Exploration of the preventive effect of S. lessoniana liver oil on cardiac markers, hematological patterns and lysosomal hydrolases in isoproterenol-induced myocardial infarction in wistar rats: a novel report
Meivelu Moovendhan*,
Palaniappan Seedevi,
Annaian Shanmugam and
Shanmugam Vairamani
Centre of Advanced Study in Marine Biology, Faculty of Marine Sciences, Annamalai University, Parangipettai 608 502, Tamil Nadu, India. E-mail: somumoo@gmail.com; moovendha85@gmail.com; Tel: +91 9788847517
First published on 28th June 2016
Abstract
The purpose of this study was to explore the in vivo cardioprotective potency of liver (digestive gland) oil from S. lessoniana on isoproterenol induced myocardial infracted wistar rats. Rats received IPH for 2 successive days (85 mg kg−1 body weight) at 24 h intervals to induce myocardial infarction at the end of the experiment. S. lessoniana liver oil was served orally at a dose of 0.05 mL per day for 45 days, then the serum and heart tissue were analysed for CK and LDH enzyme activity, then haematological, lipid profile changes was examined in the blood, histopathological examination was carried in heart tissue and the activity of β-D-glucuronidase, β-D-acetylglucosaminidase, acid phosphatase and cathepsin-D in the serum lysosomal heart fraction. The results of the present study suggested that the pre-treated animals with squid liver oil prevented isoproterenol-induced haematological changes and heart weight increases. Lysosomal membrane integrity was well protected in rats pre-treated with squid liver oil, as indicated by significantly lowered activities of lysosomal hydrolases in the serum and associated increase in their activity in the lysosomal fraction of the heart. The histopathological examination further confirmed that the cardioprotective potency of squid liver oil.
1. Introduction
Myocardial infarction (MI) and coronary heart disease are still the most important causes of death in many developing countries, and it is expected to gain a similar standing in others fairly soon.1 One of the instant effects of coronary occlusion is the severe ventricular rhythm disorders which account for most of the deaths in the hours immediately following an acute myocardial infarction. About 650![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people die each year from coronary heart disease culminating in myocardial infarction worldwide. CVD (Cardio Vascular Disease) in India causes 5 million deaths per year accounting for 25% of all mortality.2 CVD in the US continues to be an important cause of death and accounts for 36% of all deaths.3 The World Health Organization (WHO) predicts that, deaths due to circulatory system diseases to double between 1985 and 2015.4–6
000 people die each year from coronary heart disease culminating in myocardial infarction worldwide. CVD (Cardio Vascular Disease) in India causes 5 million deaths per year accounting for 25% of all mortality.2 CVD in the US continues to be an important cause of death and accounts for 36% of all deaths.3 The World Health Organization (WHO) predicts that, deaths due to circulatory system diseases to double between 1985 and 2015.4–6
While the first AHA (American Heart Association) Science Advisory “Fish consumption, fish oil, lipids, and coronary heart disease”,7 important new findings, including evidence from randomized controlled trials (RCTs) have been reported about the beneficial effects of omega-3 (or n-3) fatty acids on cardiovascular disease (CVD) in patients with pre-existing CVD as well as in healthy individuals.8 Arterial stiffness is associated with metabolic syndrome and is a predictor of cardiovascular events.9 Pulse Wave Velocity (PWV) and Augmentation Index (AIx) are measurements of arterial distensibility.10 Reducing dietary saturated fats and increasing omega-3 polyunsaturated fats (O3), especially from fish (O3), have long been known to improve vascular health and may improve measures of arterial stiffness.11
Lipids and particularly the PUFA have long been known to be essential for the maintenance of good health of any individual. Omega-3 (n-3) long-chain PUFA, including EPA and DHA are dietary fats with an array of health benefits.12 The long-chain omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) of fish oil are recognized to play important roles in reducing the risk of fatal coronary heart disease13–16 and in reducing serum levels of fasting triglycerides.17–20 Frequent intake of long-chain omega-3 fatty acids, namely docosahexaenoic (DHA; 22:6n-3) and eicosapentaenoic (EPA; 20:5n-3) acids through dietary sources, are coupled with decreased cardiovascular mortality. Most of the reputed cardioprotective effects of omega-3 fatty acids are due to their hypotriglyceridemic and anti-inflammatory effects, which reduces the risk for cardiovascular disease and myocardial infarction.21,22
Interests on the effects of fish oil on human health have been accelerated in the current years with the studies carried out by researchers.23 Such studies have reported on the variety of heart diseases was decreased in Greenland Eskimos and their relationships with the consumption of fish oil (high in ω-3 PUFA) and also the fish oil reduced the sudden cardiac death.24 EPA and DHA effectively compete with ω-3 PUFA for cyclooxygenases initiating the synthesis of prostaglandins with changed properties.25 As a marine resource, squids are nutritionally valuable regarding their possession of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), described as having hypotriacylglycerolemic, antiarrhythmic, antiatherogenic, antithrombotic and anti-inflammatory properties. Subsequently, EPA and DHA are considered as protective agents against Coronary Heart Diseases (CHD).26 The present study has been attempted to investigate the in vivo cardioprotective effect of liver oil (extracted from the squid S. lessoniana liver [digestive gland]) on ISO (isoproterenol) induced myocardial injury in male wistar rats which further evaluates the possible usefulness in ameliorating ISO-induced cardiotoxicities.
