Efficient copolymerization of ethylene with norbornene or its derivatives using half-metallocene zirconium(IV) catalysts†
Yulian Li,
Jixing Yang,
Bin Wang and
Yuesheng Li*
Tianjin Key Lab Composite & Functional Materials, School of Materials Science and Engineering, Tianjin University, Tianjin 300072, China. E-mail: ysli@tju.edu.cn
First published on 15th June 2016
Abstract
A series of half-metallocene zirconium complexes CpZr(thf)Cl2[O-2,4-tBu-6-(P-PhR)C6H2] (Cp = C5H5, 2a: R = Me, 2b: R = tBu, 2c: R = Ph, 2d: R = 4-F-Ph) have been synthesized by reacting CpZrCl3 with the corresponding ligands in THF. All the complexes were characterized by (1H, 13C and 31P) NMR spectroscopy as well as elemental analysis. X-ray structural analysis for 2a and 2d revealed a six-coordinate distorted octahedral geometry around the zirconium center in the solid state. With the activation of a suitable cocatalyst, half-metallocene zirconium complexes 2a–d were employed to catalyze ethylene polymerization and ethylene copolymerization with cycloolefins including norbornene (NBE), 5-ethylidene-2-norbornene (ENB) and 5-vinyl-2-norbornene (VNB). All the complexes exhibited high efficiency toward the copolymerizations. High comonomer incorporations up to 62% (NBE), 76% (ENB) and 40.6% (VNB), respectively, with high catalytic activities above 103 kgpolymer per molZr per h were achieved using catalyst 2c. Steric hindrance and electronic effects of the complexes or the comonomers greatly influence copolymerization behavior. Thus, catalytic activity and copolymer chain structure, including comonomer incorporation and the molecular weight, can be easily tuned over a wide range by changing catalyst and reaction conditions.
1. Introduction
Cyclic olefin copolymers (COCs) are good candidates for engineering plastics and optical apparatus, thanks to their excellent transparency, and high thermal and chemical stability relative to polyethylene or ethylene/α-olefin copolymer. These desirable properties have led to the emergence of commercially available copolymers such as TOPAS and APEL.1,2 The existence of ring strain in the structure of norbornene (NBE) and its derivatives makes them facile polymerizable, thus NBE has become one of the most active participants in ethylene/cyclo-olefin copolymerization.3In spite of commercial success, an issue that should be properly addressed in the application of COCs is their poor surface properties, resulting from the nonpolar nature of the material. As the simplest method of functionalization, copolymerization of olefin with polar monomer using early transition metal catalysts is rarely reported because of the highly oxophilic nature of polar monomer.4,5 Although this can be realized by using certain late transition metal catalysts,6 the polymerization often suffers from either low efficiency or the high cost of special comonomers. A practicable alternative is to choose comonomers bearing additional functional groups that do not interfere with the copolymerization, which allows for post-functionalization through simple transformations.7,8 5-Ethylidene-2-norbornene (ENB) and 5-vinyl-2-norbornene (VNB) satisfactorily meet these requirements.7,9–19
Catalysts play a crucial role in the production of ethylene/ENB or ethylene/VNB copolymers, and most of catalysts reported are ansa-metallocenes.20 For example, when Ph2C(Flu)(Cp)ZrCl2 (Cp = C5H5, Flu = fluorenyl) was used in ethylene/VNB copolymerization, the catalytic activity could be up to 3520 kg per molZr per h with VNB incorporation of 32 mol%.12 When used in ethylene/ENB copolymerization, the catalytic activity of rac-Et(Ind)2ZrCl2 (Et = C2H4, Ind = indenyl) reached 1645 kg per molZr per h and ENB incorporation was 38.4 mol%. In addition, some constrained geometry catalysts (CGCs) were also efficient for ethylene/VNB or ethylene/ENB copolymerization.15,21 For example, Mu and his coworkers reported a CGC-type titanium catalyst, which showed activity as high as 1320 kg per molTi per h with ENB incorporation of 50.5 mol%.15 Recently, half-metallocene, possessing the merits of easy synthesis and attractively catalytic performance, had received more and more attention.22–37 The half-metallocene scandium complexes reported by Hou and his colleagues showed excellent catalytic activity for ethylene/norbornene (NBE) and ethylene/dicyclopentadiene (DCPD) copolymerization, with comonomer incorporation of 44 and 45 mol%, respectively.38,39 Nomura and his coworkers reported that half-titanocenes containing pyrazolato ligands showed unique characteristics not only for ethylene and syndio-specific styrene polymerization, but also for the copolymerization of ethylene with 1-hexene, styrene or NBE.23
Recently, we reported a series of phosphine-phenolate based half-titanocenes, which showed high catalytic activities for ethylene/NBE copolymerization.40,41 Furthermore, half-zirconocenes supported by ligands with the similar structures showed higher catalytic activities for ethylene polymerization.42,43 These results prompted us to explore catalytic behavior of the half-zirconocenes for ethylene/ENB and ethylene/VNB copolymerization. Thus, on the basis of the reported half-zirconocenes (2c: R = Ph),42 we synthesized a series of novel phosphine-phenolate-based half-zirconocenes (CpZr(thf)Cl2[O-2,4-tBu-6-(PPhR)C6H2], 2a: R = CH3, 2b: R = tBu, 2d: R = 4-F-Ph, Scheme 1) and employed them catalyzing copolymerization of ethylene with VNB or ENB. By tuning the substitute on P atom, the influence of steric hindrance and electronic effect on copolymerization behavior was examined. As a result, high catalytic activities up to 103 kg per molZr per h and high cycloolefin incorporations were achieved.
