Comparative evaluation of enzyme-free nanoclay-ionic liquid based electrodes for detection of bioanalytes†
Nidhi Joshia,
Abhimanyu Sharmabc,
Kamla Rawat *bc,
K. Asokanc,
P. R. Solankib,
G. B. V. S. Lakshmic,
D. Kanjilalc and
H. B. Bohidar*ab
*bc,
K. Asokanc,
P. R. Solankib,
G. B. V. S. Lakshmic,
D. Kanjilalc and
H. B. Bohidar*ab
aPolymer and Biophysics Laboratory, School of Physical Sciences, Jawaharlal Nehru University, New Delhi 110067, India. E-mail: bohi0700@mail.jnu.ac.in; Fax: +91 11 2674 1837; Tel: +91 11 2670 4699
bSpecial Center for Nanosciences, Jawaharlal Nehru University, New Delhi 110067, India. E-mail: kamla.jnu@gmail.com
cInter University Accelerator Centre, New Delhi 110067, India
First published on 5th July 2016
Abstract
Enzyme-free electrodes were fabricated using nanoclay LAPONITE® and montmorillonite (MMT) and imidazolium based ionic liquids (ILs), 1-ethyl-3-methyl imidazolium chloride ([C2mim][Cl]) and 1-methyl-3-octyl imidazolium chloride ([C8mim][Cl]). Here, we have presented a set of quantitative results which conclude that these enzyme-free electrodes can be used successfully for the development of strip-sensors for detection of bioanalytes. Introduction of ILs into nanoclay dispersions resulted in the enhancement of stability of the thin film electrodes formed of the NC-IL materials. The fabricated electrodes were used for sensing various bioanalytes, such as ascorbic acid (AA), oxalic acid (OA), urea (U), citric acid (CA), glucose (Glu) and cholesterol (Chox). The influence of different alkyl chain lengths of ionic liquids, and the aspect ratio of nanoclay platelets were studied with respect to their electrochemical response to different analytes, which covered seven distinct matrices coated on the electrode surface. Further, the effect of oxygen ion beam irradiation on the electrochemical profiles of these electrodes was explored. The irradiation leads to reduction of the electrochemical properties by blocking certain active charge transfer sites. The electrochemical characterization of these electrodes was done using cyclic voltammetry (CV), Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM).
1. Introduction
Nanoclays are found to be promising materials which have a wide range of applications in different areas such as drug delivery, catalysis, catalysis supports, and water remediation.1–3 These are layered clay minerals which are known to be the main constituent of polymer composites, paints, rubbers, and depolluting agents extensively used in cosmetics, pharmaceutical formulations, petroleum products, coating materials etc. to name a few. LAPONITE® (L) and montmorillonite (MMT) are synthetic and naturally occurring clay minerals which are composed of platelets whose primary particles are discoids with anisotropic surface charge distribution. It consists of negative charge at its surface while positive charge on its rim. The disc diameter is of ∼30 nm and ∼300 nm, and thickness is of 1 nm each, for LAPONITE® (L) and montmorillonite (MMT) platelets, respectively.4,5 These nanoclays have layered structure that comprise of octahedral magnesium sheet sandwiched between two tetrahedral silica sheets.LAPONITE®, a synthetic hectorite is composed of clay platelets with monovalent cation exchange capacity of 0.74 mmol g−1. LAPONITE® when mixed with ILs ([C2mim][Cl] and [C8mim][Cl]) forms a characteristic arrested state after a certain time due to the electrostatic interaction between positively charged head-group of the IL with the negative surface charge of the LAPONITE®. Similar is the case for MMT with ILs, through the arrest dynamics is less profound. On the other hand, LAPONITE® when mixed with MMT also forms arrested phases due to the competitive interaction of positive rim with negative surface of other nanoclay resulting in house of cards formation which is a well-known mechanism for nanoclay gelation. Van Olphen proposed that the clay dispersions may form three dimensional aggregated structures which are called “house of cards” and the structure was believed to arise from electrostatic attraction between oppositely charged double layers at the edges and faces of the particles as a results of the different chemical composition of the face and edge surfaces.5 Further, the details about the gelation mechanism for these systems can be found elsewhere.6,7
The nanoclay dispersion exhibits a rich phase diagram consisting of equilibrium gel, glass and sol depending on the concentration and aging time.8–12 The advantage of using nanoclay in electrochemical sensing is due to their high surface area, thermal and mechanical stability, biocompatibility, ion exchange properties, and low cost. Due to their adsorption properties it has found wide applications in different areas, such as electrocatalysis, symmetric electro-oxidation of organic molecules and photoelectron-catalysis.13–16 LAPONITE® is found to be attractive matrix for enzyme immobilization due to the rich phase behavior such as hydrophilicity and excellent cation exchange capacity.17–19
Ionic liquid is one of the fascinating classes of compounds which are vastly used for the development of electrochemical sensor due to its high conductivity. Ionic liquids consist of large organic cations, and inorganic or organic anions with properties of good chemical and thermal stability, low volatility, conductivity, miscibility etc.20 Addition of ILs leads to the availability of nitrogen containing cations i.e. imidazolium as well as inorganic or organic anions. The two important electrochemical properties which make them novel for use in electrochemical applications are good conductivity, and large potential window.20–24 G. A. Baker et al. have found that the ionic liquids act as agents to stabilize proteins at elevated temperatures.25 Also there are some ILs which were found to destroy/damage the enzymes, thus the appropriate choice of IL is of paramount concern. Sharma et al. have used Agar-IL electrodes for the detection of glucose via glucose oxidase.26 There is a huge range of data in the literature pertaining to the electrochemical properties of ILs.