2. Material and methods
2.1. Extraction of liver oil
The liver of S. lessoniana (digestive gland) was cut into small pieces and dried in hot air oven at 50 °C. The dry powder obtained was used for liver oil extraction by following the method of Folch.27 Then the oil was stored in amber bottles at −20 °C for experiments.2.2. Experimental animals
All experiments were carried out in male albino wistar rats (140–160 g) obtained from the Central Animal House, Rajah Muthiah Institute of Health Sciences (RMMCH), Annamalai University, Tamil Nadu, India. They were housed in polypropylene cages (47 × 34 × 20 cm) lined with husk, renewed every 24 h, under a 12 h light: 12 h dark cycle at around 22 ± 2 °C temperature and had free access to drinking water and food ad libitum. The rats were fed on a standard pellet diet (Parnav Agro Industries Ltd., Maharashtra, India) with 22.02% of crude protein, 4.52% of crude oil, 3.25% of crude fibre, 7.5% of ash, 1.38% of sand silica, 0.8% of calcium, 0.6% of phosphorus, 2.46% of glucose, 1.8% of vitamins and 56.17% of nitrogen free extract (carbohydrates). The diet provided the metabolisable energy of 3000 kcal kg−1. The work was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India and approved by the Institutional Animal Ethical Committee (IAEC) as per the OECD guidelines of Annamalai University (no. 1073; dated 17. 04. 2014).2.3. Drug and chemicals
Isoproterenol obtained from Sigma chemicals (USA) and liver oil extracted from S. lessoniana liver was used. The assay kit for Creatine Kinase (CK) was purchased from COBAS Integra Life Sciences, Mumbai, India. Lactate dehydrogenase (LDH) was also purchased from COBAS Integra Life Sciences, Mumbai, India.2.4. Induction of experimental myocardial infarction
Isoproterenol (ISO) (85 mg kg−1) was dissolved in normal saline and injected subcutaneously to rats at regular interval of 24 h for two days (44th and 45th day) to induce MI in rats.282.5. Experimental design
A total of 24 rats divided into 4 groups of 6 rats in each group were used and dose of ISO and liver oil was fixed by following the previous study.28Group I: control rats: animals were allowed to have free access to a normal diet for 45 days.
Group II: liver oil – treated rats: the animals were treated with liver oil (50 μL per kg per day, p.o.) for 45 days.
Group III: ISO (MI)-induced rats: 85 mg kg−1 of ISO was injected subcutaneously only on 44th and 45th day.
Group IV: liver oil and ISO treated rats: the animals were treated with liver oil (50 μL per kg per day, p.o.) up to 43 days and 85 mg kg−1 of ISO was injected subcutaneously on the 44th and 45th day.
On 45th day (at the end), blood was collected by using capillary pipette through cardiac puncture method. The plasma (after using sodium citrate) and serum was separated and used for further analyses. Then the animals were anesthetized with sodium pentobarbital (65 mg kg−1, i.p.) and sacrificed by cervical decapitation. One rat from each group was used for the histopathological analysis of heart tissue. The heart was removed from the rats, then washed off blood with ice-chilled physiological saline and homogenized in phosphate buffer (pH 6.2). The homogenate was centrifuged and the supernatant was used for the estimation of various biochemical parameters.
The cardiac marker enzymes such as creatine kinase (CK) and lactate dehydrogenase (LDH) were assayed in serum and heart tissue (in triplicate). Other biochemical parameters such as total cholesterol, triglyceride (TG) and HDL were also estimated in both plasma and serum (in triplicate).
2.6. Cardiac marker enzymes
The cardiac marker enzymes such as CK and LDH were estimated in serum and heart tissue using the standard method.2.7. Hematological studies
The following hematological parameters were studied after collecting the blood from the experimental animals: enumeration of red blood corpuscles (RBCs), estimation of hemoglobin, determination of hematocrit value, platelet count, erythrocyte count (ESR), and enumeration of white blood corpuscles (WBCs) total count were done in the Automatic hematology analyzer (COBAS INTEGRA® 400 plus, Roche). The plasma fibrinogen level was estimated following the method of Reinhold.312.8. Assay of lysosomal hydrolysates
The lysosomal fraction of heart was isolated32 and activity of β-D-glucuronidase,33 β-D-acetylglucosaminidase,34 acid phosphatase30 and cathepsin-D35 was determined both in serum and lysosomal fraction of the heart tissue.2.9. Estimation of blood biochemical parameters
2.10. Histopathological studies
A small portion of heart tissue from each experimental group was fixed in 10% formalin. The washed tissue was dehydrated in the ascending grades of isopropanol and finally cleared in xylene. The tissue was then embedded in molten paraffin wax. Sections were cut at 5 μm thickness, stained with haematoxylin and eosin. The sections were then viewed under light microscope (400×) for the histological details and photographs were taken using a CCD camera inbuilt microscope (Motic, India).2.11. Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) using SPSS Software 9.05 followed by Duncan's multiple range test (DMRT). Results were expressed as mean ± S.D. from six rats in each group and triplicate estimates in each rat. P values at <0.05 level were considered as significant.3. Results
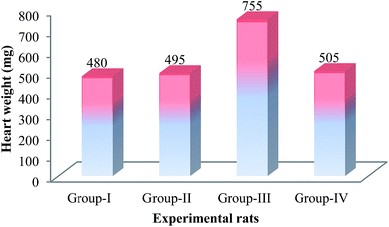
3.1. Effect of liver oil on the heart weight
