Easy-to-fill asymmetric polymeric micro-reservoirs
Vincenzo Contaldia,
Maria Giovanna Pastore Carbonebc,
Ernesto Di Maio*b,
Anastasios C. Manikas*cd and
Paolo Antonio Nettibd
aCRdC Tecnologie Scarl, Via Nuova Agnano 11, 80125 Napoli, Italy
bDipartimento di Ingegneria Chimica, dei Materiali e della Produzione Industriale, School of Engineering, University of Naples Federico II, P.le Tecchio 80, 80125 Naples, Italy. E-mail: edimaio@unina.it
cInstitute of Chemical Engineering, Foundation for Research and Technology Hellas (ICEHT/FORTH), Stadiou St., Platani, 26504, Patras, Greece. E-mail: a.manikas@iceht.forth.gr
dCenter for Advanced Biomaterials for Healthcare@CRIB, Istituto Italiano di Tecnologia (IIT), Largo Barsanti e Matteucci 53, 80125 Naples, Italy
First published on 28th June 2016
Abstract
In this work, we demonstrate the feasibility of micrometric asymmetric reservoirs made of thermoplastic polymers by using the gas foaming method, which has been recently introduced, and consists in forming bubbles in micro- or nano-metric bulk particles as is done with carbonated drinks. As this simplicity anticipates, this represents a breakthrough in the area of micro- and nanoparticles as it responds to the needs of: (i) breaking the symmetry of commonly available systems, (ii) filling the particles with a multitude of host molecules and solutions, (iii) having different shapes, (iv) having a wide range of particle dimensions, (v) having particles made of a wide range of materials. Here we report the achievement of micrometric spherical particles and of micrometric ellipsoidal particles with eccentric holes, filled with crystal violet or with quantum dots as model host molecules. Raman spectroscopy and optical and electron imaging are utilized to verify the effectiveness of the method. This study should open up the use of micro- and nano-metric reservoirs in a multitude of research areas, from biomedicine and pharmacology, to electronics, energy and optics.
1. Introduction
The fabrication of monodisperse hollow particles with controllable size and shape is currently one of the fastest growing areas of materials research. Actually, hollow nano- and micro-particles (respectively, NPs and MPs) are of great technological importance for their potential applications in catalysis, chromatography, pigments/coatings, waste removal, protection of biologically active agents, and drug delivery systems (DDSs).1–11 The chemical nature, the dimensions of the particle and the hole, the thickness and eventual aperture of the material confining the hole, all play a major role in defining the properties of these small objects, more recently engineered in ingenious, creative, sometimes exotic ways to develop into smart, active devices.12 For instance, the void space in hollow particles has been used to modulate refractive index, lower density, increase active area for catalysis, improve the particles' ability to withstand cyclic changes in volume, and to expand the assortment of imaging markers suitable for the early detection of cancer.13 Likewise, the large fraction of void space in the hollow structures has been successfully used to encapsulate and control release of sensitive materials such as drugs, cosmetics and DNA, which should be specifically targeted to particular subsets of cells.14By using optimized encapsulation technologies, the efficacy of therapeutics can be increased manyfold. Using polymeric carriers for the encapsulation of drugs has emerged as workable solution to attain both poor bio-distribution and stability of bare therapeutics.15 In fact, a large number of choices in the polymeric designs offer a direct route to optimal carrier design. In particular, the physical and chemical attributes of the polymeric carriers play a crucial role in navigating the biological barriers and hence determining the overall success of the therapy. Amongst these attributes, the shape of the carrier has been recently identified as one of the key factors that influence important biological processes, including bio-distribution and cellular uptake, in drug delivery applications. The ability to modulate these vital processes via manipulation of carrier shape has opened up new avenues such as engineering of carriers that can evade phagocytosis16 or development of long-circulating carriers.17 Carriers with shapes deviating from the conventional sphere have been identified to possess distinct pharmacokinetic properties which may be more favourable towards the therapeutic intent. The potential therapeutic benefits of using non-spherical drug delivery systems were clearly illustrated through the work of Discher and coworkers.17
The last two decades have witnessed the development of a plethora of DDSs for transporting therapeutic agents to lesion sites, micro/nano-capsules including organic,18–20 inorganic21,22 and inorganic/organic2,23 hybrid ones, presenting different sizes ranging from a few tens of nanometers up to few microns, and shape varying from the classical spherical to discoidal, hemispherical, cylindrical and conical.24 For instance, Champion et al.24 reviewed the effect of particle shape in drug delivery, evidencing both an effect on degradation and delivery profile, and on particle movement in the blood vessel, airways and intestine. Authors also evidenced that the shape of particles does influence their targeting ability and longevity: in fact, internalization (e.g. phagocytosis) of targeted particles, whether deliberated or not, may also be dictated by particle shape. It is worth mentioning that the reported studies were all performed on bulk particles (without any hollow).