The analytes studied here are ascorbic acid (AA), citric acid (CA), urea (U), cholesterol (Chox), glucose (Glu) and oxalic acid (OA), are found in human body fluids. Their deficiency in the human body lead to the different types of disorders and thus, the detection of these analytes is of considerable importance in clinical diagnostic and point of care treatment.
Here, the aim of our work was to provide a comparative study for the electrochemical properties of different NC-IL based electrodes for sensing of different analytes. The influence of oxygen ion beam irradiation to these electrodes was also studied which showed the degradation of these electrodes as far as their sensing ability was concerned, an investigation not reported so far.
2. Materials and methods
2.1. Material
LAPONITE® RD nanoclay and Cloisite-Na (MMT) were purchased from Southern Clay Products, U.S. and were used as received. The ILs, 1-ethyl-3-methyl imidazolium chloride ([C2mim][Cl]) and 1-methyl-3-octylimidazolium chloride ([C8mim][Cl]) and the analytes (cholesterol, ascorbic acid, oxalic acid, urea, citric acid and glucose) were purchased from Sigma-Aldrich, USA. Deionized water procured from Organo Biotech Laboratories, India, was used to prepare the solutions. The stock solution of the analytes was prepared by dissolving them into deionized water while the stock solution of cholesterol was prepared by dissolving it into ethanol. Indium tin oxide (ITO)-coated glass plates were obtained from Balzers, UK, (Baltracom 247 ITO, 1.1 mm thick) which has a sheet resistance and transmittance of 25 Ω sq−1 and 90% respectively. All of the experiments were performed at room temperature (20 °C). All concentrations are in % (w/v) unless otherwise stated.2.2. Preparation of nanoclay (NC) based electrodes
The required concentration of clay dispersions were prepared separately by dissolving MMT and LAPONITE® in deionized water at room temperature under constant stirring for 12 h and 2 h, respectively. However, for L-MMT electrode the individual dispersions were first prepared, and then mixed in the mixing ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and stirred vigorously for half an hour to make it homogeneous. All dispersions appeared optically clear and homogeneous to the naked eye.
1 and stirred vigorously for half an hour to make it homogeneous. All dispersions appeared optically clear and homogeneous to the naked eye.
2.3. Preparation of NC-IL based electrodes
After the preparation of LAPONITE® and MMT dispersions, the required volumes of IL ([C2mim][Cl] or [C8mim][Cl]) from the stock solutions were introduced in to the prepared nanoclay dispersion, and stirred for 15 min. A homogeneous dispersion was obtained in the case of all samples except for L-[C8mim]. In the case of L-[C8mim] dispersion, two distinct phases were immediately formed. However, only lower phase was only used that easily formed a film when deposited on electrode surface. The upper phase was glass which fails the dilution test and thus not used for sensing.After the preparation of these dispersions, typically 60 μl of the dispersion was drop-casted onto ITO plate, and kept for drying upto 24 h to form a thin films gel, and these were stored in desiccators at room temperature for further studies.