The effect of S. lessoniana liver oil on heart weight of the experimental rats is shown in Fig. 1. The heart weight of ISO (MI)-induced (Group III) rats was found to be higher (755 mg) when compared to the control (Group I) rats (480 mg). The heart weight of Group II was found to be 485 mg. In Group IV (S. lessoniana oil + ISO treated) rats, the heart weight (505 mg) was lesser when compared to that of the ISO (MI)-alone induced rats.3.2. Effect of liver oil on cardiac marker enzymes
The ISO (85 mg kg−1) (two times at regular intervals) induced group of rats (Group III) showed marked biochemical changes as well as oxidative damage in heart tissue. The effect was measured by determining the activity of CK and LDH in serum and heart tissue (Table 1). These parameters showed a significant increase in ISO-induced MI (Group III) rats with respect to control (Group I) (P < 0.05). Group IV rats reported a close to normal activity for these enzymes. The activity of these marker enzymes in Groups II rats did not reveal any significant changes when compared to that of Group I rats.| S. no. | Enzyme assays (U per mg protein) | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|---|
| a Results are mean SD for triplicate; one way ANOVA; Duncan's multiple comparison test. Values that have a different superscript letter (a, b, c, d) differ significantly (P < 0.05) with each other. Values are expressed as: serum creatine kinase (CK) and lactate dehydrogenase (LDH) IU L−1; tissue LDH mmoles pyruvate liberated per s per mg protein; tissue CK nmoles of phosphorous liberated per s per mg protein. | |||||
| 1 | Serum CK | 290.43 ± 0.35a | 305.10 ± 0.077c | 483.14 ± 0.005b | 316.62 ± 0.023d |
| 2 | Serum LDH | 72.42 ± 0.03a | 86.16 ± 0.28c | 138.16 ± 0.28b | 88.33 ± 0.57d |
| 3 | Heart CK | 191.066 ± 0.11a | 184.16 ± 0.288c | 133.86 ± 0.15b | 175.06 ± 0.11d |
| 4 | Heart LDH | 1.72 ± 0.01a | 1.98 ± 0.005c | 1.31 ± 0.02b | 1.85 ± 0.005d |
3.3. Effect of on hematological changes in experimental rats
The effect of S. lessoniana liver oil + ISO treated and ISO (MI) induced altered the various hematological parameters in the blood of experiments rats (Table 2). The RBC count, hemoglobin content, hematocrit, platelet count, ESR and fibrinogen values and WBC counts were significantly (P < 0.05) increased in ISO (MI)-treated rats when compared to the control. These values in rats treated with S. lessoniana liver oil alone were not significant when compared to control rats. In rats which received S. lessoniana liver oil and ISO (MI) administration, the RBC count, hemoglobin content, hematocrit, platelet count, ESR and fibrinogen content and WBC counts were significantly (P < 0.05) decreased when compared to rats treated with ISO (MI)-induced alone.| Sl. no. | RBCs | Hb | Platelets | HCT | ESR | WBC | Fibrinogen |
|---|---|---|---|---|---|---|---|
| a Results are mean ± SD for triplicate; one way ANOVA; Duncan's multiple comparison test. Values are expressed as: RBC count as million per cumm; hemoglobin (Hb) count as g dL−1 of blood; platelet count is expressed in as ×105 cells per cumm; erythrocytes sedimentation rate (ESR) in blood is expressed as mm h−1; fibrinogen is expressed as mg dL−1 of plasma. | |||||||
| Group I | 5.63 ± 0.461 | 13.66 ± 0.057 | 240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 0.11 000 ± 0.11 |
50 | 3.8 ± 0.31 | 8200 ± 0.47 | 252 ± 0.21 |
| Group II | 6.11 ± 0.028 | 14.13 ± 0.057 | 265![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 0.21 000 ± 0.21 |
51.5 | 4.1 ± 0.31 | 8100 ± 0.29 | 263 ± 0.28 |
| Group III | 9.13 ± 0.057 | 17.86 ± 0.057 | 340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 0.12 000 ± 0.12 |
55 | 7.3 ± 0.52 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ± 0.56 800 ± 0.56 |
342.17 ± 0.15 |
| Group IV | 5.63 ± 0.461 | 13.66 ± 0.057 | 240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 0.11 000 ± 0.11 |
51 | 3.8 ± 0.31 | 8450 ± 0.52 | 252 ± 0.21 |
3.4. Effect of liver oil on lysosomal hydrolysates of heart fraction in experimental rats
The activity of lysosomal hydrolases in serum and lysosomal fraction of heart tissue are presented in Table 3. A significant (P < 0.05) increase in the activity of lysosomal hydrolases in serum, namely β-D-glucuronidase, β-N-acetylglucosaminidase, cathepsin-D and acid phosphatase, was observed in ISO (MI) – induced rats (Group III) when compared to the control rats (Group I). S. lessoniana liver oil and ISO treated rats (Group IV) brought about significant (P < 0.05) reduction in the activity of β-D-glucuronidase, β-N-acetylglucosaminidase, cathepsin-D and acid phosphatase in serum when compared to the rats treated with ISO (MI) alone (Group III). Activity of β-D-glucuronidase, β-N-acetylglucosaminidase, cathepsin-D and acid phosphatase in lysosomal fraction of heart was found to be decreasing significantly (P < 0.05) in ISO induced rats (Group III) when compared to control rats; whereas the activity of β-D-glucuronidase, β-N-acetylglucosaminidase, cathepsin-D and acid phosphatase was significantly (P < 0.05) increased in rats pretreated with S. lessoniana liver oil followed by ISO treated rats (Group IV) when compared to the rats (Group III) treated with ISO alone.| Lysosomal enzymes | Sample | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|---|