Due to the great attractive of hollow MPs and NPs, a variety of chemical and physicochemical methods, such as heterophase polymerization combined with a sol–gel process,25–27 emulsion/interfacial polymerization,28–30 self-assembly techniques,31–33 spray-drying methods34,35 and surface living polymerization processes36–38 have been adopted to prepare polymeric or inorganic hollow spheres. Because the hollow particles produced as by the aforementioned techniques are all characterized by a completely closed shell structure, the molecules to be encapsulated can be only introduced into the capsules by diffusing through the shell, which is usually a slow and/or ineffective process. To solve this problem, some researchers have recently demonstrated the preparation of a new class of capsules, polymeric hollow particles with intended and controllable holes in their surfaces, so-called “fish bowls”.39–47 In that case, the functional material to be encapsulated can be directly loaded through the opening, which can be subsequently closed through a thermal or solvent-induced annealing process.42,46,47 However, a detailed analysis of the literature reveals that the direct loading of the host molecule in the hollow particles which can subsequently be closed is limited only to spherical particles. Producing non-spherical reservoirs by opening-loading and then closing the hole still remains an open issue. This is of paramount importance especially in drug delivery techniques since, as stated before, the shape effect is a fundamental aspect. Some of the authors have recently introduced a novel method to produce hollow micro- or nano-metric particles, of a large variety of materials, dimensions, shapes and hollow attributes, by using an environmentally friendly and cheap technology, which is very common in polymer processing and known as gas foaming. By using this technique, hollow polystyrene (PS) and poly(lactic-co-glycolic) acid particles 100 μm to 100 nm in size, spherical, ellipsoidal and discoidal in shape, were produced thus obtaining open or closed, single or multiple, variable in size hollows (Fig. 1). In comparison with available methods to produce hollow MPs and NPs, the method proposed by Orsi et al.48 offers independent control over dimension, material and shape of the particles, and number, shape and open/closed feature of the hollows. This is obtained by disjoining the particle formation stage from the hollow formation stage. In this approach, in fact, it is possible to first treat the bulk, spherical particles to confer a desired shape (ellipsoidal, discoidal, worm-like, etc.) by mechanical manipulation16 and subsequently to form the hollow by gas foaming. It is worth of note, here, that fine-tuning the gas foaming process (selection of the blowing agent mixture, of the foaming temperature and pressure, to mention the most effective process parameter) allows for the achievement of desired hollow features, such as number, shape, dimension, eccentricity (position within the particle) and open/closed. As a non-trivial consequence, asymmetric hollow particles, whose need has recently emerged in a number of technological and scientific fields, are at hand.49–52 A limit of the proposed method, however, is the stochastic nature of the foaming process and the inevitable wider distribution of results, with respect to the aforementioned common methods to obtain hollow particles. Nevertheless, when needed, sorting by means of available methods based on e.g. microfluidics53,54 could effectively be used.
 | ||
| Fig. 1 Hollow particle shapes achieved by treating the bulk spherical particle with the gas foaming method introduced by Orsi et al.48 | ||
Here we report on the fabrication of spherical and ellipsoidal micro-reservoirs (MRs) based on the gas foaming technique combined with solvent treatment and thermal annealing for the host molecule encapsulation, as referred by Im et al.42 In this work, the foregoing methodology was applied, as proof of principle, to commercial, 5 μm-PS bulk spheres in order to verify the encapsulation efficiency of PS MRs. As host molecules in this study, we used crystal violet (CV) and quantum dots (QD). The encapsulation efficiency in PS particles of different shapes was proved using a variety of spectroscopic techniques like Raman spectroscopy, Scanning Electron Microscopy/energy-dispersive X-ray spectroscopy (SEM/EDS), fluorescence microscopy. The novelty of this procedure is that, for the first time, asymmetric polymeric reservoirs, filled with a desirable host molecule, were fabricated. This fact represents a breakthrough in the production of micro-reservoirs and opens new avenues in the drug delivery research field.
