Study of mesoporous magnesium carbonate in contact with whole human blood†
Abstract
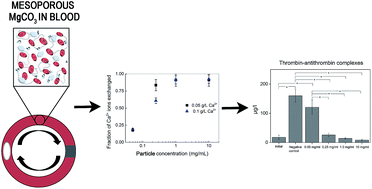
The interaction of mesoporours magnesium carbonate (Upsalite) particles (50–100 μm) with human whole blood was investigated using an in vitro loop model and the effect on the complement system, blood coagulation and red blood cell lysis was assessed. The removal of Ca2+ by Upsalite and the possible exchange with and/or release of Mg2+ were explored as well. Upsalite was found to present anticoagulant properties, most probably due to the uptake of Ca2+ by the particles. No hemolytic activity was detected at Upsalite concentrations up to 1 mg ml−1. Moderate to high levels of C3a and sC5b-9 were observed for Upsalite, however such levels were statistically different from the negative control only when the particle concentrations were 0.25 mg ml−1 and 1.0 mg ml−1, respectively. The presented findings are promising for the future development of mesoporous magnesium carbonate-based materials for biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...