| a Results are mean SD for triplicate; one-way ANOVA; Duncan's multiple comparison test. Values that have a different superscript letter (a, b, c, d) differ significantly (P < 0.05) with each other. Values are expressed as: β-D-glucuronidase – nmoles of p-nitro phenol liberated per s per 100 mg protein; β-N-acetyl glucosaminidase – ng of p-nitro phenol liberated per s per 100 mg protein; cathepsin-D nmoles of tyrosine released/s 100 mg protein; acid phosphatase – mmoles of phenol liberated per s per 100 mg protein. | |||||
| β-D-Glucuronidase | Serum | 2.75 ± 0.191 | 3.01 ± 0.21 | 4.20 ± 0.19 | 2.73 ± 0.19 |
| Heart | 12.13 ± 0.63 | 12.13 ± 0.72 | 8.01 ± 0.51 | 11.04 ± 0.51 | |
| β-N-Acetylglucosaminidase | Serum | 5.9 ± 0.181 | 5.71 ± 0.25 | 7.715 ± 0.32 | 5.01 ± 0.18 |
| Heart | 15.1 ± 0.31 | 12.9 ± 0.33 | 10.65 ± 0.32 | 11.76 ± 0.38 | |
| Cathepsin-D | Serum | 5.3 ± 0.39 | 4.013 ± 0.42 | 6.45 ± 0.38 | 5.2 ± 0.29 |
| Heart | 18.13 ± 0.41 | 19.01 ± 0.52 | 14.01 ± 0.42 | 15.50 ± 0.43 | |
| Acid phosphatase | Serum | 2.01 ± 0.34 | 1.36 ± 0.031 | 2.71 ± 0.056 | 1.786 ± 0.06 |
| Heart | 2.210 ± 0.28 | 1.967 ± 0.042 | 1.765 ± 0.041 | 2.103 ± 0.031 | |
3.5. Effect of liver oil on the level of total cholesterol, triglycerides and HDL in plasma on experimental rats
The level of total cholesterol, triglycerides and HDL in plasma on experimental rats. In ISO-induced MI (Group III) rats, the level of plasma cholesterol, triglycerides and HDL increased significantly when compared to that of control (Group I) rats (Fig. 3). In the case of HDL of Group III rats, the level decreased significantly when compared to Group I rats. In Group IV rats, the level of triglycerides and total cholesterol in plasma significantly decreased when compared to Group III rats; whereas the level of HDL significantly increased in Group IV rats when compared to Group III rats.3.6. Effect of liver oil on histopathology of heart in experimental rats
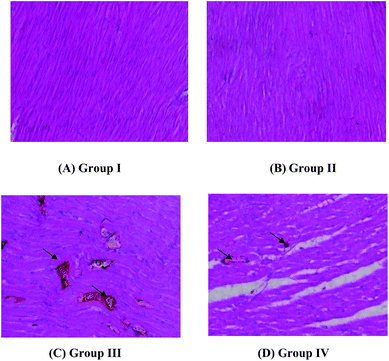
Groups I and II present normal cardiac histology [Fig. 2(A) and (B)]. Fig. 2(C) reveals the destruction and hypertrophy of cardiac muscle fibers. In the photomicrograph, the swollen and flabby muscle fibers of the ISO-induced group (Group III) can be clearly observed. The S. lessoniana liver oil + ISO treated group (Group IV) of rats (Fig. 2(D)) reported less marked changes and showed an almost normal cardiac muscle fiber. The histology of control group showed normal cardiac fibers without any infarction oedema and inflammatory cells. The heart tissue of Group II rats also showed normal cardiac fibers without any infarction, oedema and inflammatory cells. But the Group III rats showed myocardium infarcted zone with (→) oedema and inflammatory cells in the infarcted area and separation of muscle fibres, whereas the minimal histological changes are seen (→) in Group IV rats.4. Discussion
Myocardial infarction (MI) is the condition of cellular injury that results in damage and ultimately necrosis of the myocardium, which occurs as a result of imbalance between coronary blood supply and myocardial demand.38 Cardiovascular diseases are the leading cause of mortality in many parts of the world. Although modern drugs are effective in preventing cardiovascular disorders, their use is often limited because of their side effects.39Several studied reported that the cephalopods liver oil is an non-toxic and rich source of several fatty acids such as PUFA, MUFA and HUFA and it take part an major role in cardiovascular disease prevention as well as several biomedical applications and nutrition supplements.40 Cardioprotective effects of cuttlefish (S. pharaonis) liver oil in isoproterenol induced rats was reported by Sherief41 and reported that the animals fed with 1% liver oil had less incidence of induced heart attack due to the presence of EPA and DHA. In the present study, the liver oil and ISO dose was fixed previously described by Padma28 and saravanan11 for this experiment. In the present study, the cardiac hypertrophy, i.e., enlargement of the heart has been observed in Group III (ISO-induced) treated rats. Decreased heart weight in Group IV rats may remove the stimulus for hypertrophy. Nirmala and Puvanakrishnan42 also reported that the observed increase in the heart weight of ISO-induced rats might be due to the increased water content, oedematous intramuscular space and extensive necrosis of cardiac muscle fibers followed by the invasion of damaged tissue by the inflammatory cells. The increase of heart weight seen in Group III rats can be attributed to the increased protein content. Since the heart weight was found increasing in the ISO-induced group of rats, the heart weight: body weight ratio increased because the body weight in ISO-induced rats remains unchanged.43 Yogeeta44 observed the heart weight in rats pre-co-treated with ferulic acid and ascorbic acid could remove the stimulus induced by ISO for hypertrophy. Adkins45 reported that the cardioprotective effect of ω-3 PUFA results from a synergism between multiple, intricate mechanism that involve anti-inflammation, proresolving lipid mediators, modulation of cardiac ion channels, reduction of triglycerides, influence on membrane microdomains and downstream cell signaling pathways, anti-thrombotic and anti-arrhythmic effects. Similarly Micallef and Garg46 pointed out the anti-inflammatory and cardioprotective effects of Ω-3 polyunsaturated fatty acids. The results of the present study also reported the cardio-protective effect of S. lessoniana liver oil.