2. Experimental
Preparation of PS MRs
PS spherical particles (5 μm in diameter, ThermoScientific) were initially embedded in a PVA barrier film (Mowiol 40-88, Sigma-Aldrich). In particular, an aqueous solution containing PS particles (0.5% wt/vol) was added to an aqueous solution of PVA (5% wt/vol) and of glycerol (2% wt/vol), which had been previously heated at 40 °C and slowly stirred. The obtained mixture was then slowly stirred for 24 h and subsequently dried in a Teflon mold at room temperature for three days, in order to obtain a PS-embedded PVA film. Ellipsoidal PS particles were obtained following the method proposed by Mitragotri:24 strips of the PS embedded PVA film were mounted on a dynamometer (SANS 4304, China) equipped with an environmental chamber and then uniaxially stretched at 110 °C (e.g., above the glass transition temperature of PS) at a rate of 5 mm min−1. In particular, the final particles aspect ratio was modulated by applying a given stretching ratio. The as-prepared film was then processed in the foaming apparatus designed by Marrazzo et al.55 In a typical foaming experiment, the PS-embedded PVA films were loaded into a thermo-regulated pressure vessel, having a volume of 0.3 L, pressurized with the blowing agent at 16.0 MPa and 100 °C for three hours and pressure quenched at 75 MPa s−1. The pressure discharge system consists of a discharge valve (model 15-71 NFB, HiP Erie, US-PA), an electromechanical actuator (model 15-72 NFB TSR8, HiP Erie, US-PA) and an electro-valve. The pressure history was registered by using a data acquisition system (DAQ PCI6036E, National Instruments, US-TX) and a pressure transducer (model P943, Schaevitz Measurement Specialties, Hampton, US-VA). Open hollow MPs were thus recovered by dissolving the PVA film in deionized water at room temperature and then washed five times by sonicating in an ultrasonic cleaning bath for 20 min and by successively centrifuging at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 40 min. In order to fill and close the holes formed in the PS particles, a procedure based on solvent treatment and thermal annealing has been performed, as suggested by Im et al.42 According to this modified procedure, the open hollow PS MPs were dispersed in a water/toluene (1–5%) host molecule solution. In particular, crystal violet (CV) and CdSe quantum dots (CdSe-QD) have been adopted as host molecules. The mixture was then heated at 70 °C for two hours for both spherical particles and ellipsoidal ones, in order to ensure closing process. Finally, toluene was removed by washing and centrifuging the closed hollow particles three times, as already performed during the particle recovering process. The as-prepared micro-reservoirs were characterized by Scanning Electron Microscopy and energy dispersive X-ray spectroscopy or SEM/EDS, optical microscopy and Raman spectroscopy and fluorescence spectroscopy.
000 rpm for 40 min. In order to fill and close the holes formed in the PS particles, a procedure based on solvent treatment and thermal annealing has been performed, as suggested by Im et al.42 According to this modified procedure, the open hollow PS MPs were dispersed in a water/toluene (1–5%) host molecule solution. In particular, crystal violet (CV) and CdSe quantum dots (CdSe-QD) have been adopted as host molecules. The mixture was then heated at 70 °C for two hours for both spherical particles and ellipsoidal ones, in order to ensure closing process. Finally, toluene was removed by washing and centrifuging the closed hollow particles three times, as already performed during the particle recovering process. The as-prepared micro-reservoirs were characterized by Scanning Electron Microscopy and energy dispersive X-ray spectroscopy or SEM/EDS, optical microscopy and Raman spectroscopy and fluorescence spectroscopy.