2.4. Characterization methods
Electrochemical studies (Cyclic Voltammetry (CV)) were conducted using Autolab Potentiostat/Galvanostat (Eco Chemie, Netherlands) with a three-electrode cell where one is a working electrode, platinum wire was the auxiliary electrode, and Ag/AgCl was the reference electrode in a 3.3 mM Zobell's solution {3.3 mM K4Fe(CN)6 (potassium ferrocyanide), 3.3 mM K3Fe(CN)6 (potassium ferricyanide) containing 0.1 M KCl (potassium chloride)}. Fourier transform infrared (FTIR) spectroscopic studies were carried out using PerkinElmer, Spectrum BX II instrument. Scanning electron microscopy (SEM) was done using LVO 40 Zeiss instrument.3. Results and discussion
The nanoclay based thin films (L, MMT, L-MMT, L-[C2mim], L-[C8mim], MMT-[C2mim] and MMT-[C8mim]) were successfully fabricated on indium tin oxide (ITO) substrate and their electrochemical studies for different analytes (Chox, AA, OA, Glu, U and CA) were probed using various techniques. The electrochemical studies of L-MMT and L-[C2mim] electrodes towards different analytes was reported earlier where L-MMT showed maximum detection towards cholesterol (Chox) while L-[C2mim] exhibited the same towards oxalic acid (OA).27,28 The comparative electrochemical studies for other matrices have been undertaken here.Initially the parameters such as concentration, pH of the electrolyte support, frequency, scan rate etc. were investigated in order to obtain the best experimental working conditions. To determine the influence of the pH of the electrolyte support, a range of pH from 4 to 10 was studied where the highest current value were obtained for pH = 7 in the ferri/ferro cyanide solution as shown in Fig. S1 (ESI†). The scan rate was fixed at 50 mV s−1, and pH of ferri/ferro solution was fixed at 7. Ferri/ferro cyanide electrolyte was used to study the electron transfer kinetics of the matrix. The cyclic voltammogram of all the electrodes were recorded in the ferri/ferro solution (Zobell's solution) in the potential range of −0.1 to +0.4 V (Fig. 1). A well-defined pair of redox peaks, one corresponding to oxidation (Ea), and other to reduction (Ec) was observed for all the cases in the cyclic voltammogram. The first step was to optimize the concentration of different matrices by measuring their peak anodic current (Ia). The maximum value of anodic current gave the optimized concentration which was then used for electrochemical sensing of analytes. Fig. 1 shows a representative graph for optimizing the concentration of the LAPONITE® electrode where the maximum current value is obtained for concentration of 1.5% and similar concentration optimization was done for all other cases. The optimized concentrations for all the matrices are listed in Table 1. Further, electrochemical and other studies were done using the optimized concentration of the matrices.
 | ||
| Fig. 1 Cyclic voltammetric response of L-ITO electrode shown for different concentration of LAPONITE®. | ||
| S. No | Electrode matrix | copt (% w/v) | Specific analyte | (Ks) s−1 | (I*) × 108 mol cm−2 | (D) × 10−12 cm2 s−1 | Sensitivity μA mM−1 cm−2 |
|---|---|---|---|---|---|---|---|
| 1 | L | 1.50 | CA | 0.04 | 0.09 | 0.05 | 9.71 |
| 2 | MMT | 3.00 | OA | 0.01 | 0.08 | 0.03 | 10.1 |
| 3 | L-[C2mim] | 0.04 | OA | 0.08 | 0.23 | 0.13 | 8.01 |
| 4 | L-[C8mim] | 0.01 | AA | 0.31 | 0.09 | 0.04 | 3.26 |
| 5 | MMT-[C2mim] | 0.01 | Chox | 0.24 | 0.05 | 0.01 | 8.96 |
| 6 | MMT-[C8mim] | 0.03 | Chox | 0.17 | 0.04 | 0.01 | 3.09 |
| 7 | L-MMT | 1.25 | Chox | 0.02 | 0.05 | 0.002 | 0.96 |
Now, the scan rate dependence on the redox peaks of different electrodes was investigated. Fig. 2(A) and (B) shows the response of some representative electrodes with the scan rate varying from 10–100 mV s−1 in ferri/ferro solution at pH 7. It was found that with the increase in scan rate, the anodic (Ia) and cathodic (Ic) current increased while the peak potential remain unchanged. The linear behavior of both the currents with square root of scan rate led us to conclude that the process is diffusion controlled.29 Also, based on the value of slope obtained from log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ia versus log
Ia versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E, it was further concluded that the electrochemical process was diffusion controlled. For adsorption controlled process, the slope is 1 whereas for diffusion controlled process slope is 0.5.30 In our case, the slope was found in between 0.3 and 0.6 and confirms the diffusion controlled process in the electrode. On comparing the nanoclay (NC) electrode with NC-IL electrode, lesser value of current was obtained in NC-IL, but the stability of the electrode was found more due to the electrostatic and hydrophobic interactions occurring due to IL. The stability of these electrodes was due to introduction of IL where self-assembly of IL coated LAPONITE® platelets led to gelation transition. Thus, there was a clear tread off.
E, it was further concluded that the electrochemical process was diffusion controlled. For adsorption controlled process, the slope is 1 whereas for diffusion controlled process slope is 0.5.30 In our case, the slope was found in between 0.3 and 0.6 and confirms the diffusion controlled process in the electrode. On comparing the nanoclay (NC) electrode with NC-IL electrode, lesser value of current was obtained in NC-IL, but the stability of the electrode was found more due to the electrostatic and hydrophobic interactions occurring due to IL. The stability of these electrodes was due to introduction of IL where self-assembly of IL coated LAPONITE® platelets led to gelation transition. Thus, there was a clear tread off.
 | ||
| Fig. 2 Variation of (A) anodic (Ia) and (B) cathodic (Ic) current with square root of scan rate showing the diffusion controlled process of charge transfer. | ||
The peak current as a function of square rate of scan rate in range of 10–100 mV s−1 was found to be linear with regression equation given for both anodic and cathodic response as follows:
| Ia (μA)L/ITO = 0.98 μA + 8.65 (μA mV s−1)1/2, R2 = 0.99 | (1a) |
| Ic (μA)L/ITO = −4.04 μA − 6.74 (μA mV s−1)1/2, R2 = 0.99 | (1b) |
| Ia (μA)L-[C2mim]/ITO = 0.48 μA + 2.38 (μA mV s−1)1/2, R2 = 0.99 | (2a) |
| Ic (μA)L-[C2mim]/ITO = −1.38 μA − 1.71 (μA mV s−1)1/2, R2 = 0.99 | (2b) |
| Ia (μA)MMT/ITO = 15.2 μA − 8.74 (μA mV s−1)1/2, R2 = 0.97 | (3a) |
| Ic (μA)MMT/ITO = −12.5 μA − 8.07 (μA mV s−1)1/2, R2 = 0.97 | (3b) |
| Ia (μA)L-MMT/ITO = 15.0 μA − 13.6 (μA mV s−1)1/2, R2 =0.98 | (4a) |
| Ic (μA)L-MMT/ITO = −11.4 μA − 12.9 (μA mV s−1)1/2, R2 = 0.98 | (4b) |
The surface concentration of the electrode could be estimated from current versus potential plot using the Brown–Anson model which is based on the equation given as31
 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), I* is the surface concentration of electrode (mol cm−2) and A is surface area of the electrode (0.25 cm2), V is the scan rate and R is gas constant (8.314 J mol−1 K−1) and T = 300 K.
485 C mol−1), I* is the surface concentration of electrode (mol cm−2) and A is surface area of the electrode (0.25 cm2), V is the scan rate and R is gas constant (8.314 J mol−1 K−1) and T = 300 K.
The diffusion coefficient (D) value of the redox species from the electrolyte to the electrode was calculated using the Randles–Sevcik equation given by31
| Ip = (2.69 × 105)n3/2AD1/2CV1/2 | (6) |
 | (7) |
The next step is to determine the sensitivity of the particular electrode towards different analytes and discussed briefly below.
3.1. Oxalic acid based sensor
Oxalic acid (OA) is one of the components found in kidney calculus. Due to immiscibility of calcium oxalate in water, the crystal cores of calcium oxalate grow into kidney and urethral calculi and thus cause kidney stones. Thus, its determination in food and urine is of most importance. In our earlier work, it was established that L-[C2mim] electrode was used as oxalic acid sensor.273.2. Cholesterol based sensor
Quantification of cholesterol (Chox) in blood is one of the regular practices for the diagnosis and prevention of several kinds of cardiac diseases, hypertension, cerebral thrombosis, metabolic disorder etc. Thus, its determination has received considerable attraction in clinical diagnostic. The content of cholesterol in human serum is in the range of 5.17 mM that constitute of 30% sterol and 70% esterified with fatty acids. Here, we have used a series of nanoclay-IL electrodes to observe their response towards this analyte out of which three L-MMT, MMT-[C2mim] and MMT-[C8mim] electrodes showed maximum cholesterol detection potential. The L-MMT electrode showed cholesterol detection which was discussed in our earlier work.28 The effect of all the analytes on the response of MMT-[C2mim] and MMT-[C8mim] electrode was recorded.3.3. Citric acid based sensor
Citric acid (CA) is found in fruit juices and beverages. The concentration of citric acid in citrus food lies in the range of 0.005 mol L−1 to 0.30 mol L−1. It is used as a flavoring and preservative in food and beverages industries.3.4. Ascorbic acid based sensor
The wide use of ascorbic acid (AA) in food and drinks industry is due to its anti-oxidant properties. Besides this, it is important in several human metabolic processes thus it is used in pharmaceuticals, clinical and food industry. It helps in cell development, healing of injuries and burns as well as synthesis of blood vessels, bones, cartilage etc. In human blood plasma and urine, the normal concentration ranges between 0.6 and 1.5 mg per 100 mL.The electrochemical response of various electrodes was investigated as a function of concentration in range 1–20 mM of different analytes at 50 mV s−1 scan rate. At a fixed scan rate of 50 mV s−1, the variation of the anodic (Ia) and cathodic (Ic) peak current with different analyte concentration in the range of 1–20 mM was investigated (Fig. 3 and Fig. S2 (ESI†)). However, for representation we have presented only three cases which are shown in Fig. 3. It was observed that with increasing concentration of analyte, the magnitude of both the anodic and cathodic peak currents increased. The calibration curves were compared for all the electrodes for different analytes. On addition of analytes into the ferro/ferri electrolyte solution it gets diffused first, and then gets evenly distributed in the solution under stirring, followed by diffusion into the electrode matrix. The proposed MMT with IL electrode had good stability due to the presence of ILs, and this enabled its use for cholesterol detection due to the binding of hydroxyl group of cholesterol with MMT-IL matrix. The electrode offered some favorable sites for transferring the electron from electrolyte to electrode, and vice versa which accelerated the electron transfer kinetics. These electrodes provide the maximum electrocatalytic activity towards the particular analyte in the detection range of 1–20 mM.