The results of the present study reveal that the administration of ISO (two times at interval of 24 hours in the last two days [44th and 45th day] of the experiment) increased the level of serum cardiotoxicity indices such as CK and LDH which might be due to their release from the damaged heart tissue into the blood stream as also noticed in the earlier studies.47,48 Geetha49 observed a positive correlation between the concentration of enzymes and the number of necrotic cells present in the cardiac tissue. A rise in all this enzymes content accompanying heart failure has been reported earlier Saravanan.50 The present study clearly reports the severe myocardial damage induced by ISO which is indicated by diminished CK activity in the cardiac tissue of Group III treated rats. The reduced necrotic changes in S. lessoniana liver oil-treated animals could be the reason for the decreased activities of the marker enzymes, namely CK and LDH in Group IV rats (treated oil + ISO). The findings of this study further substantiate the favorable action exerted by S. lessoniana liver oil in curtailing inflammatory sequelae that disturb the normal cellular enzymatic machinery.
Based on the close relationship between cardiovascular risk factors and hemorheology,51 it has been postulated that both atherogenesis and blood rheology might have some common denominator. An increase in blood viscosity occurs with acute myocardial infarction (AMI), which is attributable to hemoconcentration. During AMI there is an increase in the number of erythrocytes, hemoglobin content, reticulocyte quantity, hematocrit indices, mass of circulating erythrocytes and intensity of erythropoiesis.52 The significant increase in RBC, hemoglobin content and hematocrit values following ISO-induced when compared to control is in accordance with52 during MI. The treatment with S. lessoniana liver oil in ISO-induced rats resulted in a significant decrease in red blood cell count, hemoglobin content, platelet count and hematocrit values, compared to rats treated with ISO induced alone. But Padma and Devi43 also reported a significant increase in RBC, WBC, platelet count, hemoglobin content and hematocrit values in ISO-induced (MI) rats when compared to control rats and in fish oil treated rats; they observed that all the above factors were similar to that of the control rats. Thus the results of the present study revealed that the S. lessoniana liver oil reduced the blood viscosity by decreasing the RBC count, hemoglobin content, etc.
Lysosomal enzymes are important mediators of myocardial infarction (MI) and their release into the cytoplasm stimulates the formation of inflammatory mediators such as oxygen radicals and prostaglandins. As lysosomal hydrolases have been implicated in the propagation of the cellular injury during the early stages of MI, this action may be crucial in protecting the heart against ischemic damage. The results of the present study showed an increase in the activity of lysosomal enzymes (β-D-glucuronidase, β-N-acetylglucosaminidase and cathepsin-D and acid phosphatase) in serum with a concomitant decrease in the activity of β-D-glucuronidase, β-D-N-acetylglucosaminidase, acid phosphatase and cathepsin-D in the lysosomal fraction of heart in the ISO-induced rats. Whereas the Group IV rats (oil + ISO-induced) showed a decrease in the activity of β-D-glucuronidase, β-D-N-acetylglucosaminidase, acid phosphatase and cathepsin-D in serum and correspondingly the above lysosomal enzyme activity were found higher similar to that of control. The results of the present study are found consistent with the previous report by Sathish48 i.e., the oral administration of nicorandil (2.5 mg kg−1) and amlodipine (5.0 mg kg−1) significantly decreased the activity of lysosomal enzymes in serum and significantly increased the activities of β-D-glucuronidase and cathepsin-D in lysosomal fraction of ISO-induced rats. This might be due to inhibiting the release of lysosomal enzymes by decreasing membrane damage, thereby enhancing the stability of lysosomes.53 Similarly Rajadurai and Prince54 reported that the activity of lysosomal enzymes (β-glucuronidase, β-D-glucosamidase, cathepsin-B and cathepsin-D) were significantly increased in serum and heart of ISO-induced rats, but the activity of β-glucuronidase and cathepsin-D were decreased significantly in the lysosomal fraction of the heart. In the study of Sherief41 was proved the cardioprotective effect of cuttlefish liver oil in isoproterenol induced the myocardial infracted rats and the research team strongly revealed that the DHA and EPA of the cuttlefish liver oil is responsible for cardioprotective effect through the cardiac marker enzymes activity and the same cardiac markers activity was noticed in fish oil treated rats experiment was done by Padma.28