Raman spectroscopy
The Raman spectra were excited with a diode laser nm operating at 532 nm. A 100×/0.25 LWD objective was used to focus the laser beam into the well plate. The Raman spectra were acquired with a DXR Raman spectrometer from Thermofischer Scientific with 5.0 mW laser power. The acquisition time for our experiments was 10 s. The Rayleigh line was removed from the collected Raman scattering using a holographic notch filter in the collection path. Raman images were obtained using a Raman point-mapping method with a 100× objective lens. A computer-controlled x–y translational stage was scanned in 8 μm × 7 μm area with a step size of 500 nm.Scanning electron microscopy
SEM experiments for both images and elemental analysis, experiments were performed with a field emission gun scanning electron microscope (FEG-SEM; Carl Zeiss Ultra plus) while PS particles were coated with gold (Cressington 208HR, high resolution sputter coater). The elemental analysis involves the interaction of the primary beam with atoms in the sample, which causes shell transitions that result in the emission of an X-ray. The emitted X-ray has an energy characteristic of the parent element. Detection and measurement of the energy permits elemental analysis (energy dispersive X-ray spectroscopy or EDS).Fluorescence spectroscopy
Fluorescence images were acquired with an Olympus IX81 inverted fluorescence microscope equipped with a 100× objective (Olympus, Tokyo, Japan). The samples were analyzed by using a 592 nm depletion laser, and detecting the emitted fluorescence between 480 and 550 nm.3. Results and discussion
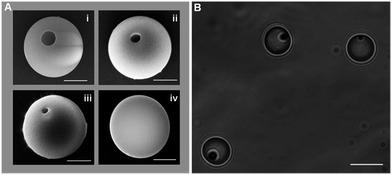
The method of producing hollows in MPs by gas foaming described in Orsi et al.48 has proved suitable for achieving particles of a large variety of materials, dimensions, shapes and hollow attributes, as it is shown in Fig. 1. In the reported process, in particular, gas bubble form at the periphery of the particle (due to heterogeneous bubble nucleation at the particle surface) and, as a result, the porosity is eccentric, leading to asymmetrical hollow particles. In the present contribution, we move on the development of the method to achieve non-symmetric MRs filled with a desirable host molecule. To do so, we introduced the filling and the closing steps in the sequences of operations involved in the method. Despite the stochastic nature of foaming (it is impossible to know where bubbles will be located when opening a carbonated drink), a wide literature on polymer foaming has consolidated a vast knowledge on the effect of processing variables on the final foam density and morphology.56 In our case, in our tiny samples, we actually observed same effects of the processing variables on the particle size, hole position and dimension,60 proving both the versatility and the robustness of the present approach. In this paper, we will focus on one particle size and a single foaming condition giving a hole shape suitable for being subsequently filled and closed. Fig. 2 reports the whole manufacturing sequence, by starting from the bulk (without any hollow), spherical MPs, ending to the spherical or ellipsoidal asymmetric MRs. In particular, PS spherical bulk MPs, readily available in the market of several dimensions, or easily produced in the lab, were embedded in a poly vinyl alcohol (PVA) film (by drying the mixture of a PVA/water solution and MPs/water emulsion) to form a MPs-embedded PVA composite film. This film, as it has been reported,48 permits easy handling and, most importantly, stress transfer to the particle thus allowing to modify particle shape24 (see Fig. 2). For instance, by uniaxially stretching the composite film above the glass transition temperature of PS, ellipsoidal particles are easily obtained. Foaming is then performed on the composite film containing the spherical or deformed MPs, by solubilization of a blowing agent at high pressure and temperature, followed by a pressure quench, to obtain hollow MPs, in a manner similar to how hollows (i.e. bubbles) are formed in a carbonated drink. The water-soluble PVA film is then water washed and the hollow MPs are ready for further treatment, which is the subject of the present contribution. Hollow MPs are immersed in a filling solution (in particular, CV–water solution or CdSe QD–water solution) and kept under sonication. Finally, in order to close the hollow particle and thus obtaining MRs, a swelling agent (toluene in the case of PS particles) is added to the solution which is then thermal treated for an adequate time in order to superficially close the hollows.42 It is worth of note, here, that QDs cytotoxicity represents a major concern in in vivo applications57,58 while, in this case, it is totally shielded by the PS reservoir and should not be