 | ||
| Fig. 3 Calibration plot for anodic current versus analyte concentration for (A) MMT-[C8mim], (B) L-[C8mim] and (C) LAPONITE®. Representative error bars are shown. | ||
In all the cases, the magnitude of both the current increased on the addition of analytes while the maximum change in current was found for a particular analyte only (see Table 1). These calibration plots were used for determining sensitivity which gives the measure of sensing a particular analyte through a given matrix. It was revealed from the electrochemical response studies that the current response increases with successive addition of analytes which may perhaps due to the NC and NC-IL based network that is found to acts as acceptor of electron generated during re-oxidation of particular analyte and transferred to electrode via ferri/ferro conversion.
The NC and NC-IL based electrodes thus offers a favorable environment for different analytes facilitating the electron transfer from electrode to electrolyte.
The sensitivity of the electrode could be calculated through the slope of the calibration curve. In case of MMT with ILs, MMT-[C2mim] and MMT-[C8mim], these electrodes exhibited maximum sensitivity of 8.96 and 3.09 μA mM−1 cm−2 for cholesterol. In these cases, presence of cholesterol enhanced the electron transfer kinetics, and thus provided the best environment for the transfer of electrons. The presence of ILs provides both hydrophobic and electrostatic interaction to nanoclay but the shorter alkyl chain which is [C2mim] lead to higher detection value compared to longer alkyl chain due to low hydrophobicity. Thus, both MMT-IL electrodes showed satisfactory electrocatalytic activities towards the oxidation of Cholesterol. The electrodes without ILs were found to have very small transfer rate constant as well as they are less stable compared to those with ILs.
The interesting thing noticed was that the MMT electrodes show the maximum sensitivity for oxalic acid (OA) while MMT with IL shows the maximum for cholesterol (Chox). Introducing ILs lead to introduction of the hydrophobicity in the electrodes, and tune its property to detect (bind to) the cholesterol due to the presence of hydroxyl group in its structure. Thus, it would be able to interact with hydrogen atoms of NC-IL matrices with cholesterol through hydrogen bonding leading to diffusion of analytes and fast response kinetics of electron transfer. Similarly, the electrochemical studies for other matrices were undertaken and the maximum sensitivity for a particular analyte was clearly noticed. The same trend was also found for LAPONITE®-IL based electrodes. The LAPONITE® as well as LAPONITE® with ILs shows the maximum detection for different acidic analytes. The binding of LAPONITE® with ILs in case of high alkyl chain leads to formation of two phases one of which is gel while other is glass. The electrochemical studies were done in the gel state of L-[C8mim] because the glass state violated the dilution test i.e. showed melting on addition of solvent. The use of ILs in the nanoclay leads to lesser sensitivity for the detection of analyte, but enhance the physical stability of the electrode.
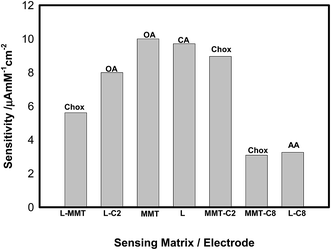
Of all the cases, the L, L-[C2mim] and MMT-[C2mim] shows maximum sensitivity towards CA, OA and Chox, respectively. The presence of ILs with nanoclay provided enhanced electron transfer rate constant. On the basis of these calculated parameters, the short alkyl chain of IL provides more electrocatalytic behavior compared to high alkyl chain due to less hydrophobicity. Thus, the complete summary provided in Table 1 allow us to arrive at the following conclusions. In short, we have extensively studied an array of seven NC and NC-IL electrodes using electrochemical technique and found the maximum sensitivity for a particular electrode towards specific analytes in the detection range of 1–20 mM shown in Fig. 4. Introducing ILs into the nanoclay provided a robust platform for the detection of metabolites. Also the detection of glucose (Glu) and urea (U) was not found in any of these cases. It was thus concluded that the higher sensitivity obtained using [C2mim] in the detection range of 1–20 mM can be attributed to the presence of less hydrophobic interaction with the nanoclay.