In the present study, a significant increase in the level of total cholesterol and triglycerides in plasma was observed in Group III rats than in Group II rats, these changes could be due to enhanced lipid biosynthesis by cardiac cAMP and an as also observed by He55 who reported that rats fed with fish oil diet showed significant elevations in aortic and plasma cholesterol and that increase in aortic inversely correlates with plasma HDL levels. The Group III (ISO-induced) rats showed increase in the plasma lipoprotein level; this was restored by Group IV (S. lessoniana liver oil + ISO) rats. Isoproterenol enhances the free radical formation, which may cause the cellular cholesterol deposition through increasing cholesterol biosynthesis, by decreasing cholesteryl ester hydrolysis and by reducing cholesterol efflux.56
The results of the present study is similar to that of Saranya56 who reported the high level of total cholesterol and triglycerides in ISO-induced rats and decrease in the HDL level in blood plasma and the above parameters showed an inverse relationship in rutin and ellagic acid + ISO-induced rats. In the present study, the cardiac toxicity induced by ISO is further confirmed by the abnormal histology in heart tissues of rat. Cardiac muscle hypertrophy is obvious in Group III rats where the muscle fibers are swollen and flabby in structure due to the toxicity of the ISO. Toxicity manifestation in the heart cells is revealed by the crowding cardiocytes with vascular congestion, suggestive of congestive heart failure. In S. lessoniana liver oil + ISO-induced (Group IV) rats, the histology showed lesser damage which is near similar to that of the normal rats when compared to ISO-induced rats (Group III). Saravanan50 reported an aberrant cardiac histology after ISO treatments, where in they observed marked oedema, swelling of myocardial fibres in the heart section. Similarly Barwin Vino57 noted the same above histological details mentioned above in ISO-induced and mucopolysaccharide treated + ISO-induced rats.
5. Conclusions
In the present study demonstrate the ability of S. lessoniana liver oil to modulate hematological parameters and maintain lysosomal membrane integrity, thereby offering multifaceted protection to the heart and it has the ability to prevent several heart diseases also.Conflict of interest
The authors declare no conflict of interest.Acknowledgements
Authors are thankful to the Dean and Director, CAS in Marine Biology, Faculty of Marine Sciences, Annamalai University for providing all necessary facilities. Two authors (M. M. and A. S.) are also thankful to Centre for Marine Living Resources and Ecology (CMLRE), Ministry of Earth Sciences, Kochi for financial assistance.References
- S. Padmavati, Prevention of heart disease in India in the 21st century: Need for a concerted effort, N. Engl. J. Med., 2004, 350, 2438–2440 CrossRef PubMed.
- R. Tanuja, R. Srinath, S. Mario Vaz, D. Donna, C. W. Prabhakaran, J. S. Walter and A. Alberto, Diet and risk of ischaemic heart disease in India, Am. J. Clin. Nutr., 2004, 79, 582–592 Search PubMed.
- NHLBI, Fact Book Fiscal Year 2008, National Institutes of Health, National Heart, Lung, and Blood Institute, 2009, available from: http://www.nhlbi.nih.gov/about/ Search PubMed.
- R. A. O. Bulatao and P. W. Stephens, Demographic estimates and projections, by region, 1970–2015, in Disease control priorities in developing countries, ed. D. T. Jamison and W. H. Mosley, World Bank, Washington, DC, 1990 Search PubMed.
- K. S. Reddy, Cardiovascular disease in India, World Health Stat., 1993, 46, 101–107 CAS.
- K. S. Reddy and S. Yusuf, Emerging epidemic of cardiovascular disease in developing countries, Circulation, 1998, 97, 596–601 CrossRef CAS PubMed.
- N. J. Stone, Fish consumption, fish oil, lipids, and coronary heart disease, Circulation, 1996, 94, 2337–2340 CrossRef CAS PubMed.
- R. M. Krauss, R. H. Eckel and B. Howard, AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association, Circulation, 2000, 102, 2284–2299 CrossRef CAS PubMed.
- M. S. Ahn, J. Y. Kim, Y. J. Youn, S. Y. Kim, S. B. Koh, K. Lee, B. S. Yoo, S. H. Lee, J. Yoon, J. K. Park and K. H. Choe, Cardiovascular parameters correlated with metabolic syndrome in a rural community Cohort of Korea: The Arirang Study, J. Med. Sci., 2010, 25, 1045–1052 CAS.
- G. F. Mitchell, Arterial stiffness and wave reflection: Biomarkers of cardiovascular risk, Artery Res., 2009, 3, 56–64 CrossRef PubMed.
- M. F. Chong, S. Lockyer, C. J. Saunders and J. A. Lovegrove, Long chain n-3 PUFArich meal reduced postprandial measures of arterial stiffness, Clin. Nutr., 2009, 29, 678–681 CrossRef PubMed.