harmful. Furthermore, the surface closing method of the PS hollow particle by toluene, reported in the present contribution, could be substituted by a milder method, as different hollow particle-forming polymers could be used and different closing agent and conditions, correspondingly.46,47 In Orsi et al.,48 for example, we reported the formation of poly(lactic-co-glycolic) acid hollow micrometric particles, which swells at room temperature with water. Fig. 3A(i–iv) shows, for a foamed 5 μm-PS particle, the effect of this last closing treatment, conducted with 2% toluene–water solution at 70 °C, on the morphology of the hollow. In particular, from the SEM images, it is possible to observe how the initial 1 μm-aperture of the inner hollow (an open, accessible porosity) progressively reduces with treatment time, and finally closes after 120 min ca. It is worth of note, here, that spherical MR could be achieved by conducting the same closing process at 80 °C. Conversely, at 80 °C ellipsoidal MRs were subjected to a shape change towards the spherical, more stable shape. Furthermore, by SEM image analysis of the hole rim, we observed an increase of the hole rim thickness during the treatment, which may be ascribed to the tendency of the system to reduce the sharp curvature (and surface energy) of the initial rim formed during foaming. By extrapolating this thickness at closure time it is possible to evaluate a minimum thickness of the MR shell of 200 nm ca.A summary investigation of the closed particles by optical microscopy suggests the presence of an inner, closed porosity (Fig. 3B). Similar closing treatment on ellipsoidal MPs also provides ellipsoidal MRs (see Fig. 4). Scanning electron and optical microscopy provide a first indication of the effectiveness of the closing process to produce a closed porosity. Further and more incisive proofs can be obtained by filling the MRs with a host molecule, which can be directly detected by means of spectroscopic techniques. A first example is represented by crystal violet (CV), a dye of use in diagnostics, which can be easily detected by Raman spectroscopy even at low concentration levels. After filling MRs with CV and adequately washing them, a preliminary investigation was performed by collecting randomly Raman spectra from the MRs, in order to verify any presence of CV inside the MRs. Raman spectra of the commercial PS beads, of the CV–water solution and of the filled MRs are reported in Fig. 5A. As highlighted, some of the spectral features of CV (at 1621 cm−1, assigned to the ring C–C stretching, 725 cm−1, assigned to the out of plane vibration of ring C–H and 915 cm−1 assigned to ring skeletal vibration59) clearly distinguish in the spectra of the MRs after filling procedure, thus proving the presence of CV inside the MRs.
 | ||
| Fig. 4 SEM images of PS hollow ellipsoidal particles before (A) and after (B) closing process (scale bar 2 μm). Inset: group of ellipsoidal MRs (scale bar 10 μm). | ||
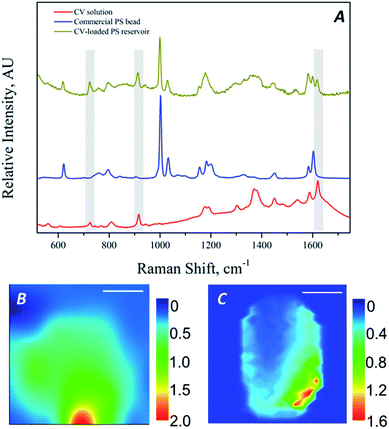 | ||
| Fig. 5 Raman spectra of CV solution, commercial PS beads and CV-loaded PS MRs (A); Raman imaging of spherical (B) and ellipsoidal (C) MRs filled with CV solution (scale bar 2 μm). | ||
Quantitative analysis of Raman spectra provided important information about the concentration of host molecules encapsulated in the PS MRs, which was found to be 0.05% w/w for spherical MRs treated in a 0.14 mM CV water/toluene solution. The analysis was performed on the bases of a calibration curve for CV solution in water/toluene and by neglecting the absorption of scattered light due to the PS thin shell. Furthermore, in order to investigate the spatial distribution of the CV inside the MRs, Raman imaging of the fabricated MRs has been performed; in particular, the spatial distributions of the ratio of the intensities of the peaks at 1621 cm−1 and 1600 cm−1 (respectively attributed to CV and PS characteristic vibrational modes) are reported in Fig. 5B and C, respectively for spherical and ellipsoidal MRs. For both the shapes of MRs, the Raman mapping shows that CV is mostly distributed in a confined, eccentric zone of the particle, thus proving not only the effective encapsulation of the investigated host molecules in the closed cavity but also the eccentricity of the cavity itself, being the MRs asymmetric.
Cadmium selenide quantum dots (CdSe-QD), which are generally used as bioactive fluorescent probes in sensing, imaging, immunoassay, and other diagnostics applications, were also adopted as host molecule for the investigation of MRs. CdSe-QD were encapsulated in both spherical and ellipsoidal MRs using a similar filling/closing procedure, followed by adequately washing. In this case the detection of the host molecule was performed by using SEM/EDS and confocal microscopy.