2. Experimental section
2.1 General procedure and materials
All manipulation of air- and/or moisture-sensitive compounds was carried out under nitrogen atmosphere by using standard Schlenk techniques or in an Mbraun glove box. All solvents were purified from an Mbraun SPS system. All 1H, 13C and 31P NMR spectra of ligands and complexes were recorded on a Bruker-400 MHz spectrometer at ambient temperature, with CDCl3 or d6-DMSO as the solvent (dried by MS 4 Å). Moreover, the 1H and 13C NMR spectra were taken TMS (trimethylchlorosilane) as an internal reference, however, H3PO4 was employed as an external reference for 31P NMR spectra. 1H and 13C NMR spectra of polymer samples were obtained on the same Bruker-400 MHz spectrometer at 135 °C, with o-C6D4Cl2 as a solvent. The molecular weights (MWs) and the polydispersities of the polymer samples were determined at 150 °C by a PL-GPC 220 type high-temperature chromatograph equipped with three PL gel 10 μm Mixed-B LS type columns. 1,2,4-Trichlorobenzene (TCB) was employed as the solvent at a flow rate of 1.0 mL min−1. The calibration was made by the polystyrene standard Easi Cal PS-1 (PL Ltd.).CpZrCl3 and trityltetrakis(pentauorophenyl)borate ([Ph3C][B(C6F5)4]) were purchased from Aldrich and used without further purification. Triisobutylaluminium (AliBu3) and modified methylaluminoxane (MMAO, 7% aluminium heptane solution) were purchased from Akzo Nobel Chemical Inc. Dried-MAO was prepared by removing toluene and Al(CH3)3 in MAO. Commercial ethylene was directly used for polymerization without further purification. ENB and VNB were stirred over CaH2 for 24 h and distilled under N2 before use. The NBE was stirred with sodium (Na) for 8 h and distilled under N2 before use. The other reagents and solvents were commercially available.
2.2 Typical copolymerization procedure
Copolymerizations were carried out under atmospheric pressure in toluene in a 150 mL glass reactor equipped with a mechanical stirrer. The total volume of the solution was 30 mL. The reactor was saturated with ethylene firstly, then charged with comonomer, prescribed volume of toluene and AliBu3 sequentially. After equilibration at the desired polymerization temperature for 5 min, toluene solution of the catalyst was added, and the polymerization was initiated by the addition of [Ph3C][B(C6F5)4] to the reactor. After a desired period of time, the reactor was vented. The resultant copolymers were precipitated from hydrochloric acid/ethanol (volume ratio: 1/50), filtered, washed three times with ethanol, then dried in vacuum at 60 °C to a constant weight.2.3 Density functional theory calculations
Density functional theory (DFT) calculations were used for optimizing active species of catalysts and finding out the differences between these four catalysts in coordination space. Geometry optimizations were performed using the local density approximation augmented with Beckes nonlocal exchange correction and Perdews nonlocal correction.44,45 A triple SOT basis set was used for Zr atom, whereas all other atoms were described by a double-ζ plus polarization SOT basis. The 1s electrons of the C, P and O atoms, as well as the 1s–2p electrons of Zr atom, were treated as frozen core. Finally, first-order scalar relativistic corrections were added to the total energy of the system.2.4 Synthesis of complex 2a, 2b and 2d
The ligand 1a–1d was synthesized according to the literature method,46 and directly used for next procedures. Then the remaining steps to get the catalysts 2a–b and 2d were similar to that of 2c.423. Results and discussion
3.1 Synthesis and characterization
Complex 2c was synthesized and characterized in our previous report, but it was not used to catalyze ethylene/cycloolefins copolymerization. Similar to the synthesis of complex 2c, novel phosphine-phenolate-based half-zirconocenes 2a–b and 2d have been prepared in moderate yields (50.0–66.7%) by treating CpZrCl3 with phosphine-phenolate sodium salt (1.0 equiv.), which was prepared from the corresponding ligands with NaH, as shown in Scheme 1. Pure samples were collected from the chilled concentrated mixture of THF and hexane solution placed in a freezer (−30 °C). These complexes were identified by 1H, 13C and 31P NMR spectra as well as elemental analyses. The 1H NMR spectra showed no complexity and the integration of complexes confirms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Cp groups to phosphine-phenolate ligands. The resonances ascribed to the protons in THF were observed at around δ 3.72 and 1.63 ppm, which were up-field from those in the corresponding free THF in CDCl3, indicating that the THF molecular coordinated to the zirconium center. The chemical shift values of these four complexes in the 31P NMR spectra were different, because of different substitutes on phosphorus (P) atom. The steric hindrance and electron effect of these ligands are quite different. Their steric hindrance was decreased in the order: 2b > 2c ∼ 2d > 2a.
1 ratio of Cp groups to phosphine-phenolate ligands. The resonances ascribed to the protons in THF were observed at around δ 3.72 and 1.63 ppm, which were up-field from those in the corresponding free THF in CDCl3, indicating that the THF molecular coordinated to the zirconium center. The chemical shift values of these four complexes in the 31P NMR spectra were different, because of different substitutes on phosphorus (P) atom. The steric hindrance and electron effect of these ligands are quite different. Their steric hindrance was decreased in the order: 2b > 2c ∼ 2d > 2a.
Block microcrystals of 2a and 2d suitable for X-ray crystallographic analyses were grown from the chilled concentrated THF–hexane solution mixtures. The crystallographic data together with the collection and refinement parameters are summarized in Table S1.† Selected bond distances and angles for 2a and 2d are listed in Table 1. Structures for 2a and 2d are shown in Fig. 1 and S1 (see ESI†).
