (BU) were carried out by the DFT method at the B3LYP/6-31(d) level with Gaussian 09 program package. Since the trifluoromethyl groups probably do not significantly affect the electronic properties of the BU,17 they were excluded in the calculation of the mechanism clarification. In fact, we calculated the BU of PyCH36FPI with and without the trifluoromethyl groups at the same level, as can be seen in Fig. S8.† Without disturbing the distribution, a slight difference could be found for the energy levels of the molecular orbitals distributed on the corresponding diamine moieties. For instance, the LUMO+2 and HOMO energy levels of the BU without trifluoromethyl groups were just about the LUMO+4 and HOMO energy levels of the BU with trifluoro methyl groups, respectively. Therefore, the results of the simplified but reasonable molecular simulation for the BU of PyCH36FPI are displayed in Fig. 10. The HOMO is predominantly distributed on the pyrrole moiety, while the LUMO is located on the phthalimide moiety, suggesting that the pyrrole moieties serve as electron donor and the phthalimide moieties serve as electron acceptor in PyCH36FPI. The higher frontier orbitals, LUMO+1 and LUMO+2, are distributed over the BU.
 | ||
| Fig. 10 The calculated frontier molecular orbitals of the basic unit of PyCH36FPI (left) and the plausible electronic transitions induced by an electric field (right). | ||
The plausible electronic transitions induced by an electric field are presented in Fig. 10 as well, with the aid of further theoretical calculations for the oscillator strengths and transition contributions by the time-dependent DFT (TD-DFT) method (Table 3). As the HOMO → LUMO transition possesses an oscillator strength of 0.0007 which is quite low, electrons accumulated at HOMO are more inclined to hop up to LUMO+1 and LUMO+2, because these transitions possess much larger value of oscillator strength. When sufficient energy is conducted, electrons at the HOMO of PyCH36FPI accumulate and shift to LUMO+1 or/and LUMO+2 to result in an excited state. As LUMO+1 and LUMO+2 delocalize over the BU, the electrons at the excited states are capable of relaxing easily to the LUMO which locates at a higher electron affinity phthalimide moiety, via internal transition to from a CT state. Besides, the formation of the CT state may also derive directly from the HOMO to the LUMO, but it's very unlikely due to the mentioned low oscillator strength.
| Polyimide | Excited state | Oscillator strength | Orbitals | Contribution |
|---|---|---|---|---|
| a Prohibited transitions are not listed. | ||||
| PyCH36FPI | 1 | 0.0007 | HOMO → LUMO | 0.99 |
| 2 | 0.3321 | HOMO → LUMO+1 | 0.99 | |
| 7 | 0.3607 | HOMO → LUMO+2 | 0.85 | |
| HOMO → LUMO+4 | 0.14 | |||
| PyCF36FPI | 1 | 0.0007 | HOMO → LUMO | 0.99 |
| 2 | 0.3565 | HOMO → LUMO+1 | 0.78 | |
| HOMO → LUMO+2 | 0.21 | |||
| 9 | 0.6259 | HOMO → LUMO+2 | 0.05 | |
| HOMO → LUMO+4 | 0.93 | |||
| PyCPh36FPI | 1 | 0.0007 | HOMO → LUMO | 0.99 |
| 3 | 0.2730 | HOMO → LUMO+1 | 0.99 | |
| 14 | 0.5544 | HOMO → LUMO+2 | 0.19 | |
| HOMO → LUMO+3 | 0.75 | |||
These processes are consistent with the absorption spectrum of PyCH36FPI in solution. The onset absorption wavelength at 374 nm (3.31 eV) arises from the HOMO → LUMO transition (2.78 eV), and the two primary absorption peaks at 329 nm (3.77 eV) and 307 (4.04 eV) nm correspond respectively to the HOMO → LUMO+1 transition (3.76 eV) and the HOMO → LUMO+2 transition (4.43 eV). These results are in absolute accordance with the conclusions in the optical properties section, where the absorption tail is assigned to the CT transition and the absorption peak at 307 nm is assigned to the local transition of the pyrrole moieties.