FTIR spectroscopy was used to investigate the structural changes occurring due to interaction of analyte with the electrode matrix. The FTIR spectrum of bare MMT-[C8mim] was compared with MMT-[C8mim] with cholesterol in Fig. 5(A). Cholesterol exhibited different peaks at 790, 1037, 1380 and 1471 cm−1 corresponding to C–H, C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. The change in peaks in the region 1100–1700 cm−1 shows the binding of cholesterol to the MMT-[C8mim] network which is noticed from the occurrence of various peaks rising from the CH–O, O–H or C
C bonds. The change in peaks in the region 1100–1700 cm−1 shows the binding of cholesterol to the MMT-[C8mim] network which is noticed from the occurrence of various peaks rising from the CH–O, O–H or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond formation. Addition of analyte decreased the broadness of certain peaks, and also shifting of some peaks was observed.
O bond formation. Addition of analyte decreased the broadness of certain peaks, and also shifting of some peaks was observed.
 | ||
| Fig. 5 FTIR spectrum for (A) MMT-[C8mim], (B) L-[C8mim] and (C) LAPONITE® with their specific analyte. | ||
Ascorbic acid is known to behave as vinylogons carboxylic acid where the double bond electron, hydroxyl group lone pair, and lactone ring carbonyl double bond are present. The FTIR spectrum of bare L-[C8mim] was compared with L-[C8mim]-ascorbic acid that exhibited bands at 3000–3500 cm−1 which correspond to OH stretching while peak at around 1640 cm−1 corresponds to OH deformation (Fig. 5(B)). The peaks at 2850 and 2950 cm−1 are ascribed to C–H stretching vibration of methyl and methylene group. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch vibration of the imidazolium ring was observed at 1589 cm−1. Addition of ascorbic acid resulted in changes in the spectra in terms of disappearance of various bands, or in the decrease of their breadth due to the interaction of ascorbic acid with L-[C2mim].
C stretch vibration of the imidazolium ring was observed at 1589 cm−1. Addition of ascorbic acid resulted in changes in the spectra in terms of disappearance of various bands, or in the decrease of their breadth due to the interaction of ascorbic acid with L-[C2mim].
Citric acid (CA) is a weak organic tribasic acid with hydroxyl and carboxyl group present in it and the spectrum of LAPONITE® in the presence of citric acid shows the increase in peak broadness at 1654 cm−1 that correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations in carboxyl group. Also, the shifting in the peak from 1414 to 1434 cm−1 demonstrates the certain bond formation due to citric acid with LAPONITE®. The clear differences were detected in spectra of LAPONITE® with LAPONITE®–citric acid due to the stronger bond formation of CA with the nanoclay.
O stretching vibrations in carboxyl group. Also, the shifting in the peak from 1414 to 1434 cm−1 demonstrates the certain bond formation due to citric acid with LAPONITE®. The clear differences were detected in spectra of LAPONITE® with LAPONITE®–citric acid due to the stronger bond formation of CA with the nanoclay.
SEM images were recorded for all these matrices so as to study the surface morphology of the electrodes. Fig. 6 depicts the SEM images of different electrodes for the particular analytes. The addition of metabolites into the matrix leads to modification of the surfaces, and shows the binding of the particular analyte with the matrix.
 | ||
| Fig. 6 SEM Images for the (A) LAPONITE® (B) MMT (C) L-[C8mim] (D) MMT-[C2mim] (E) MMT-[C8mim] with their specific analyte and (F) Irradiated MMT-[C2mim]. | ||
Scheme 1(A) shows the fabrication of bare nanoclay (i.e. LAPONITE® and MMT) electrodes which results in formation of house of cards networks while Scheme 1(B) shows the schematics for the fabrication of the matrices formed through different combination of nanoclay and Ionic Liquids. Clay particles are known to form house of cards structures via face(−)/edge(+) attraction in acidic medium and band-like structures are formed via cation-mediated face(−)/face(−) attraction in alkaline medium. The schematics for L-[C2mim] and L-MMT were shown in earlier work.27,28 The nanoclay with ionic liquid results in formation of network due to hydrophobic and electrostatic interactions and used for detection of different analytes. The gel network formation mechanism is well described in our earlier work so these schematics take account only of electrochemical properties.6,7
 | ||
| Scheme 1 Schematics for the preparation of (A) only NC based electrodes and (B) NC-IL based electrodes. | ||
The electrodes were stored under ambient conditions in desiccators when not in use. Their response was monitored by examining their redox current for a period of one week. The cyclic voltagrams exhibited by the electrodes showed storage stability up to 4 days typically.