| 2a | 2d | |
|---|---|---|
| Bond distances in Å | ||
| Zr–O(1) | 2.033(2) | 2.020(3) |
| Zr–O(2) | 2.309(2) | 2.292(3) |
| Zr–P(1) | 2.7599(9) | 2.8353(11) |
| Zr–Cl(1) | 2.4929(9) | 2.4878(12) |
| Zr–Cl(2) | 2.4806(9) | 2.4817(12) |
| P(1)–C(6/1) | 1.805(3) | 1.806(4) |
| P(1)–C(15) | 1.828(3) | 1.834(4) |
| P(1)–C(21) | 1.823(4) | 1.844(4) |
| O(1)–C(1/6) | 1.363(4) | 1.352(3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Bond angles in ° | ||
| O(1)–Zr–P(1) | 70.70(6) | 69.74(8) |
| Cl(1)–Zr–Cl(2) | 91.00(3) | 91.26(4) |
| Zr–O(1)–C(1/6) | 135.62(19) | 137.6(2) |
| Zr–P(1)–C(6/1) | 98.55(11) | 97.19(13) |
| O(1)–Zr–Cl(1) | 148.24(7) | 148.79(8) |
| O(1)–Zr–Cl(2) | 87.72(6) | 90.43(9) |
| P(1)–Zr–Cl(1) | 78.10(3) | 80.43(3) |
| P(1)–Zr–Cl(2) | 76.94(3) | 76.33(4) |
| O(2)–Zr–Cl(1) | 84.69(5) | 81.98(8) |
| O(2)–Zr–Cl(2) | 153.31(6) | 153.60(8) |
| O(2)–Zr–O(1) | 82.43(8) | 82.78(11) |
| O(2)–Zr–P(1) | 76.39(6) | 77.39(7) |
| P(1)–C(6/1)–C(1/6) | 114.2(2) | 114.3(3) |
| C(15)–P(1)–C(21) | 101.89(16) | 104.15(18) |
 | ||
| Fig. 1 Molecular structure of 2a with thermal ellipsoids at 30% probability level. Hydrogen atoms are omitted for clarity. | ||
When the centroid of the cyclopentadienyl ring is considered to be a single coordination site, all complexes adopt a six-coordinate, distorted octahedral geometry around the zirconium center, similar to the reported catalyst 2c.42 For 2a and 2d, the equatorial positions are occupied by the oxygen atom of the chelating phosphine-phenolate ligand, the oxygen atom of THF and two chlorine atoms. The cyclopentadienyl ring and the P atom are coordinated at axial positions (Fig. 1 and S1†). Moreover, in complexes 2a and 2d, P(1) is trans to the Cp ring, while O(1) and O(2) are cis to the Cp ring. The Zr–P bond distance in 2d is longer than that in 2a, because of the electron withdrawing property of F, resulting in weaker interaction between Zr and P atom. Similarly, the Zr–O(1 or 2) distances in 2d are both shorter than those in 2a to compensate the deficiency of electron donation property of phosphorus moiety. The bond angle of C(15)–P(1)–C(21) in 2d is larger than that in 2a because of the repellence between the two benzene rings connected on P atom in 2d.
3.2 Ethylene polymerization
Ethylene polymerizations by CpZr(thf)Cl2[O-2,4-tBu-6-(P-PhR)C6H2] (2a–d) in the presence of MMAO were firstly examined to explore the effect of the steric hindrance and electron effect near the catalytic active centre on the polymerization behavior, and the typical results are depicted in Table 2. In all, complexes 2a–d showed moderate catalytic activity towards ethylene polymerization, yielding polymers with low molecular weight (MW) (Table 2). Catalyst 2a and 2b with electron donating group on P atom showed higher activity at 75 °C, while the catalytic activity of 2c and 2d were much higher at 50 °C. Therefore, it can be concluded that increasing the electron donating ability of P atom can improve the stability of active species at high temperature. However, at 75 °C, the MWs of polymers produced by 2c and 2d with electron withdrawing groups are higher than those obtained by 2a and 2b (run 2, 7, 10 and 13 in Table 2). Catalyst 2a and 2b may have more stable active species, affording more chance for aluminum alkyls or β-H to compete with monomers. All catalysts show the lowest activities at 100 °C, suggesting the active species under this condition are not stable. The influence of electronic and steric properties of these four catalysts on catalytic activity is irregular. At 50 °C, the activity is decreased in the order: 2c (1600 kg per molZr per h) > 2b (560 kg per molZr per h) > 2d (380 kg per molZr per h) > 2a (182 kg per molZr per h). Perhaps, these catalysts are heavily influenced by the amount of alkyl-aluminum and their optimal conditions are different.| Run | Catalyst | Temp. (°C) | Yield (g) | Activity (kg per molZr per h) | Mwd (×100) | PDId |
|---|---|---|---|---|---|---|
| a Conditions: catalyst 3 μmol, MMAO 3 mmol, ethylene 1 atm, Vtotal = 30 mL, reaction for 10 min.b d-MAO (prepared by removing toluene and AlMe3 from ordinary MAO) 3 mmol.c AliBu3 1 mmol, [Ph3C][B(C6F5)4] 6 μmol.d Determined by GPC at 150 °C in 1,2,4-C6Cl3H3 vs. narrow PS standards. | ||||||
| 1 | 2a | 50 | 0.091 | 182 | 890 | 1.27 |
| 2 | 2a | 75 | 0.37 | 740 | 890 | 1.35 |
| 3b | 2a | 75 | 0.15 | 300 | 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.93 |
| 4c | 2a | 75 | 0.20 | 400 | 5400 | 2.50 |
| 5 | 2a | 100 | 0.046 | 92 | 1050 | 1.40 |
| 6 | 2b | 50 | 0.28 | 560 | 930 | 1.32 |
| 7 | 2b | 75 | 0.38 | 760 | 680 | 1.14 |
| 8 | 2b | 100 | 0.046 | 92 | 690 | 1.15 |
| 9 | 2c | 50 | 0.80 | 1600 | 960 | 1.28 |
| 10 | 2c | 75 | 0.14 | 280 | 1720 | 2.07 |
| 11 | 2c | 100 | 0.043 | 86 | 1390 | 1.48 |
| 12 | 2d | 50 | 0.19 | 380 | 920 | 1.28 |
| 13 | 2d | 75 | 0.095 | 190 | 1110 | 1.65 |
| 14 | 2d | 100 | Trace | — | — | — |
The kind of cocatalyst also exhibits a profound effect on polymerization catalysis behavior. For example, when the polymerization was carried out by 2a in the presence of dry MAO (d-MAO), the MWs of the resultant polymers increased greatly (run 2 and 3). This result suggests that chain transfer to aluminum alkyls is crucial among different chain-transfer pathways for phosphine-phenolate-based half-zirconocenes here. The molecular weight of the polymer was largely determined by the chain transfer reaction. The components of these three kinds of cocatalysts are different. MMAO contains both the free Al(CH3)3 and AliBu3. The alkylation of catalyst and chain transfer to aluminum alkyls are very easy. However, d-MAO contains little of free Al(CH3)3. Thus, alkylation and chain transfer reactions are both difficult to occur. The condition to AliBu3/[Ph3C][B(C6F5)4] is compromised. The chain transfer to aluminum alkyls was relatively difficult owning to larger steric hindrance relative to Al(CH3)3. Therefore, polymers with highest molecular weight were obtained when using d-MAO as cocatalyst.