- L. Su, M. Wang, S. T. Yin, H. L. L. Wang, L. Chen, G. Sun and D. Y. Ruan, The interaction of selenium and mercury in the accumulations and oxidative stress of rat tissues, Ecotoxicol. Environ. Saf., 2008, 70, 483–489 CrossRef CAS PubMed.
- P. M. Kris-Etherton, W. S. Harris and L. J. Appel, Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease, Circulation, 2002, 106, 2747–2757 CrossRef PubMed.
- K. He, Y. Song, M. L. Daviglus, K. Liu, L. Van Horn, A. R. Dyer and P. Greenland, Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies, Circulation, 2004, 109, 2705–2711 CrossRef PubMed.
- D. Mozaffarian and E. B. Rimm, Fish intake, contaminants, and human health: evaluating the risks and the benefits, J. Am. Med. Assoc., 2006, 296, 1885–1899 CrossRef CAS PubMed.
- K. Musa Veloso, Impact of low v. moderate intakes of long-chain n-3 fatty acids on risk of coronary heart disease, Br. J. Nutr., 2011, 106, 1129–1141 CrossRef CAS PubMed.
- E. M. Balk, M. Asad and P. K. Nanjundan, Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review, Atherosclerosis, 2006, 189, 19–30 CrossRef CAS PubMed.
- A. C. Skulas Ray, J. P. Vanden Heuvel, P. R. Wagner and S. G. West, Omega-3 fatty acid concentrates in the treatment of moderate hypertriglyceridemia, Expert. Opin. Pharmacother., 2008, 9, 1237–1248 CrossRef CAS PubMed.
- EFSA, EFSA Panel on Dietetic Products. European Food Safety Authority, EFSA J., 2009, 7, 26 Search PubMed.
- K. Musa Veloso, Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid dose-dependently reduce fasting serum triglycerides, Nutr. Rev., 2010, 68, 155–167 CrossRef PubMed.
- A. Grey and M. Bolland, Clinical trial evidence and use of fish oil supplements, JAMA Intern. Med., 2013, 210, 1–4 Search PubMed.
- D. F. Richard, C. Oszust, H. Guillaume, D. Millart, C. Laurent Maquin, P. Broud, F. Bausero and F. Visioli, Infusion of docosahexaenoic acid protects against myocardial infarction, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2014, 90, 139–143 CrossRef CAS PubMed.
- J. Dyerberg, H. O. Bang and N. Hjorne, Fatty acid composition of the plasma lipids in Greenland Eskimos, Am. J. Clin. Nutr., 1975, 28, 958–966 CAS.
- R. B. Shekelle, L. Missell and O. Paul, Fish consumption and mortality from coronary heart disease, N. Engl. J. Med., 1985, 313, 814–820 Search PubMed.
- K. A. Jorgensen and J. Dyerberg, Platelets and atherosclerosis, Adv. Nutr. Res., 1983, 5, 57–75 CAS.
- V. Wijendran and K. C. Hayes, Dietary n-6 and n-3 fatty acid balance and cardiovascular health, Annu. Rev. Nutr., 2004, 24, 597–615 CrossRef CAS PubMed.
- J. Folch, M. Lees and G. H. S. Stanley, Simple method for isolation and purification of total lipids from animal tissues, J. Biol. Chem., 1957, 226, 497–507 CAS.
- V. V. Padma and C. S. S. Devi, Cardioprotective effect of fish oil on isoproterenol induced myocardial infarction in rats, J. Food Lipids, 2009, 16, 19–32 CrossRef CAS.
- S. Okinaka, H. Kumogai, S. Ebashi, H. Sugitha, H. Momoi and Y. Toyokura, Serum creatine phosphokinase activity in progressive muscular dystrophy and neuromuscular diseases, Arch. Neurol., 1961, 4, 520–525 CrossRef CAS PubMed.
- J. King, The Dehydrogenases or Oxidoreductases, Lactate Dehydrogenase, Practical Clinical Enzymology, Van Nostrand, Co. Ltd., London, UK, 1965, pp. 83–93 Search PubMed.
- J. G. Reinhold, Standard methods in clinical chemistry, ed. M. Reiner, Academic Press, New York, 1953, p. 88 Search PubMed.
- R. Wattiaux, Mammalian Cell Membranes, ed. G. A. Jamiesson and D. M. Robinson, Butterworth 2, London, UK, 1977, pp. 165–166 Search PubMed.
- B. Hultberg, J. Linsten and S. Sjoblad, Molecular form and activities of glycosidase in culture amniotic fluid cell, Biochem. J., 1976, 55, 599–605 CrossRef.
- J. C. Moore and J. E. Morris, A simple automated colorimetric method for the determination of N-acetyl glucosaminidase, Ann. Clin. Biochem., 1982, 19, 157–159 CrossRef CAS PubMed.
- A. I. Sapolsky, R. D. Altman and D. S. Howell, Cathepsin D activity in normal and osteoarthritic human cartilage, Fed. Proc., 1973, 32, 1489–1493 CAS.
- A. Zlatkis, B. Zak and G. J. Boyle, A simple method for determination of serum cholesterol, J. Clin. Med., 1953, 41, 486–492 CAS.