3.5. Effect of irradiation
It was thought imperative to process these electrodes to low-energy ion beam irradiation to observe if any enhancement in the electrochemical responses was possible. Implantations of ions often augment the charge transfer kinetics of electrodes. These samples were irradiated using Table Top 50 kV Ion accelerator which is one of the low energy ion beam facility (LEIBF) available at IUAC (Inter University Accelerator Centre, New Delhi, India).33 Ion irradiation was performed on these films using 50 keV O2+ ion beam with variable fluence ranging from 5 × 1012 to 5 × 1015 ion per cm2 and further we studied the electrochemical profiling of these exposed electrodes using cyclic voltammetry (CV). In the irradiated samples, the two sharp peaks one corresponding to oxidation and other corresponding to reduction were obtained. Following the same procedure of analysis as discussed earlier it was found that the irradiation of these nanoclay-ionic liquid electrodes by oxygen ion beam result in damaging of the electrochemical properties of these electrodes. Different parameter which was earlier calculated for the matrix has been now evaluated for after irradiation at different fluence and are listed in Table ST1–ST6 (ESI†) and the results shows decrease in different parameters i.e. charge transfer constant, diffusion coefficient etc. The bombardment of oxygen ion beam into electrodes blocked the active sites which were earlier responsible for electron transfer kinetics. These blockages reduce the sensitivity for sensing of the particular analyte by the matrix. Increasing the ion fluence results in diminution of sensitivity of the electrode (Fig. 7). The diffusion of the oxygen ion into the active sites of the electrodes results in hindrance for electron to transfer from electrolyte to electrode and vice versa. Higher ion fluence completely destroyed the conductivity of electrode which was well known for many cases in the literature. Thus, implantation using oxygen ion beam can cause the degradation of the electrodes. | ||
| Fig. 7 Sensitivity for oxygen irradiated electrode as a function of fluence for (A) LAPONITE® and (B) L-[C2mim][Cl]. | ||
A representative FTIR plot for one of these irradiated samples (L-[C8mim][Cl]) were shown in Fig. 8. These bare irradiated electrodes were compared with the electrodes having analyte. The suppression of various bands was observed due to the oxygen ion beam irradiation. Irradiation blocks most of the active sites thus appearance of new peaks was not observed in the case of irradiated electrode.
 | ||
| Fig. 8 FTIR representation of the non-irradiated and irradiated L-[C8mim] without and with analyte ascorbic acid (AA). | ||
The aspect ratio of the nanoclay as well as length of alkyl chain of IL was responsible for the electrochemical response to a particular analyte. It was found that as compared to nanoclay, the presence of IL enhanced the stability of the electrode. As observed from the data the magnitude of anodic and cathodic peak current was less in case of IL than the bare nanoclay but both [C2mim] and [C8mim] provides stability to the electrode. The advantage of our method compared to others was that the prepared electrodes made no use of enzymes. Thus, these biocompatible and enzyme-free electrodes of nanoclay and IL could be used to detect different bioanalytes to a very good accuracy, and this paved the way for the design of strip-based sensors.
4. Conclusion
We have developed a range of seven enzyme-free electrodes for the electrochemical detection of different bioanalytes using nanoclay and ionic liquid as base materials. The sensitivity for the particular analytes decreased on the introduction of IL, but enhanced the stability of these electrodes. It was found that the alkyl chain length of ILs affected the electron transfer kinetic process of the electrodes. The [C2mim] having shorter alkyl chain length enhanced the transfer kinetics from electrode to electrolyte and vice versa in comparison to [C8mim] electrode having longer alkyl chain length. The modification noticed in the FTIR spectra confirmed the interaction of matrix with the analytes. The advantage of electrochemical sensing using the non-enzymatic materials for the detection of various metabolites over the enzyme was stability, simplicity, inexpensive as well as biodegradablity. Also the effect of oxygen ion beam irradiation onto the electrodes was investigated. The sensitivity of the electrodes towards the particular analyte was found to decrease due to the vacant sites occupied by oxygen or their radical. At higher ion fluence the irradiation caused severe damage to the electrode resulting in much lesser sensitivity. We have not performed any experiment on clinical samples using our electrodes. The present study merely addresses the application potential of clay based electrodes for the detection of bioanalytes. This study will be extended to include clinical samples in future.Acknowledgements
NJ acknowledges CSIR, Government of India for Senior Research Fellowship. KR is thankful to Department of Science and Technology, Government of India-Inspire Faculty Award. We are thankful to the Advanced Research Instrumentation Facility (AIRF) of the University for allowing us access to the SEM and FTIR facility and Inter University Accelerator Centre (IUAC) for oxygen ion beam irradiation facility.References
- C. Viseras, P. Cerezo, R. Sanchez, I. Salcedo and C. Aguzzi, Appl. Clay Sci., 2010, 48, 291–295 CrossRef CAS.
- H. A. Patel, R. S. Somani, H. C. Bajaj and R. V. Jasra, Bull. Mater. Sci., 2006, 29, 133–145 CrossRef CAS.
- S. Wang, Y. Wu, R. Guo, Y. Huang, S. Wen, M. Shen, J. Wang and X. Shi, Langmuir, 2013, 29, 5030–5036 CrossRef CAS PubMed.