The 1H NMR spectra of ethylene polymers were collected to investigate characteristics of the oligomers (Fig. S2 in ESI†). It turns out that the characteristic peaks of terminal methyl can be observed clearly for polymers (runs 2 and 4). Moreover, the chemical shifts corresponding to double bonds can only be seen with appropriate augmented. Therefore, chain transfer to aluminum alkyls and β-H elimination can both exist in the polymerization system contained aluminum alkyls. Chain transfer to aluminum alkyls is the main way in this polymerization system. When AliBu3/[Ph3C][B(C6F5)4] is used as the cocatalyst, ethylene polymerization by 2a also produces polymers with higher MWs compared with MMAO (run 2 and 4 in Table 2). When d-MAO or AliBu3/[Ph3C][B(C6F5)4] as the cocatalysts, the catalytic activities reduced half. Taking all these into consideration, we choose AliBu3/[Ph3C][B(C6F5)4] as the cocatalyst, because the catalytic activity and MWs of copolymers are both satisfying.
3.3 Ethylene/NBE copolymerization
Ethylene copolymerizations with NBE catalyzed by 2a–d/AliBu3/[Ph3C][B(C6F5)4] were carried out with different NBE concentrations in feed and at different reaction temperature. Typical results are shown in Table 3, and all of them produced relatively high MW copolymers (Mw = 35.0–170.3 kg per molZr per h) with relative narrow and unimodal molecular weight distributions (MWDs, PDI = 1.26–2.43). When conducted at 75 °C, catalyst 2a with a methyl group on P atom displayed the highest activity. The catalytic activity decreases in the order: 2a (4020 kg per molZr per h) > 2d (3900 kg per molZr per h) > 2c (3700 kg per molZr per h) > 2b (2180 kg per molZr per h). Therefore, this result indicated that increasing the steric bulk of phosphorus moiety could reduce the catalytic activity evidently. The NBE incorporations for the four catalysts are decreased in the order: 2d (46.9 mol%) > 2c (42.0 mol%) > 2a (38.0 mol%) > 2b (32.1 mol%). Notably, when the comonomer in feed is increased to 1.0 M, the NBE incorporation for catalyst 2b only enhanced 7.2%, which is obviously different from the other catalysts. The lowest incorporation capability of 2b is also ascribed to the bulk substituent on P atom. In homopolymerization, the polymerization behaviors of 2b are comparative to those of 2a and 2d. Thus, the steric hindrance was not a main factor in determining the catalytic activity of catalyst in ethylene homopolymerization. Therefore, introducing cyclic comonomers with easier coordination and incorporation capabilities into the polymerization system can adjust polymerization.41 From the viewpoint of electronic effect, we can assume that the more electron-withdrawing property of the substitute on P atom, the copolymers with higher NBE incorporation will be produced. All these copolymers exhibit high MW, and their variation tendency is in accordance with that of the incorporation (run 1–4, Table 3). Some DFT calculations about the differences of 2a–d in coordination space and electronic properties can be seen in the ESI.† The DFT calculations show that the coordination space is decreased in the order: 2a ∼ 2c ∼ 2d ≫ 2b, while as to electron withdrawing capabilities: 2d > 2c > 2a > 2b. These DFT calculations are in accordance with our experimental results.| Run | Catalyst | Temp. (°C) | NBE (mol L−1) | Incorp.c (mol%) | Yield (g) | Activity (kg per molZr per h) | Mwd (×103) | PDId |
|---|---|---|---|---|---|---|---|---|
| a Conditions: catalyst 3 μmol, AliBu3 1 mmol, [Ph3C][B(C6F5)4] 6 μmol, ethylene 1 atm, Vtotal = 30 mL, reaction for 10 min.b AliBu3 1.5 mmol.c Established by 13C NMR spectra at 135 °C with o-C6D4Cl2 as a solvent (run 1, 3, 4 and 9–11 in Table 4), while (run 2, 5–8) established by 13C NMR spectra at 25 °C with CDCl3 as a solvent, referred to the literatures.52–59d Determined by GPC at 150 °C in 1,2,4-C6Cl3H3 vs. narrow PS standards. | ||||||||
| 1 | 2a | 75 | 0.5 | 38.0 | 2.01 | 4020 | 70.0 | 1.60 |
| 2 | 2b | 75 | 0.5 | 32.1 | 1.09 | 2180 | 55.3 | 1.58 |
| 3 | 2c | 75 | 0.5 | 42.0 | 1.85 | 3700 | 118.0 | 1.95 |
| 4 | 2d | 75 | 0.5 | 46.9 | 1.95 | 3900 | 170.3 | 1.96 |
| 5 | 2b | 50 | 0.5 | 28.1 | 0.64 | 1280 | 57.5 | 1.77 |
| 6 | 2b | 75 | 1.0 | 39.3 | 1.57 | 3140 | 60.4 | 1.26 |
| 7b | 2b | 75 | 0.5 | 37.4 | 1.34 | 2680 | 35.0 | 1.59 |
| 8 | 2b | 100 | 0.5 | 39.4 | 0.74 | 1480 | 38.2 | 1.58 |
| 9 | 2c | 50 | 0.5 | 37.2 | 1.09 | 2180 | 107.0 | 2.20 |
| 10 | 2c | 75 | 1.0 | 62.0 | 3.57 | 7140 | 166.0 | 1.69 |
| 11 | 2c | 100 | 0.5 | 57.1 | 1.43 | 2860 | 74.9 | 2.43 |
Since catalysts 2b and 2c are different in steric hindrance and electronic effect, these two typical catalysts are used to explore the effect of the reaction parameters like reaction temperature, Al/Zr molar ratio, and concentration of comonomer in the feed on the copolymerization behaviors. As the reaction temperature increases from 50 °C to 100 °C, the catalytic activity of both catalysts increased firstly and then decreased, while NBE incorporations are always increased. Thus, to this kind of catalysts, it is relatively difficult to proceed polymerization at 50 °C in this ethylene/NBE copolymerization system. Because the processes about removing alkyls by Lewis acid and the formation of four-membered-ring transition state are both endothermic.41 The higher temperature would decrease the solubility of ethylene in toluene, resulting in higher comonomers incorporation. Therefore, the effect of raising temperature on comonomer incorporation is similar to that of increasing comonomer concentration in feed. For both catalysts, the catalytic activity, comonomer