In Fig. 6 are presented the EDS spectra collected from micro-particles (MPs), which were first incubated in CdSe-QD solution and adequately washed, and MRs produced as by the proposed method. The elemental microanalysis (Table 1) shows that, for both the spherical and the ellipsoidal MPs, no traces of Se and Cd were observed; while large amount of those elements was detected in the case of the MRs. Finally, fluorescence microscopy was exploited for one more experimental proof of the asymmetric encapsulation.
| Sample | C | O | Na | Al | Si | K | Ti | Cl | Zn | Ca | Se | Cd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spherical MPs | 77.51 | 18.73 | 1.14 | 0.2 | 1.99 | 0.29 | 0.14 | — | — | — | — | — |
| Spherical MRs | 66.83 | 21.99 | 1.38 | — | 2.19 | — | — | 0.47 | — | 0.29 | 2.39 | 4.36 |
| Ellipsoidal MPs | 75.87 | 17.77 | 1.88 | 0.2 | 3.12 | 0.65 | 0.18 | 0.33 | — | — | — | — |
| Ellipsoidal MRs | 44.73 | 27.54 | 1.35 | — | 4.74 | 0.78 | — | 1.05 | 1.24 | — | 10.1 | 11.15 |
As for SEM/EDS analysis, fluorescence microscopy images were acquired from both CdSe-filled spherical MRs and commercial PS beads that were first incubated in CdSe-QD solution, according to the procedure proposed herein, and then adequately washed. Results of these investigations are shown in Fig. 7, where images of the commercial PS bead and the MR acquired by both optical and fluorescence confocal microscopy are reported. Fluorescence emission of CdSe-QD can be observed only in the images collected from filled MR; in particular, the emission is confined in eccentric zones of the MR, thus providing a further proof of the asymmetrical encapsulation of the host molecule. No fluorescence emission can be observed from the commercial PS beads which had experienced the same solvent/annealing treatment: this observation is worthy of note as it proves that the washing procedure is efficient and removes all CdSe-QD that may remain adsorbed on the surface of the particle after the incubation.
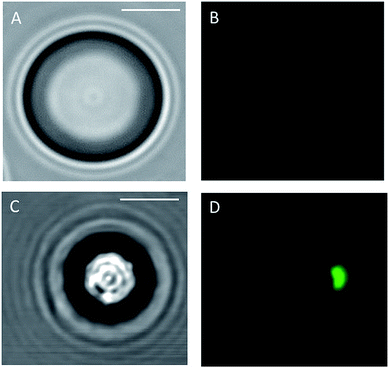 | ||
| Fig. 7 Optical and fluorescence images of spherical particle incubated with CdSe solution (respectively, (A) and (B)) and of CdSe-filled MRs (respectively, (C) and (D)) (scale bar 2 μm). | ||
4. Conclusions
Asymmetric polymeric micro-reservoirs have been produced by using gas foaming technique followed by solvent treatment and thermal annealing. The proposed methodology was applied, as proof of principle, to commercial 5 μm-PS spheres, and was adopted to produce spherical and ellipsoidal micro-reservoirs. These were filled with crystal violet and cadmium selenide quantum dots, and several techniques, such as Raman spectroscopy, scanning electron microscopy/energy-dispersive X-ray spectroscopy and fluorescence microscopy, were used to prove the effectiveness of the encapsulation and to verify the distribution of the host molecule in the micro-reservoirs, which was proved to be asymmetrical. The novelty of this methodology consists in the direct loading of the desirable host molecule in the asymmetric hollow particles and the subsequent closing (which was, so far, limited only to spherical particles with concentric hollow). Furthermore, as also shown in a previous work,48 the proposed methodology can be easily extended to a wide range of dimensions also at the nano-scale. This fact represents a breakthrough in the production of micro-reservoirs and opens new avenues in the drug delivery research field, and the shape, size and nature of the MR, the release profile and/or opening event, all may be designed to allow for proper hosting, protection and deliver of the host molecule.Acknowledgements
The authors thank Manlio Colella for his support during the SEM analysis.Notes and references
- F. Caruso, Adv. Mater., 2001, 13, 11–22 CrossRef CAS.
- F. Caruso, R. A. Caruso and H. Mohwald, Science, 1998, 282, 1111–1114 CrossRef CAS PubMed.
- P. Jiang, J. F. Bertone and V. L. Colvin, Science, 2001, 291, 453–457 CrossRef CAS PubMed.
- S. Kidambi, J. H. Dai, J. Li and M. L. Bruening, J. Am. Chem. Soc., 2004, 126, 2658–2659 CrossRef CAS PubMed.
- J. Y. Kim, S. B. Yoon and J. S. Yu, Chem. Commun., 2003, 790–791, 10.1039/b211714b.
- Y. S. Li, J. L. Shi, Z. L. Hua, H. R. Chen, M. L. Ruan and D. S. Yan, Nano Lett., 2003, 3, 609–612 CrossRef CAS.