- http://www.byk.com/fileadmin/byk/additives/product_groups/rheology/former_rockwood_additives/technical_brochures/BYK_B-RI21_LAPONITE_EN.pdf.
- H. Van Olphen, An Introduction to Clay colloid chemistry, Wiley Interscience, New York, 1977 Search PubMed.
- N. Joshi, K. Rawat, V. K. Aswal and H. B. Bohidar, Colloids Surf., A, 2014, 461, 66–75 CrossRef CAS.
- N. Joshi, K. Rawat and H. B. Bohidar, J. Phys. Chem. B, 2014, 118, 6329–6338 CrossRef CAS PubMed.
- M. Dijkstra, J. P. Hansen and P. A. Madden, Phys. Rev. Lett., 1995, 75, 2236–2239 CrossRef CAS PubMed.
- H. Z. Cummins, J. Non-Cryst. Solids, 2007, 353, 3891–3905 CrossRef CAS.
- D. Bonn, H. Tanaka, G. Wegdam, H. Kellay and J. Meunier, Europhys. Lett., 1998, 45, 52–57 CrossRef.
- B. Ruzicka, E. Zaccarelli, L. Zulian, R. Angelini, M. Sztucki, A. Moussaid, T. Narayanan and F. Sciortino, Nat. Mater., 2011, 10, 56–60 CrossRef CAS PubMed.
- B. Ruzicka, L. Zulian and G. Ruocco, Langmuir, 2006, 22, 1106–1111 CrossRef CAS PubMed.
- N. Oyama and F. C. Anson, J. Electroanal. Chem. Interfacial Electrochem., 1986, 199, 467–470 CrossRef CAS.
- H. Y. Liu and F. C. Anson, J. Electroanal. Chem. Interfacial Electrochem., 1985, 184, 411–417 CrossRef CAS.
- P. K. Ghosh, A. H. Mau and A. J. Bard, J. Electroanal. Chem. Interfacial Electrochem., 1984, 169, 315–317 CrossRef CAS.
- A. Yamagishi and A. Aramata, J. Electroanal. Chem. Interfacial Electrochem., 1985, 191, 449–452 CAS.
- A. Senillou, N. Jaffrezic, C. Martelet and C. Cosnier, Anal. Chim. Acta, 1999, 401, 117–124 CrossRef CAS.
- D. Shan, C. Mousty, S. Cosnier and S. L. Mu, Electroanalysis, 2003, 15, 1506–1512 CrossRef CAS.
- S. Cosnier, C. Mosty, C. Gondran and A. Lepellec, Mater. Sci. Eng., C, 2006, 26, 442–447 CrossRef CAS.
- P. Wassercheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef.
- A. A. J. Torriero and A. M. Bond, in Electroanalytical Chemistry research Trend, ed. K. Hayaski, Nova science Publisher Inc., New York, 2009, pp. 1–63 Search PubMed.
- H. Liu, Y. Liu and J. Li, Phys. Chem. Chem. Phys., 2010, 12, 1685–1697 RSC.
- D. Wei and A. Ivaska, Anal. Chim. Acta, 2008, 607, 126–135 CrossRef CAS PubMed.
- P. Sun and D. W. Armstrong, Anal. Chim. Acta, 2010, 661, 1–16 CrossRef CAS PubMed.
- G. A. Baker, S. N. Baker, S. Pandey and F. V. Bright, Analyst, 2005, 130, 800–808 RSC.
- A. Sharma, K. Rawat, P. R. Solanki and H. B. Bohidar, Anal. Methods, 2015, 7, 5876–5885 RSC.
- N. Joshi, K. Rawat, P. R. Solanki and H. B. Bohidar, Sensing and Bio-Sensing Research, 2015, 5, 105–111 CrossRef.
- N. Joshi, K. Rawat, P. R. Solanki and H. B. Bohidar, Biochem. Eng. J., 2015, 102, 69–73 CrossRef CAS.
- A. Kaushik, P. R. Solanki, A. A. Ansari, G. Sumana, S. Ahmad and B. D. Malhotra, Sens. Actuators, B, 2009, 138, 572–580 CrossRef CAS.
- R. N. Goyal, V. K. Gupta and N. Bachheti, Anal. Chim. Acta, 2007, 597, 82–89 CrossRef CAS PubMed.
- A. J. Bard and L. R. Faulkner, A digital simulation model for electrochromic processes at WO3 electrodes, Wiley, New York, 1980 Search PubMed.
- E. Laviron, J. Electroanal. Chem., 1979, 101, 19–28 CrossRef CAS.
- R. Kumar, R. Ahuja, C. P. Safvan and A. Roy, Proceeding of the Indian particle Accelerator conference, 2013, pp. 1–3 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11514d |
| This journal is © The Royal Society of Chemistry 2016 |