incorporation and the MW of the copolymers were all enhanced with increasing concentration of comonomer in feed (compared run 2 with 6 or 3 with 10, Table 3). Hence, the MWs of copolymers can be both influenced by temperature and comonomer incorporation. Higher temperature can result in lower MWs because of the increased chain transfer reaction. However, higher comonomer incorporation will retard the chain transfer reaction, resulting higher molecular weight. For catalyst 2b with electron-donating group at P atom, the increase in reaction temperature leads to a decrease in MWs of the resultant copolymers. However, the MW of copolymer produced by 2c at 75 °C is higher than that produced at 50 °C in the same concentration of NBE (run 3 and 9, Table 3), thanks to higher NBE incorporation at 75 °C. Moreover, 2c shows both high activity reached 7140 kg per molZr per h and high comonomer incorporation up to 62 mol% at 75 °C (run 10, Table 3). The increase in Al/Zr ratio for catalyst 2b causes a slightly increase in comonomer incorporation as well as catalytic activity and a large extent decrease in the MW (run 2 and 7 in Table 3). Thus, chain-transfer to aluminum alkyls cannot be neglected for 2b in ethylene/NBE copolymerization. The effect of the amount aluminum alkyls on copolymerization is very complicated, all kinds of parameters can be influenced with different degree, which is similar to the case reported previously.50,51
The typical 13C NMR spectra of the copolymers (Fig. 2 and S3†) show that the microstructure formed by catalyst 2b possesses isolated and alternated NBE units without NBE continue sequences when the incorporation is below 50 mol% (Fig. 2a). With the increasing of NBE incorporation, more alternative NBE units will be traced (Fig. S3†). The NBE incorporation of copolymers catalyzed by 2c can reach to higher than 50 mol%. Compared with Fig. 2a, the 13C NMR spectrum of the copolymer with NBE incorporation of 57.1% shows new peaks assigned to NBE diads and triads (Fig. 2b), which is similar to the case reported previously.56,60,61
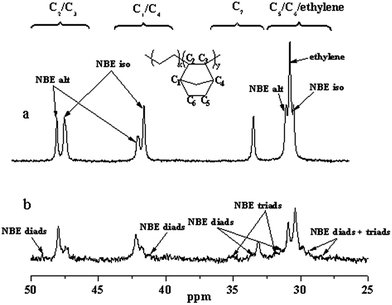 | ||
| Fig. 2 13C NMR spectra of ethylene/NBE copolymers produced by different catalysts (a, run 6, 39.3 mol%; b, run 11, 57.1 mol% in Table 3). | ||
Compared with ethylene homopolymerization, catalytic activity and MW of the resultant polymers in copolymerization can be both substantially increased because of the introduction of comonomers. We assume that the cycloolefins are easier to coordinate and be incorporated in comparison with ethylene, similar to our previous reported literature.41 The more bulky size of cycloolefins can inhibit chain transfer effectively, which results in producing the higher MW copolymers.
3.4 Ethylene/ENB copolymerization
Copolymerizations of ethylene with ENB catalyzed by complexes 2a–d were subsequently explored under the similar conditions. All the catalytic systems employed are able to copolymerize ethylene with ENB and produce copolymers with high MWs and unimodal MWDs. The typical polymerization results are summarized in Table 4.| Run | Catalyst | Temp. (°C) | ENB (mol L−1) | Yield (g) | Activity (kg per molZr per h) | Incorp.c (mol%) | Mwd (×103) | PDId |
|---|---|---|---|---|---|---|---|---|
| a Conditions: catalyst 3 μmol, AliBu3 1 mmol, [Ph3C][B(C6F5)4] 6 μmol, ethylene 1 atm, Vtotal = 30 mL, reaction for 10 min.b AliBu3 1.5 mmol.c Comonomer incorporation (mol%) established by 1H NMR spectra at 135 °C with o-C6D4Cl2 as a solvent (run 1, 3, 4 and 9–12 in Table 5), while that of the others (run 2, 5–8) established by 1H NMR spectra at 25 °C with CDCl3 as a solvent, referred to the literatures.12,13,17d Determined by GPC at 150 °C in 1,2,4-C6Cl3H3 vs. narrow polystyrene standards.e Reaction for 6 min. | ||||||||
| 1 | 2a | 75 | 0.5 | 2.50 | 5000 | 32.1 | 124.8 | 1.77 |
| 2 | 2b | 75 | 0.5 | 1.40 | 2800 | 28.8 | 50.1 | 1.54 |
| 3 | 2c | 75 | 0.5 | 2.45 | 4900 | 40.4 | 180.0 | 1.40 |
| 4 | 2d | 75 | 0.5 | 2.45 | 4900 | 39.4 | 192.4 | 1.69 |
| 5 | 2b | 50 | 0.5 | 0.74 | 1480 | 23.6 | 95.3 | 1.64 |
| 6b | 2b | 75 | 0.5 | 0.55 | 1100 | 32.3 | 59.7 | 1.64 |
| 7 | 2b | 75 | 1.0 | 2.27 | 4540 | 37.5 | 96.0 | 1.55 |
| 8 | 2b | 100 | 0.5 | 0.74 | 1480 | 34.7 | 41.7 | 1.58 |
| 9 | 2c | 50 | 0.5 | 2.80 | 5600 | 30.3 | 96.0 | 1.62 |
| 10b | 2c | 75 | 0.5 | 2.40 | 4800 | 35.8 | 17.4 | 1.73 |
| 11e | 2c | 75 | 1.0 | 2.41 | 8033 | 76.6 | 347.0 | 1.91 |
| 12 | 2c | 100 | 0.5 | 2.20 | 4400 | 58.0 | 134.0 | 1.86 |
The catalyst 2b with the bulky tert-butyl group on the P atom exhibited lower activity compared with the other catalysts under the same conditions (run 1–4, Table 4). Moreover, ethylene/ENB copolymers produced by 2b also contain lower ENB content and have lower MWs than those of other copolymers obtained by 2a and 2c–d. Similar to the ethylene/NBE copolymerization, the catalysts 2c–d with electron-withdrawing group on the P atom displayed higher comonomer incorporation capacities than 2a–b with electron donating group on the P atom. Catalysts 2b and 2c are also chosen to explore the effect of polymerization parameters like reaction temperature, Al/Zr ratio, and comonomer concentration in the feed on copolymerization behaviors. It is interesting that the catalytic activities of catalyst 2b at 50 °C and 100 °C are the same and are almost half of that at 75 °C. However, the copolymer produced at 100 °C show higher ENB incorporation and lower MW compared with copolymer obtained at 50 °C. The reason about the influence of temperature on catalytic activity and comonomers incorporation is the same as E/NBE copolymerization.
For catalyst 2c with electron withdrawing substitute, the catalytic activity decreases with the increase of polymerization temperature, on account of unstable active species at high temperature. However, it is worth noting that the molecular weight produced at 100 °C is higher than that at 50 °C, thanks to higher comonomer incorporation at 100 °C. For both catalysts, increasing the ENB concentration can largely improve catalytic activity, ENB incorporation and MW. If more AliBu3 is used initially, MWs of copolymers produced by catalyst 2c decrease substantially without too much change in catalytic activity and ENB incorporation (run 3 and 10). However, the increase of Al/Zr ratio causes a decrease in catalytic activity and an increase in the incorporation and MW for 2c (run 2 and 6). Therefore, chain transfer to aluminum alkyls is an important way of chain transfer in the copolymerization by 2c, but aluminum alkyls do not play a crucial role in chain transfer for catalyst 2b. It confirms the conclusion again that aluminum alkyls do play important and complicated role for these catalysts in ethylene/NBE-derivates copolymerization, similar to the situation previously reported.50,51
It is also noteworthy that the ethylene/ENB copolymers afforded by catalyst 2b can be dissolved in THF and CHCl3 at room temperature, while those produced by catalyst 2c can not. This may be determined by the MW and microstructure of copolymers, which is the same as E/NBE copolymers. From the typical 1H NMR spectra of ENB and ethylene/ENB copolymer, only encyclo double bond participates in addition polymerization, which configuration is similar with that of comonomer (Fig. 3). Moreover, cross-linking can be ignored because of the narrow and unimodal molecular weight distributions of E/ENB copolymers (PDI < 2.0) and good solubility in certain conditions.
 | ||
| Fig. 3 1H NMR spectra of ENB in CDCl3 and poly(E-co-ENB) sample in o-C6D4Cl2 at 135 °C (run 12 in Table 4). | ||
3.5 Ethylene/VNB copolymerization
Ethylene undergoes facile copolymerization with VNB in toluene under different conditions. The typical results of ethylene/VNB copolymerization catalyzed by 2a–d/Al(iBu3)/[Ph3C][B(C6F5)4] systems under different conditions are summarized in Table 5. Complex 2a with methyl substitute on P atom shows the highest catalytic activity (run 1, Table 5). However, introducing the tert-butyl group on P atom (2b) seriously decreased the activity under the same conditions (run 2). Similarly, catalysts with more electron-withdrawing group on P moiety exhibited higher VNB incorporation capacities.| Run | Catalyst | Temp. (°C) | VNB (mol L−1) | Yield (g) | Activity (kg per molZr per h) | Incorp.c (mol%) | Mwd (×103) | PDId |
|---|---|---|---|---|---|---|---|---|
| a Conditions: catalyst 3 μmol, AliBu3 1 mmol, [Ph3C][B(C6F5)4] 6 μmol, ethylene 1 atm, Vtotal = 30 mL, reaction for 10 min.b AliBu3 1.5 mmol.c Established by 1H NMR spectra at 25 °C with CDCl3 as a solvent except for run 10, which was established by 1H NMR spectra at 135 °C with o-C6D4Cl2 as a solvent, referred to the literatures.12,13,17d Determined by GPC at 150 °C in C6Cl3H3 vs. narrow polystyrene standards. | ||||||||
| 1 | 2a | 75 | 0.5 | 2.30 | 4600 | 28.2 | 40.0 | 1.75 |
| 2 | 2b | 75 | 0.5 | 0.63 | 1260 | 18.6 | 37.4 | 1.77 |
| 3 | 2c | 75 | 0.5 | 2.24 | 4480 | 34.7 | 43.0 | 2.00 |
| 4 | 2d | 75 | 0.5 | 2.08 | 4160 | 37.8 | 46.7 | 1.65 |
| 5 | 2b | 50 | 0.5 | 0.69 | 1380 | 13.4 | 26.7 | 1.65 |
| 6 | 2b | 75 | 1.0 | 0.28 | 560 | 21.2 | 88.4 | 1.69 |
| 7b | 2b | 75 | 0.5 | 0.41 | 820 | 18.0 | 16.9 | 1.56 |
| 8 | 2b | 100 | 0.5 | 0.19 | 380 | 24.3 | 18.0 | 1.60 |
| 9 | 2c | 50 | 0.5 | 2.50 | 5000 | 30.3 | 48.9 | 2.16 |
| 10 | 2c | 75 | 1.0 | 3.03 | 6060 | 40.8 | 155.0 | 1.79 |
| 11b | 2c | 75 | 0.5 | 2.10 | 4200 | 23.6 | 72.0 | 1.92 |
| 12 | 2c | 100 | 0.5 | 0.54 | 108 | 40.6 | 48.6 | 1.63 |
Therefore, we chose catalyst 2b and 2c to examine the influence of different conditions on copolymerization. At elevated temperatures, the catalytic activities of 2b and 2c will decrease, especially when the reaction temperature is 100 °C. However, higher VNB incorporations are achieved because of lower solubility of ethylene in toluene at higher temperature. Notably, the MW of copolymer produced by 2b at 75 °C is higher than that obtained at 50 °C (run 2 and 9 in Table 5) and the MW of copolymer afforded by 2c at 100 °C is higher than that obtained at 75 °C (run 3 and 12, Table 5). This result is the same as ethylene/VNB copolymers. For both catalysts, the increase in VNB concentration in feed from 0.5 M to 1.0 M can cause an increase in catalytic activity, MW and incorporation (run 2 vs. 6 and 3 vs. 10). Nonetheless, a slightly degree of increment in VNB incorporation is found, compared with ethylene/ENB and ethylene/NBE copolymerization. Meanwhile, monomer incorporation and the MW of the E/VNB copolymers were both much lower than those of E/ENB or E/NBE copolymers. Similar results were found in our previous reports.11 It may be ascribed to the different exocyclic double bond in VNB. Perhaps, the vinyl double bond in VNB can interact with the active species remotely, resulting in smaller incorporation space and easier chain transfer reaction. These results strongly indicated that the minor difference in structures of catalysts and comonomers can both affect the polymerization behaviors.