- L. B. Foster and R. T. Dunn, Stable reagents for determination of serum triglycerides by a calorimetric Hantzsch condensation method, Clin. Chem., 1973, 196, 336–340 Search PubMed.
- J. Wang, H. Bo, X. Meng, Y. Wu, Y. Bao and Y. Li, A simple and fast experimental model of myocardial infarction in the mouse, Tex. Heart Inst. J., 2006, 33, 290–293 Search PubMed.
- M. Rajadurai and P. S. M. Prince, Preventive effect of naringin on lipid peroxides and antioxidants in Isoproterenol-induced cardiotoxicity in wistar rats: biochemical and histopathological evidences, Toxicology, 2006, 228, 259–268 CrossRef CAS PubMed.
- J. R. Nair, D. Pillai, S. M. Joseph, P. Gomathi, P. V. Senan and P. M. Sherief, Cephalopod research and bioactive substances, Indian J. Geo-Mar. Sci., 2011, 40(1), 13–27 CAS.
- P. M. Sherief, S. M. Joseph, J. R. Nair and M. C. George, Cardio protective effect of cuttle fish liver oil in isoproterenol administered rats, International symposium on Atherosclerosis, University of Kerala, Trivandrum, 2004, p. 26 Search PubMed.
- C. Nirmala and R. Puvanakrishnan, Effect of curcumin on certain lysosomal hydrolases in Isoproterenol induced myocardial infarction in rats, Biochem. Pharmacol., 1996, 51, 47–51 CrossRef CAS PubMed.
- V. V. Padma, C. S. Shyamala Devi and K. M. Ramkumar, Effect of fish oil pretreatment on isoproterenol-induced changes in myocardial membrane phospholipids, Nutrition, 2006, 22, 1171–1176 CrossRef PubMed.
- S. K. Yogeeta, A. Gnanapragasam, S. Senthilkumar, R. Subhashini and T. Devaki, Synergistic salubrious effect of ferulic acid and ascorbic acid on membrane bound phosphatases and lysosomal hydrolases during experimental myocardial infarction in rats, Life Sci., 2006, 80, 258–263 CrossRef CAS PubMed.
- Y. Adkins and D. S. Kelley, Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids, J. Nutr. Biochem., 2010, 21, 781–792 CrossRef CAS PubMed.
- M. A. Micallef and M. L. Garg, Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals, Atherosclerosis, 2009, 204, 476–482 CrossRef CAS PubMed.
- A. P. Ithayarasi, V. N. Padmavathy and C. S. Devi, Effect of alpha-tocopherol on isoproterenol induced myocardial infarction in rats-electrocardiographic biochemical and histological evidences, Indian J. Physiol. Pharmacol., 1996, 40(4), 297–302 CAS.
- V. Sathish, K. K. Ebenezar and T. Devaki, Synergistic effect of nicorandil and amlodipine on lysosomal hydrolases during experimental myocardial infarction in rats, Biomed. Pharmacother., 2003, 57, 309–313 CrossRef CAS.
- A. Geetha, R. Sanker, T. Manar and C. S. Shyamala Devi, Alpha Tocopherol reduces doxorubicin induced toxicity in rats, histological and biochemical evidence, Indian J. Physiol. Pharmacol., 1990, 34, 94–100 CAS.
- R. Saravanan, Bioactive compounds from marine bivalve mollusc (Isolation, Purification, characterization & anticoagulant activity of glycosaminoglycans and heparin sulfate from marine scallop, Amussium pleuronectus) and its cardioprotective effect on Isoproterenol-induced myocardial infarction in male wistar rats, Ph.D. thesis, Annamalai University, India, 2010, pp. 1–165 Search PubMed.
- E. Ernst, T. Weihmayr, M. Schmid, M. Baumann and A. Matria, Cardiovascular risk factors and hemorheology, Atherosclerosis, 1986, 59, 263–269 CrossRef CAS PubMed.
- A. N. Tarasov, Changes of the red blood indicators in the acute period of myocardial infarct, Kardiol. Pol., 1976, 16, 129–131 CAS.
- S. J. Roy and P. S. M. Prince, Protective effects of sinapic acid on lysosomal dysfunction in Isoproterenol induced myocardial infarcted rats, Food Chem. Toxicol., 2012, 50, 3984–3989 CrossRef CAS PubMed.
- M. Rajadurai and S. M. Prince, Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats, Toxicology, 2007, 12(2–3), 178–188 CrossRef PubMed.
- K. He, Fish, long-chain omega-3 polyunsaturated fatty acids and prevention of cardiovascular disease eat fish or take fish oil supplement, Prog. Cardiovasc. Dis., 2009, 52, 95–114 CrossRef CAS PubMed.
- P. Saranya, R. Rajesh Kumar and M. Rajadurai, Protective effect of rutin and ellagic acid on isoproterenol-induced myocardial infarction in wistar rats, J. Environ. Appl. Biores., 2012, 67, 12–14 Search PubMed.
- A. Barwin Vino, Bioactive compounds from cephalopod mollusc (isolation, characterization and in vivo antioxidant activity of glycosaminoglycans from cuttlefish Sepia brevimana Steenstrup, 1875 and its cardioprotective effect on isoproterenol-induced myocardial infarction in male wistar rats), Ph.D. thesis, Annamalai University, India, 2010, pp. 130–181 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |