Enhanced mechanical properties of olefin block copolymer by adding a quaternary ammonium salt functionalized graphene oxide
Abstract
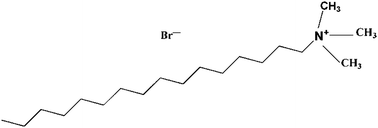
As a promising substitute for traditional thermoplastic elastomers, olefin block copolymers (OBCs) exhibit a marvelous application potential in many fields. To further improve the mechanical properties of OBCs, in this paper, quaternary ammonium salt cetyltrimethyl ammonium bromide (CTAB) was firstly introduced as a modifier for the non-covalent functionalization of graphene oxide (GO), then the modified GO (CTAB-GO) was used as a reinforcing agent for OBCs. With the incorporation of CTAB, a fine dispersion of GO in organic solvent and later in the OBC matrix have been achieved. As a result, the mechanical performance is largely improved, e.g., the increases of tensile strength, elongation at break and Young's modulus are 30%, 13% and 78%, respectively, with 1.0 wt% CTAB-GO incorporation. It is also observed that CTAB-GO exhibits a stronger nucleation ability on crystallization of OBC than pristine GO, which leads to a higher crystallization temperature and smaller size of crystals.

 Please wait while we load your content...
Please wait while we load your content...