- T. C. Wang, R. E. Cohen and M. F. Rubner, Adv. Mater., 2002, 14, 1534–1537 CrossRef CAS.
- Y. L. Wang, L. Cai and Y. N. Xia, Adv. Mater., 2005, 17, 473–477 CrossRef CAS.
- Y. L. Wang and Y. N. Xia, Nano Lett., 2004, 4, 2047–2050 CrossRef CAS.
- X. L. Xu and S. A. Asher, J. Am. Chem. Soc., 2004, 126, 7940–7945 CrossRef CAS PubMed.
- Z. Z. Yang, Z. W. Niu, Y. F. Lu, Z. B. Hu and C. C. Han, Angew. Chem., Int. Ed., 2003, 42, 1943–1945 CrossRef CAS PubMed.
- X. Zhao and P. Liu, Mol. Pharm., 2014, 11, 1599–1610 CrossRef CAS PubMed.
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- S. De Koker, L. J. De Cock, P. Rivera-Gil, W. J. Parak, R. A. Velty, C. Vervaet, J. P. Remon, J. Grooten and B. G. De Geest, Adv. Drug Delivery Rev., 2011, 63, 748–761 CrossRef CAS PubMed.
- A. S. Hoffman, J. Controlled Release, 2008, 132, 153–163 CrossRef CAS PubMed.
- J. A. Champion and S. Mitragotri, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 4930–4934 CrossRef CAS PubMed.
- Y. Geng, P. Dalhaimer, S. Cai, R. Tsai, M. Tewari, T. Minko and D. E. Discher, Nat. Nanotechnol., 2007, 2, 249–255 CrossRef CAS PubMed.
- W. Meier, Chem. Soc. Rev., 2000, 29, 295–303 RSC.
- X. P. Qiu, S. Leporatti, E. Donath and H. Mohwald, Langmuir, 2001, 17, 5375–5380 CrossRef CAS.
- Y. Wang, V. Bansal, A. N. Zelikin and F. Caruso, Nano Lett., 2008, 8, 1741–1745 CrossRef CAS PubMed.
- Y. Chen, H. Chen, L. Guo, Q. He, F. Chen, J. Zhou, J. Feng and J. Shi, ACS Nano, 2010, 4, 529–539 CrossRef CAS PubMed.
- T. Zhang, J. Ge, Y. Hu, Q. Zhang, S. Aloni and Y. Yin, Angew. Chem., Int. Ed., 2008, 47, 5806–5811 CrossRef CAS PubMed.
- Y. F. Zhu, J. L. Shi, W. H. Shen, X. P. Dong, J. W. Feng, M. L. Ruan and Y. S. Li, Angew. Chem., Int. Ed., 2005, 44, 5083–5087 CrossRef CAS PubMed.
- J. A. Champion, Y. K. Katare and S. Mitragotri, J. Controlled Release, 2007, 121, 3–9 CrossRef CAS PubMed.
- S. E. A. Gratton, P. D. PohhauS, J. Lee, I. Guo, M. J. Cho and J. M. DeSimone, J. Controlled Release, 2007, 121, 10–18 CrossRef CAS PubMed.
- A. Imhof, Langmuir, 2001, 17, 3579–3585 CrossRef CAS.
- I. Tissot, J. P. Reymond, F. Lefebvre and E. Bourgeat-Lami, Chem. Mater., 2002, 14, 1325–1331 CrossRef CAS.
- P. J. Bruinsma, A. Y. Kim, J. Liu and S. Baskaran, Chem. Mater., 1997, 9, 2507–2512 CrossRef CAS.
- C. E. Fowler, D. Khushalani and S. Mann, Chem. Commun., 2001, 2028–2029, 10.1039/b104879c.
- R. K. Rana, Y. Mastai and A. Gedanken, Adv. Mater., 2002, 14, 1414–1418 CrossRef CAS.
- B. M. Discher, Y. Y. Won, D. S. Ege, J. C. M. Lee, F. S. Bates, D. E. Discher and D. A. Hammer, Science, 1999, 284, 1143–1146 CrossRef CAS PubMed.
- M. S. Wendland and S. C. Zimmerman, J. Am. Chem. Soc., 1999, 121, 1389–1390 CrossRef CAS.
- M. Q. Zhao, L. Sun and R. M. Crooks, J. Am. Chem. Soc., 1998, 120, 4877–4878 CrossRef CAS.
