Using water-mimic organic compounds to activate guest inclusion by initially dry beta-cyclodextrin†
Abstract
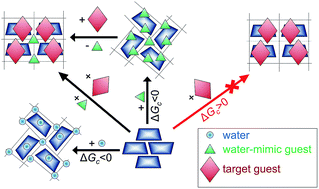
Optimal conditions were found enabling anhydrous beta-cyclodextrin (bCD) to include target guests using small monofunctional organic compounds instead of water. Structural criteria were specified for organic substances with such water-mimic behavior. For this, a thermodynamic description of guest and water inclusion by initially dry bCD in binary systems was given using experimentally determined vapor sorption isotherms. These data perform a cooperative inclusion of each water-mimic guest and water with phase transition, and give the values of inclusion and hydration Gibbs energy, respectively. The observed inclusion cooperativity in binary systems with bCD defines a specific size-exclusion effect banning monofunctional organic compounds from entering the dry bCD phase if they exceed a threshold value of molecular size parameter near that of acetone. For larger guests, this threshold was shown to be removed in ternary systems by simultaneous inclusion with water-mimic guests or by solid-phase exchange of such guests. As well as water, water-mimic organic compounds activate the inclusion of target guests by initially dry bCD just by forming ternary clathrates, thus making all other hypotheses on the role of water and its mimics in this inclusion process excessive. These procedures may be useful for practical purposes when the presence of water does not give good results in clathrate preparation with bCD. This may provide a way for developing new techniques for the preparation of beta-cyclodextrin clathrates with various organic compounds.


 Please wait while we load your content...
Please wait while we load your content...