We subsequently found that the PyCF36FPI and PyCPh36FPI systems underwent similar electronic transition processes with slight differences. As evidenced by the calculated oscillator strength and transition assignment in Table 3, for PyCF36FPI system, the accumulated electrons were more inclined to be excited to LUMO+4 rather than LUMO+2 under sufficient energy. As the molecular distributions of PyCF36FPI depicted in Fig. S9,† the LUMO+4 was mainly distributed on the diamine moiety, while the LUMO+2 was widespread on the pendent group, resulting in a higher contribution to the excited state formation of the HOMO to LUMO+4 transition. Taking on the into account the energy barriers (Fig. 11) in PyCF36FPI of the main transition with large oscillator strength, 3.9 eV for HOMO to LUMO+1, and 4.63 eV for HOMO to LUMO+4, the higher threshold voltage for PyCF36FPI-containing device could be well explained, since PyCH36FPI possessed lower transition barriers, i.e. 3.76 eV for HOMO to LUMO+1, and 4.43 eV for HOMO to LUMO+2. Likewise, for PyCPh36FPI system, the barriers were 3.72 eV for HOMO to LUMO+1, and 4.60 eV for HOMO to LUMO+3. On the other hand, the lowest ranking for the threshold voltage of PyCPh36FPI could be chalked up to the comprehensive effects of the conjugated donor properties as well as the steric hindrance effect.
Conclusions
Three novel polyimides Py6FPIs containing pyrrole moiety with different pendent groups were synthesized. The yellowish PI films were tough and flexible with highly thermal stability. The simple memory devices with the configuration of ITO/Py6FPIs/Al were fabricated by spin-coating. Nonvolatile bistable WORM characteristics were observed under an applied electric field to the devices. They showed highly stable performances with different turn-on threshold voltages and ON/OFF current ratios depending on the pendent groups. It appeared that the threshold voltages were tuneable trough modulating the electron affinity of the pendent group for these pyrrole-containing polyimides. In addition, electronic absorption spectra and molecular simulations were used to help clarify the CT processes and understand the electrical switching behaviors. These results suggest that the memory behaviors of the PI-based devices can be significantly affected and adjusted by the nucleophilicity of the affiliated pendent groups of the PI chain structure. The newly synthesized polyimides herein promise the potential applications for the future alternative memory technologies.Experimental
Materials
Trifluoroacetic acid, p-toluidine, triphenylmethyl chloride, 2-bromo-1-(4-nitrophenyl)ethan-1-one, 4-(trifluoro-ethyl)niline, and hydroxymethanesulfinic acid monosodium salt dihydrate (rongalite) were purchased from Aladdin and used as received. 4,4′-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA) were purchased from National Pharmaceutical Group Chemical Reagent Co., Ltd. and heated at 150 °C under vacuum for 12 h before use. Tetrabutylammonium perchlorate (TBAP) and ferrocene was obtained from Alfa Aesar and used as received. N,N-Dimethylformamide (DMF) purchased from Aladdin was dried over calcium hydride for 12 h and distilled under reduced pressure. All other reagents were analytical grade and used as received from commercial sources, unless otherwise mentioned.Instrumentation
1H NMR spectra of intermediates and monomers were recorded on a Varian Mercury-plus 300 spectrometer, while 1H NMR spectra of polymers and all 13C NMR spectra were recorded on a Varian Unity Inova 500 NB spectrometer. All samples were measured in a solution of deuterated dimethyl sulfoxide (DMSO-d6) with tetramethylsilane (TMS) as an internal standard. Mass spectra were performed on a Thermo EI mass spectrometer (DSQ II). Elemental analysis was run in a CHNS elemental analyzer. Infrared spectra (IR) were analyzed by a BRUKER TENSOR 27 Fourier-transform infrared (FT-IR) spectrometer. The inherent viscosities (ηinh) of polyimides were obtained at a solid content of 0.5 wt% in N-methyl-2-pyrrolidone (NMP) at 30 °C on an Ostwald viscometer. Wide-angle X-ray diffraction (WAXD) measurements were performed on Rigaku SmartLab X-ray diffractometer at a scanning rate of 10° min−1. Ultraviolet-visible (UV-vis) absorption spectra were obtained on a Hitachi UV-Vis spectrophotometer (U-3900). For the thin film spectra, PIs were dissolved in NMP and filtered through membrane microfilters with a pore size of 0.22 μm, spin-coated at a speed rate of 1500 to 2000 rpm for 10 s onto quartz plates and baked at 150 °C for 3 h under vacuum. The PI solution spectra were measured at a concentration of approximate 2 × 10−5 M before the absorbance was normalized. Thermogravimetric analyses (TGA) were carried out on a TA thermal analyzer (Q50) under a nitrogen atmosphere at a heating rate of 20 °C min−1. Differential scanning calorimetry (DSC) curves were obtained with a NETZSCH thermal analyzer (DSC 204) at a heating rate of 10 °C min−1 under a nitrogen flow. The dynamic mechanical (DMA) spectra were determined by a TA DMA 2980 analyzer in tensile mode at an amplitude of 20 μm, a preload force of 0.01 N and a force track of 125% at a heating rate of 5 °C min−1. Thermomechanical analysis (TMA) was conducted with a TA TMA Q400 analyzer at a preload force of 0.05 N and a heating rate of 10 °C min−1. Cyclic voltammetry (CV) measurements were performed on a Bio-Logic VMP-300 multi-channel electrochemical workstation using a three-electrode cell in 0.1 M solution of TBAP in acetonitrile (CH3CN). The PI thin film spin-coated onto an ITO glass substrate was used as a working electrode and a platinum disk was used as an auxiliary electrode. All cell potentials were taken with the use of a Ag/AgCl, KCl (sat.) reference electrode. A solution of ferrocene (5 mM) was used as an external reference for calibration. All scans were run at a rate of 50 mV s−1. Atomic force micrographs (AFM) of the PI thin films on the device surface were obtained by a Bruker Multimode 8 microscope. The thickness of the vacuum evaporated Al electrodes and the spin-coated PI thin films was measured with a Kosaka Laboratory Ltd. Surfcorder ET150 step profiler.Synthesis of the monomers and polyimides
The general synthesis routes of the diamines and the corresponding polyimides are outlined in Schemes 1 and 2, respectively. The intermediate 1,4-bis(4-nitrophenyl)butane-1,4-dione (2) was synthesized by the coupling reaction of 2-bromo-1-(4-nitrophenyl)ethan-1-one (1) with rongalite in DMF according to a previously reported procedure.12 Another intermediate 4-tritylaniline was obtained by a one-step facile reaction between aniline and triphenylmethyl chloride according to the ref. 13.Synthesis of 2,5-bis(4-nitrophenyl)-1-p-tolyl-1H-pyrrole (PyCH3DN)
Monoamine p-toluidine (2.79 g, 26 mmol) was added to a suspension of compound 2 (6.56 g, 20 mmol) in methanol (80 mL) and acetic acid (80 mL). The mixture was stirred at 90 °C for 18 h under argon. After cooling to room temperature, the resulting precipitate was filtered and washed by ethanol. The crude product was subsequently purified by silica-gel column chromatography using ethyl acetate/n-hexane (v/v = 1/4) as an eluent to afforded PyCH3DN as a light brown powder (6.60 g, 82.6%). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 8.06–8.03 (d, 4H), 7.29–7.26 (d, 4H), 7.23–7.20 (d, 2H), 7.10–7.07 (d, 2H), 6.82 (s, 2H), 2.34 (s, 3H). MS (EI, m/z): [M]+ calcd for C23H17N3O4: 399.12. Found: 399. IR (KBr, ν, cm−1): 1587 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, stretching), 1520 (NO2, νas), 1333 (NO2, νs).
N, stretching), 1520 (NO2, νas), 1333 (NO2, νs).
Synthesis of 2,5-bis(4-nitrophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrrole (PyCF3DN)
Monoamine 4-(trifluoromethyl)aniline (4.19 g, 26 mmol) was added to a suspension of compound 2 (6.56 g, 20 mmol) in toluene (120 mL) and trifluoroacetic acid (5.70 g, 50 mmol). The mixture was stirred at 110 °C for 24 h under argon. After cooling to room temperature, the resulting precipitate was filtered and washed by ethanol. The crude product was subsequently purified by silica-gel column chromatography using ethyl dichloromethane/n-hexane (v/v = 1/2) as an eluent to afforded PyCF3DN as a yellow powder (7.24 g, 79.9%). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 8.10–8.07 (d, 4H), 7.79–7.76 (d, 2H), 7.43–7.40 (d, 2H), 7.29–7.23 (d, 4H), 6.85 (s, 2H). MS (EI, m/z): [M]+ calcd for C23H14F3N3O4: 453.09. Found: 453. IR (KBr, ν, cm−1): 1589 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, stretching), 1522 (NO2, νas), 1331 (NO2, νs), 1167 (C–F, νs).