The increase in Al/Zr ratio for catalyst 2b gave rise to a decrease both in catalytic activity and MW of the copolymer (run 2 and 7, Table 5). Thus, chain transfer to aluminum alkyls was serious in ethylene/VNB copolymerization for catalyst 2b. As for 2c, more AliBu3 in feed will result in higher MW and lower comonomer incorporation with unobviously change in the catalytic activity (run 3 and 11, Table 5). The 1H NMR spectra of VNB and typical E/VNB copolymers are shown in the Fig. 4. Obviously, only encyclo double bond can be region-selectively incorporated into the polymer chains. All the copolymers produced by catalysts 2b and 2c can be dissolved in THF and CHCl3 at room temperature. All the aforementioned results suggest that cross-linking can be ignored and this phosphine-phenolate-based half-zirconocenes showed high regioselectivity to VNB.
 | ||
| Fig. 4 1H NMR spectra of VNB in CDCl3 and a sample of ethylene/VNB copolymer in CDCl3 (run 8, 24.3% in Table 5). | ||
4. Conclusions
The phosphine-phenolate-based half-zirconocenes 2a–d with different substitutes on phosphorus atom show moderate activities in ethylene polymerization, affording polymers with low molecular weights. However, they are good candidates for ethylene/ENB, ethylene/VNB and ethylene/NBE copolymerization. The titanium analogues would be poisoned partly by the extra vinyl and ethylidene double bond in VNB and ENB respectively, resulting in moderate activity about 5 × 105 kg per molTi per h. Steric hindrance of the ligands in catalysts plays a more important role in catalytic activity, and the catalytic activity decreases in the order: 2a (methyl) > 2c (phenyl) ∼ 2d (4-F-phenyl) ≫ 2b (tert-butyl). Electron effect of the substitute on the phosphorus atom play a decisive role in comonomer incorporation capability, thus the catalysts bearing more electron withdrawing ligands showed higher comonomer incorporation ability and produce copolymers with higher molecular weights. The three kinds of comonomers NBE, ENB and VNB possess different steric hindrance and reactivity, resulting in different copolymerization behaviors under same conditions. The obtained copolymers with pendent double bonds are amenable to post-functionalization through epoxidation reactions or thio–ene click reaction to introduce polar groups into the polyolefins. As far as we know, this phosphine-phenolate-based half-zirconocenes are the rare examples among half-metallocenes which can catalyze ethylene/cyclic-olefin copolymerization with high efficiency and region-selectivity.Acknowledgements
The authors are grateful for financial support by the National Natural Science Foundation of China (No. 21234006).References
- M. J. Brekner, F. Osan, J. Rohrmann and M. Antberg, Process for the preparation of chemically homogeneous cycloolefin copolymers, US Patent, 5, 324, 801, 1994.
- Chem. Eng. News, 2007, 85(24), 17 Search PubMed.
- I. Tritto, L. Boggioni and D. R. Ferro, Coord. Chem. Rev., 2006, 250, 212–241 CrossRef CAS.
- H. Terao, S. Ishii, M. Mitani, H. Tanaka and T. Fujita, J. Am. Chem. Soc., 2008, 130, 17636–17637 CrossRef CAS PubMed.
- Z. Chen, J. F. Li, W. J. Tao, X. L. Sun, X. H. Yang and Y. Tang, Macromolecules, 2013, 46, 2870–2875 CrossRef CAS.
- M. J. Yanjapappa and S. Sivaram, Prog. Polym. Sci., 2002, 27, 1347–1398 CrossRef.
- M. Hong, S. R. Liu, B. X. Li and Y. S. Li, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2499–2506 CrossRef CAS.
- M. Hong, J. Y. Liu, B. X. Li and Y. S. Li, Macromolecules, 2011, 44, 5659–5665 CrossRef.
- A. Malmberg and B. Löfgren, J. Appl. Polym. Sci., 1997, 66, 35–44 CrossRef CAS.
- M. Mortazavi, H. Arabi, S. Ahmadjo, M. Nekoomanesh and G. H. Zohuri, J. Appl. Polym. Sci., 2011, 122, 1838–1846 CrossRef CAS.
- J. Y. Liu, S. R. Liu, L. Pan and Y. S. Li, Adv. Synth. Catal., 2009, 351, 1505–1511 CrossRef CAS.
- H. Lasarov and T. T. Pakkanen, Macromol. Chem. Phys., 2000, 201, 1780–1786 CrossRef CAS.
- S. Marathe and S. Sivaram, Macromolecules, 1994, 27, 1083–1086 CrossRef CAS.
- Y. Y. Long, Y. X. Wang, J. Y. Liu, X. F. Li and Y. S. Li, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 4626–4638 CrossRef CAS.
- H. C. Li, J. C. Li, Y. T. Zhang and Y. Mu, Polymer, 2008, 49, 2839–2844 CrossRef CAS.
- I. N. Meshkova, A. N. Shchegolikhin, E. V. Kiseleva and L. A. Novokshonova, Polym. Sci., Ser. B, 2015, 57, 77–84 CrossRef CAS.
- I. Kim, React. Funct. Polym., 2001, 49, 197–204 CrossRef CAS.
- K. Nomura, B. K. Bahuleyan, S. Zhang, P. M. V. Sharma, S. Katao, A. Igarashi, A. Inagaki and M. Tamm, Inorg. Chem., 2014, 53, 607–623 CrossRef CAS PubMed.
- T. Hasan, T. Ikeda and T. Shiono, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4581–4587 CrossRef CAS.
- X. F. Li and Z. M. Hou, Coord. Chem. Rev., 2008, 252, 1842–1869 CrossRef CAS.