- M. Iida, T. Sasaki and M. Watanabe, Chem. Mater., 1998, 10, 3780–3782 CrossRef CAS.
- Y. F. Lu, H. Y. Fan, A. Stump, T. L. Ward, T. Rieker and C. J. Brinker, Nature, 1999, 398, 223–226 CrossRef CAS.
- C. Perruchot, M. A. Khan, A. Kamitsi, S. P. Armes, T. von Werne and T. E. Patten, Langmuir, 2001, 17, 4479–4481 CrossRef CAS.
- T. von Werne and T. E. Patten, J. Am. Chem. Soc., 2001, 123, 7497–7505 CrossRef CAS PubMed.
- Q. Y. Zhou, S. X. Wang, X. W. Fan, R. Advincula and J. Mays, Langmuir, 2002, 18, 3324–3331 CrossRef CAS.
- R. A. Cox, J. M. Creasey and M. C. Wilkinson, Nature, 1974, 252, 468–469 CrossRef CAS.
- T. Douglas and M. Young, Nature, 1998, 393, 152–155 CrossRef CAS.
- A. R. Goodall, M. C. Wilkinson and J. Hearn, J. Colloid Interface Sci., 1975, 53, 327–331 CrossRef CAS.
- S. H. Im, U. Y. Jeong and Y. N. Xia, Nat. Mater., 2005, 4, 671–675 CrossRef PubMed.
- J. R. Millman, K. H. Bhatt, B. G. Prevo and O. D. Velev, Nat. Mater., 2005, 4, 98–102 CrossRef CAS PubMed.
- C. Nardin, J. Widmer, M. Winterhalter and W. Meier, Eur. Phys. J. E: Soft Matter Biol. Phys., 2001, 4, 403–410 CrossRef CAS.
- F. Li, Y. Liu, C.-B. Qu, H.-M. Xiao, Y. Hua, G.-X. Sui and S.-Y. Fu, Polymer, 2015, 59, 155–165 CrossRef CAS.
- W. Yin and M. Z. Yates, Langmuir, 2008, 24, 701–708 CrossRef CAS PubMed.
- W. Yin and M. Z. Yates, J. Colloid Interface Sci., 2009, 336, 155–161 CrossRef CAS PubMed.
- S. Orsi, E. Di Maio, S. Iannace and P. A. Netti, Nano Res., 2014, 7, 1018–1026 CrossRef CAS.
- J. Ye, L. Lagae, G. Maes, G. Borghs and P. Van Dorpe, Opt. Express, 2009, 17, 23765–23771 CrossRef CAS PubMed.
- S. Xing, Y. Feng, Y. Y. Tay, T. Chen, J. Xu, M. Pan, J. He, H. H. Hng, Q. Yan and H. Chen, J. Am. Chem. Soc., 2010, 132, 9537–9539 CrossRef CAS PubMed.
- N. R. Jana, Small, 2005, 1, 875–882 CrossRef CAS PubMed.
- D. Seo and H. Song, J. Am. Chem. Soc., 2009, 131, 18210–18211 CrossRef CAS PubMed.
- D. F. Chen, W. H. Li, H. Du and M. Li, J. Nanosci. Nanotechnol., 2012, 12, 3035–3039 CrossRef CAS PubMed.
- O. Jakobsson, C. Grenvall, M. Nordin, M. Evander and T. Laurell, Lab Chip, 2014, 14, 1943–1950 RSC.
- C. Marrazzo, E. Di Maio, S. Iannace and L. Nicolais, J. Cell. Plast., 2008, 44, 37–52 CrossRef CAS.
- C. Marrazzo, E. Di Maio and S. Iannace, J. Cell. Plast., 2007, 43, 123–133 CrossRef CAS.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. I. Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165–1170 CrossRef CAS PubMed.
- T. S. Hauck, R. E. Anderson, H. C. Fischer, S. Newbigging and W. C. W. Chan, Small, 2010, 6, 138–144 CrossRef CAS PubMed.
- E. J. Liang, X. L. Ye and W. Kiefer, J. Phys. Chem. A, 1997, 101, 7330–7335 CrossRef CAS.
- V. Contaldi, L. Imperato, S. Orsi, E. Di Maio, P. A. Netti and S. Iannace, J. Appl. Polym. Sci. Search PubMed , submitted.
| This journal is © The Royal Society of Chemistry 2016 |