N, stretching), 1522 (NO2, νas), 1331 (NO2, νs), 1167 (C–F, νs).
Synthesis of 2,5-bis(4-nitrophenyl)-1-(4-tritylphenyl)-1H-pyrrole (PyCPh3DN)
PyCPh3DN was synthesized by using 4-tritylaniline as monoamine in the similar procedure as PyCF3DN to give a yellowish orange solid (9.95 g, 79.3%). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 8.05–8.02 (d, 4H), 7.34–7.25 (m, 10H), 7.22–7.08 (m, 13H), 6.86 (s, 2H). MS (EI, m/z): [M]+ calcd for C41H29N3O4: 627.22. Found: 627. IR (KBr, ν, cm−1): 1589 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, stretching), 1514 (NO2, νas), 1333 (NO2, νs).
N, stretching), 1514 (NO2, νas), 1333 (NO2, νs).
4,4′-(1-p-Tolyl-1H-pyrrole-2,5-diyl)dianiline (PyCH3DA)
A yellow powder (2.78 g, 82.0%). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 7.06–7.04 (d, 2H), 6.85–6.83 (d, 2H), 6.67–6.64 (d, 4H), 6.34–6.31 (d, 4H), 6.10 (s, 2H), 4.96 (s, 4H, NH2), 2.27 (s, 3H). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 21.10, 107.84, 113.83, 121.42, 129.31, 129.52, 129.59, 135.78, 136.63, 137.24, 147.42. MS (EI, m/z): [M]+ calcd for C23H21N3: 339.17. Found: 339. IR (KBr, ν, cm−1): 3464, 3378 (N–H, ν), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, stretching), 1283 (C–N, stretching). Anal. calcd for C23H21N3: C, 81.38; H, 6.24; N, 12.38. Found: C, 80.94; H, 6.19; N, 12.30.
N, stretching), 1283 (C–N, stretching). Anal. calcd for C23H21N3: C, 81.38; H, 6.24; N, 12.38. Found: C, 80.94; H, 6.19; N, 12.30.
4,4′-(1-(4-(Trifluoromethyl)phenyl)-1H-pyrrole-2,5-diyl)dianiline (PyCF3DA)
A light yellow crystalline solid (3.26 g, 75.3%). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 7.64–7.61 (d, 2H), 7.15–7.12 (d, 2H), 6.65–6.62 (d, 4H), 6.36–6.34 (d, 4H), 5.04 (s, 4H, NH2). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 108.72, 113.90, 120.68, 126.08, 127.44, 127.69, 129.86, 130.25, 135.72, 143.32, 147.80. MS (EI, m/z): [M]+ calcd for C23H18F3N3: 393.15. Found: 393. IR (KBr, ν, cm−1): 3412, 3314 (N–H, v), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, stretching), 1333 (C–F, νas), 1178 (C–F, νs). Anal. calcd for C23H18F3N3: C, 70.22; H, 4.61; N, 10.68. Found: C, 70.41; H, 4.58; N, 10.61.
N, stretching), 1333 (C–F, νas), 1178 (C–F, νs). Anal. calcd for C23H18F3N3: C, 70.22; H, 4.61; N, 10.68. Found: C, 70.41; H, 4.58; N, 10.61.
4,4′-(1-(4-Tritylphenyl)-1H-pyrrole-2,5-diyl)dianiline (PyCPh3DA)
A light yellow crystalline solid (4.98 g, 87.7%). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 7.34–7.19 (m, 9H), 7.05–6.95 (m, 10H), 6.65–6.62 (d, 4H), 6.36–6.33 (d, 4H), 6.14 (s, 2H), 5.05 (s, 4H, NH2). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 64.61, 107.67, 113.72, 121.10, 126.73, 128.21, 129.00, 129.39, 131.05, 131.41, 135.53, 137.96, 145.77, 146.48, 147.51. MS (EI, m/z): [M]+ calcd for C41H33N3: 567.27. Found: 567. IR (KBr, ν, cm−1): 3454, 3379 (N–H, v), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, stretching), 1290 (C–N, stretching). Anal. calcd for C41H33N3: C, 86.74; H, 5.86; N, 7.40. Found: C, 86.43; H, 5.86; N, 7.34.
N, stretching), 1290 (C–N, stretching). Anal. calcd for C41H33N3: C, 86.74; H, 5.86; N, 7.40. Found: C, 86.43; H, 5.86; N, 7.34.
Preparation of polyimides (Py6FPIs)
The synthesis of polyimide PyCH36FPI was used as an example to illustrate the general synthetic procedure. To a solution of PyCH3DA (0.3249 g, 0.9570 mmol) in 5 mL freshly distilled anhydrous DMF, dianhydride 6FDA (0.4251 g, 0.9570 mmol) was added in one portion. The mixture was stirred at room temperature under argon for 8 h to afford a viscous poly(amic acid) solution. The poly(amic acid) was subsequently coated on a clean dry glass plate, followed by thermal imidization in a vacuum oven at 80 °C/1 h, 150 °C/1 h, 230 °C/1 h and 300 °C/1 h. After cooling to room temperature, the plate was soaked in warm water for a few minutes and the polyimide film (approximate 40 μm) was peeled off automatically.PyCH36FPI
1H NMR (500 MHz, DMSO-d6, δ, ppm): 8.23–8.08 (d, 2H), 7.96–7.84 (d, 2H), 7.76–7.65 (s, 1H), 7.51–6.84 (m, 12H), 6.69–6.42 (s, 2H), 2.37–2.17 (s, 3H). FT-IR (ν, cm−1): 1782 (imide, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, asymmetrical stretching), 1717 (imide, C
O, asymmetrical stretching), 1717 (imide, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, symmetrical stretching) and 1366 (C–N, stretching). Inherent viscosity, ηinh = 1.19 dL g−1.
O, symmetrical stretching) and 1366 (C–N, stretching). Inherent viscosity, ηinh = 1.19 dL g−1.
PyCF36FPI
1H NMR (500 MHz, DMSO-d6, δ, ppm): 8.18–8.10 (d, 2H), 7.96–7.88 (d, 2H), 7.80–7.74 (d, 2H), 7.73–7.68 (s, 2H), 7.48–7.41 (d, 2H), 7.35–7.28 (d, 4H), 7.24–7.11 (d, 4H), 6.69–6.57 (s, 2H). FT-IR (ν, cm−1): 1784 (imide, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, asymmetrical stretching), 1721 (imide, C
O, asymmetrical stretching), 1721 (imide, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, symmetrical stretching) and 1364 (C–N, stretching). Inherent viscosity, ηinh = 0.94 dL g−1.
O, symmetrical stretching) and 1364 (C–N, stretching). Inherent viscosity, ηinh = 0.94 dL g−1.
PyCPh36FPI
1H NMR (500 MHz, DMSO-d6, δ, ppm): 8.24–8.15 (d, 2H), 8.00–7.92 (d, 2H), 7.79–7.71 (s, 2H), 7.54–6.86 (m, 27H), 6.68–6.54 (s, 2H). FT-IR (ν, cm−1): 1784 (imide, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, asymmetrical stretching), 1717 (imide, C
O, asymmetrical stretching), 1717 (imide, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, symmetrical stretching) and 1366 (C–N, stretching). Inherent viscosity, ηinh = 1.06 dL g−1.
O, symmetrical stretching) and 1366 (C–N, stretching). Inherent viscosity, ηinh = 1.06 dL g−1.
Fabrication and characterization of the memory devices
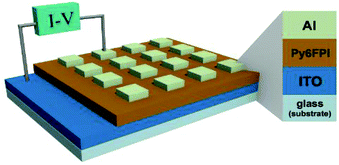
The figuration of memory device fabricated with Py6FPI as active layer is shown in Fig. 12. A glass substrate deposited with ITO was precleaned by ultra-sonication with water, acetone, and isopropanol each for 20 min. Homogenous Py6FPI solutions were prepared in NMP and filtered via membrane microfilters with a pore size of 0.22 μm. The polymer films were prepared by spin-coated the solution onto the precleaned ITO glass substrate at a speed rate of 1500 rpm for 12 s and baked at 150 °C for 3 h under vacuum. The thicknesses of the smooth PI thin films were in the range of 65–70 nm with a root-mean-square roughness (Rq) about 1.5 nm over an area of 1.0 × 1.0 μm2. Finally, an aluminium top electrode with a thickness of 110 nm was deposited onto the polyimide film surface through a shadow mask by thermal evaporation un-der vacuum. The current–voltage (I–V) measurements of the memory device was performed by a Keithley 4200-SCS semiconductor parameter analyzer equipped with a Keithley 4205-PG2 arbitrary waveform pulse generator.Molecular simulation
Molecular simulation was carried out with the Gaussian 09 program package. The molecular geometry, molecular orbitals and dipole moment of the basic unit (BU) in the polyimide molecular structure were calculated and optimized by means of the density functional theory (DFT), using the Becke's three-parameter hybrid density functional method in conjunction with Lee–Yang–Parr's correction functional (B3LYP) method, and the 6-31G(d) basic set. The time-dependent DFT (TD-DFT) was additionally applied in the calculation of the oscillator strengths and transition contributions. For all the simulations, vibration frequencies were calculated analytically to ensure the minimum total energy of the optimized molecular geometry.Acknowledgements
The financial support by the National 973 Program of China (No. 2014CB643605), the National 863 Program of China (No. 2015AA033408), the National Natural Science Foundation of China (No. 51373204, 51233008, and J1103305), the Science and Technology Project of Guangdong Province (No. 2015B090915003 and 2015B090913003), the Doctoral Fund of the Ministry of Education of China (No. 20120171130001), and the Fundamental Research Funds for the Central Universities (No. 161gzd08) are gratefully acknowledged.Notes and references
- (a) Q. D. Ling, D. J. Liaw, C. Zhu, D. S. H. Chan, E. T. Kang and K. G. Neoh, Prog. Polym. Sci., 2008, 33, 917–978 CrossRef CAS; (b) P. Heremans, G. H. Gelinck, R. Müller, K. J. Baeg, D. Y. Kim and Y. Y. Noh, Chem. Mater., 2011, 23, 341–358 CrossRef CAS; (c) Q. D. Ling, D. J. Liaw, E. Y. H. Teo, C. Zhu, S. H. Chan, E. T. Kang and K. G. Neoh, Polymer, 2007, 48, 5182–5201 CrossRef CAS; (d) Y. Yang, J. Ouyang, L. Ma, R. J. H. Tseng and C. W. Chu, Adv. Funct. Mater., 2006, 16, 1001–1014 CrossRef CAS.
- (a) K. S. Yook, J. Y. Lee, S. H. Kim and J. Jang, Appl. Phys. Lett., 2008, 92, 223305 CrossRef; (b) Y. S. Lai, C. H. Tu, D. L. Kwong and J. S. Chen, Appl. Phys. Lett., 2005, 87, 122101 CrossRef; (c) Q. D. Ling, W. Wang, Y. Song, C. X. Zhu, D. S. H. Chan, E. T. Kang and K. G. Neoh, J. Phys. Chem. B, 2006, 110, 23995–24001 CrossRef CAS PubMed; (d) E. Y. H. Teo, Q. D. Ling, Y. Song, Y. P. Tan, W. Wang, E. T. Kang, D. S. H. Chan and C. Zhu, Org. Electron., 2006, 7, 173–180 CrossRef CAS.
- (a) J. Lee and O. Kim, Jpn. J. Appl. Phys., 2011, 50, 06GF01 CrossRef; (b) S. Möller, C. Perlov, W. Jackson, C. Taussig and S. R. Forrest, Nature, 2003, 426, 166–169 CrossRef PubMed; (c) U. S. Bhansali, M. A. Khan, D. K. Cha, M. N. AlMadhoun, R. P. Li, L. Chen, A. Amassian, I. N. Odeh and H. N. Alshareef, ACS Nano, 2013, 7, 10518–10524 CrossRef CAS PubMed.
- Q. D. Ling, E. T. Kang, K. G. Neoh, Y. Chen, X. D. Zhuang, C. Zhu and D. S. H. Chan, Appl. Phys. Lett., 2008, 92, 143302 CrossRef.
- (a) G. F. Tian, D. Z. Wu, L. Shi, S. L. Qi and Z. P. Wu, RSC Adv., 2012, 2, 9846–9850 RSC; (b) Q. Lai, Z. Zhu, Y. Chen, S. Patil and F. Wudl, Appl. Phys. Lett., 2006, 88, 133515 CrossRef; (c) R. J. Tseng, J. Huang, J. Ouyang, R. Kaner and B. Yang, Nano Lett., 2005, 5, 1077–1080 CrossRef CAS PubMed; (d) B. Koo, H. Beak and J. Cho, Chem. Mater., 2012, 24, 1091–1099 CrossRef CAS; (e) B. W. Zhu, H. Wang, Y. Q. Liu, D. P. Qi, Z. Y. Liu, H. Wang, J. C. Yu, M. Sherburne, Z. H. Wang and X. D. Chen, Adv. Mater., 2016, 28, 1559–1566 CrossRef CAS PubMed; (f) A. Van Breemen, T. Zaba, V. Khikhlovskyi, J. Michels, R. Janssen, M. Kemerink and G. Gelinck, Adv. Funct. Mater., 2015, 25, 278–286 CrossRef CAS.
- (a) S. L. Qi, H. Iida, L. L. Liu, S. Irle, W. P. Hu and E. Yashima, Angew. Chem., Int. Ed., 2013, 52, 1049–1053 CrossRef CAS PubMed; (b) S. K. Hwang, J. M. Lee, S. Kim, J. S. Park, H. I. Park, C. W. Ahn, K. J. Lee, T. Lee and S. O. Kim, Nano Lett., 2012, 12, 2217–2221 CrossRef CAS PubMed; (c) G. Liu, Q. D. Ling, E. Y. H. Teo, C. X. Zhu, D. S. H. Chan, K. G. Neoh and E. T. Kang, ACS Nano, 2009, 3, 1929–1937 CrossRef CAS PubMed; (d) D. I. Son, T. W. Kim, J. H. Shim, J. H. Jung, D. U. Lee, J. M. Lee, W. I. Park and W. K. Choi, Nano Lett., 2010, 10, 2441–2447 CrossRef CAS PubMed; (e) X. D. Zhuang, Y. Chen, G. Liu, P. P. Li, C. X. Zhu, E. T. Kang, K. G. Noeh, B. Zhang, J. H. Zhu and Y. X. Li, Adv. Mater., 2010, 22, 1731–1735 CrossRef CAS PubMed; (f) T. Mosciatti, S. Bonacchi, M. Gobbi, L. Ferlauto, F. Liscio, L. Giorgini, E. Orgiu and P. Samorì, ACS Appl. Mater. Interfaces, 2016, 8, 6563–6569 CrossRef CAS PubMed.
- (a) D. J. Liaw, K. L. Wang, Y. C. Huang, K. R. Lee, J. Y. Lai and C. S. Ha, Prog. Polym. Sci., 2012, 37, 907–974 CrossRef CAS; (b) Y. W. Liu, C. Qian, L. J. Qu, Y. N. Wu, Y. Zhang, X. H. Wu, B. Zou, W. X. Chen, Z. Q. Chen, Z. G. Chi, S. W. Liu, X. D. Chen and J. R. Xu, Chem. Mater., 2015, 27, 6543–6549 CrossRef CAS.
- (a) C. J. Chen, H. J. Yen, W. C. Chen and G. S. Liou, J. Mater. Chem., 2012, 22, 14085–14093 RSC; (b) D. M. Kim, Y. G. Ko, J. K. Choi, K. Kim, W. Kwon, J. Jung, T. H. Yoon and M. Ree, Polymer, 2012, 53, 1703–1710 CrossRef CAS; (c) C. J. Chen, H. J. Yen, W. C. Chen and G. S. Liou, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3709–3718 CrossRef CAS; (d) C. L. Liu, T. Kurosawa, A. D. Yu, T. Higashihara, M. Ueda and W. C. Chen, J. Phys. Chem. C, 2011, 115, 5930–5939 CrossRef CAS; (e) T. Kuorosawa, C. C. Chueh, C. L. Liu, T. Higashihara, M. Ueda and W. C. Chen, Macromolecules, 2010, 43, 1236–1244 CrossRef CAS; (f) Y. L. Liu, Q. D. Ling, E. T. Kang, K. G. Neoh, D. J. Liaw, W. T. Liou, C. X. Zhu and D. S. H. Chan, J. Appl. Phys., 2009, 105, 044501 CrossRef; (g) Q. D. Ling, F. C. Chang, Y. Song, C. X. Zhu, D. J. Liaw, D. S. H. Chan, E. T. Kang and K. G. Neoh, J. Am. Chem. Soc., 2006, 128, 8732–8733 CrossRef CAS PubMed.