- H. Lasarov and T. T. Pakkanen, Macromol. Rapid Commun., 2001, 22, 434–438 CrossRef.
- K. Nomura and J. Y. Liu, Dalton Trans., 2011, 40, 7666–7682 RSC.
- K. Nomura, H. Fukuda, S. Katao, M. Fujiki, H. J. Kim, D.-H. Kim and I. Saeed, Macromolecules, 2011, 44, 1986–1998 CrossRef.
- K. Nomura, H. Fukuda, H. Matsuda, S. Katao and S. Patamma, J. Organomet. Chem., 2015, 798, 375–383 CrossRef CAS.
- J. Y. Liu, P. Tao, Y. X. Wang and Y. S. Li, RSC Adv., 2014, 4, 19433–19439 RSC.
- W. Z. Zhao and K. Nomura, Macromolecules, 2016, 49, 59–70 CrossRef CAS.
- K. C. Jayaratne, R. J. Keaton, D. A. Henningsen and L. R. Sita, J. Am. Chem. Soc., 2000, 122, 10490–10491 CrossRef.
- K. C. Jayaratne and L. R. Sita, J. Am. Chem. Soc., 2000, 122, 958–959 CrossRef.
- R. J. Keaton, K. C. Jayaratne, D. A. Henningsen, L. A. Koterwas and L. R. Sita, J. Am. Chem. Soc., 2001, 123, 6197–6198 CrossRef CAS PubMed.
- W. Zhang and L. R. Sita, J. Am. Chem. Soc., 2008, 130, 442–443 CrossRef PubMed.
- K. Nomura, S. Pengoubol and W. Apisuk, RSC Adv., 2016, 6, 16203–16207 RSC.
- H. Tsurugi, K. Yamamoto, R. Rochat and K. Mashima, Polym. J., 2015, 47, 2–17 CrossRef CAS.
- K. Nomura, N. Naga, M. Miki and K. Yanagi, Macromolecules, 1998, 31, 7588–7597 CrossRef CAS.
- K. Nomura, K. Oya, T. Komatsu and Y. Imanishi, Macromolecules, 2000, 33, 3187–3189 CrossRef CAS.
- K. Nomura, T. Komatsu and Y. Imanishi, Macromolecules, 2000, 33, 8122–8124 CrossRef CAS.
- K. Nomura, K. Oya and Y. Imanishi, J. Mol. Catal. A: Chem., 2001, 174, 127–140 CrossRef CAS.
- K. Nomura, H. Okumura, T. Komatsu and N. Naga, Macromolecules, 2002, 35, 5388–5395 CrossRef CAS.
- X. F. Li, J. Baldamus and Z. M. Hou, Angew. Chem., Int. Ed., 2005, 44, 962–965 CrossRef CAS PubMed.
- X. F. Li and Z. M. Hou, Macromolecules, 2005, 39, 6767–6769 CrossRef.
- X. Y. Tang, J. Y. Liu and Y. S. Li, Catalysts, 2013, 3, 261–275 CrossRef CAS.
- X. Y. Tang, Y. X. Wang, B. X. Li, J. Y. Liu and Y. S. Li, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1585–1594 CrossRef CAS.
- X. Y. Tang, Y. Y. Long, Y. X. Wang, J. Y. Liu and Y. S. Li, Dalton Trans., 2014, 43, 222–230 RSC.
- X. Y. Tang, Y. X. Wang, S. R. Liu, J. Y. Liu and Y. S. Li, Dalton Trans., 2013, 42, 499–506 RSC.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS.
- J. P. Perdew, Phys. Rev. B, 1986, 33, 822–8824 Search PubMed.
- V. T. Trepohl, R. Fröhlich and M. Oestreich, Tetrahedron, 2009, 65, 6150–6518 CrossRef.
- R. J. Long, V. C. Gibson, A. J. P. White and D. J. Williams, Inorg. Chem., 2006, 45, 511–513 CrossRef CAS PubMed.
- R. J. Long, V. C. Gibson and A. J. P. White, Organometallics, 2008, 27, 235–245 CrossRef CAS.
- L. P. He, J. Y. Liu, Y. G. Li, S. R. Liu and Y. S. Li, Macromolecules, 2009, 42, 8566–8570 CrossRef.
- Y. X. Chen and T. J. Marks, Chem. Rev., 2000, 100, 1391–1434 CrossRef PubMed.
- W. Kaminsky, Macromolecules, 2012, 45, 3289–3297 CrossRef.
- D. Ruchatz and G. Fink, Macromolecules, 1998, 31, 4669–4673 CrossRef CAS PubMed.
- D. Ruchatz and G. Fink, Macromolecules, 1998, 31, 4681–4683 CrossRef PubMed.
- A. L. Mcknight and R. M. Waymouth, Macromolecules, 1999, 32, 2816–2825 CrossRef CAS.
- I. Tritto, L. Boggioni, J. C. Jansen, K. Thorshaug, M. C. Sacchi and D. R. Ferro, Macromolecules, 2002, 35, 616–623 CrossRef CAS.
- D. Ruchatz and G. Fink, Macromolecules, 1998, 31, 4674–4680 CrossRef PubMed.
- B. Y. Lee, Y. H. Kim, Y. C. Won, J. W. Han, W. H. Suh, I. S. Lee, Y. K. Chung and K. H. Song, Organometallics, 2002, 21, 1500–1503 CrossRef CAS.
- E. S. Cho, U. G. Joung, B. Y. Lee, H. Lee, Y. W. Park, C. H. Lee and D. M. Shin, Organometallics, 2004, 23, 4693–4699 CrossRef.
- R. Tanaka, I. Kamei, Z. G. Cai, Y. S. Nakayama and T. Shiono, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 685–691 CrossRef CAS.
- A. Provasoli, D. R. Ferro, I. Tritto and L. Boggioni, Macromolecules, 1999, 32, 6697–6706 CrossRef CAS.
- I. Tritto, C. Marestin, L. Boggioni, L. Zetta, A. Provasoli and D. R. Ferro, Macromolecules, 2000, 33, 8931–8944 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1475551 and 1475552. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra11501b |
| This journal is © The Royal Society of Chemistry 2016 |