- (a) L. Shi, G. F. Tian, H. B. Ye, S. L. Qi and D. Z. Wu, Polymer, 2014, 55, 1150–1159 CrossRef CAS; (b) Y. L. Liu, K. L. Wang, G. S. Huang, C. X. Zhu, E. S. Tok, K. G. Neoh and E. T. Kang, Chem. Mater., 2009, 21, 3391–3399 CrossRef CAS; (c) H. J. Yen, C. J. Chen and G. S. Liou, Adv. Funct. Mater., 2013, 23, 5307–5316 CrossRef CAS; (d) Y. W. Liu, Y. Zhang, Q. Lan, S. W. Liu, Z. X. Qin, L. H. Chen, C. Y. Zhao, Z. G. Chi, J. R. Xu and J. Economy, Chem. Mater., 2012, 24, 1212–1222 CrossRef CAS; (e) Y. W. Liu, Y. Zhang, Q. Lan, Z. X. Qin, S. W. Liu, C. Y. Zhao, Z. G. Chi and J. R. Xu, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1302–1314 CrossRef CAS; (f) C. J. Chen, C. L. Tsai and G. S. Liou, J. Mater. Chem. C, 2014, 2, 4374–4378 RSC.
- N. H. You, C. C. Chueh, C. L. Liu, M. Ueda and W. C. Chen, Macromolecules, 2009, 42, 4456–4463 CrossRef CAS.
- (a) K. L. Wang, Y. L. Liu, I. H. Shin, K. G. Neoh and E. T. Kang, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5790–5800 CrossRef CAS; (b) K. L. Wang, Y. L. Liu, J. W. Lee, K. G. Neoh and E. T. Kang, Macromolecules, 2010, 43, 7159–7164 CrossRef CAS; (c) D. M. Kim, S. Park, T. J. Lee, S. G. Hahm, K. Kim, J. C. Kim, W. Kwon and M. Ree, Langmuir, 2009, 25, 11713–11719 CrossRef CAS PubMed; (d) J. H. Wu and G. S. Liou, ACS Appl. Mater. Interfaces, 2015, 7, 15988–15994 CrossRef CAS PubMed; (e) L. Shi, H. B. Ye, W. L. Liu, G. F. Tian, S. L. Qi and D. Z. Wu, J. Mater. Chem. C, 2013, 1, 7387–7399 RSC.
- E. K. Kemppainen, G. Sahoo, A. Valkonen and P. M. Pihko, Org. Lett., 2012, 14, 1086–1089 CrossRef CAS PubMed.
- D. K. Zhang, Y. G. Wen, Y. Xiao, G. Yu, Y. L. Liu and X. H. Qian, Chem. Commun., 2008, 39, 4777–4779 RSC.
- (a) A. J. Campbell, D. D. C. Bradley and D. G. Lidzey, J. Appl. Phys., 1997, 82, 6326–6342 CrossRef CAS; (b) S. Menzel, U. Bottger, M. Wimmer and M. Salinga, Adv. Funct. Mater., 2015, 25, 6306–6325 CrossRef CAS.
- S. M. Sze, Physics of Semiconductor Devices, Wiley, New York, 2nd edn, 1981 Search PubMed.
- M. Pope and C. E. Swenberg, Electronic Process in Organic Crystals and Polymers, Oxford University Press, New York, 2nd edn, 1999 Search PubMed.
- J. P. LaFemina, Chem. Phys. Lett., 1989, 159, 307–309 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR results of all the intermediate products; FTIR spectra, 1H NMR, WAXD patterns, thermal properties of the Py6FPI films; UV-vis absorption spectra of the diamines; molecular simulation results in details; experimental and fitted I–V curves for PI-devices. See DOI: 10.1039/c6ra11615a |
| This journal is © The Royal Society of Chemistry 2016 |