Acellular dermal matrix from one-day-old mouse skin on adult scarless cutaneous wound repair by second harmonic generation microscopic imaging
Xue Hana,
Hanping Liua,
Maosheng Chena,
Li Gongc,
Hongwen Pangd,
Xiaoyuan Deng*ab and
Ying Jin*a
aMOE Key Laboratory of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou, Guangdong, China. E-mail: xydeng12@126.com; jinying@scnu.edu.cn
bResearch Resources Center, South China Normal University, Guangzhou, Guangdong, China
cInstrumental Analysis and Research Center, Sun Yat-Sen University, Guangzhou, Guangdong, China
dGuangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, Guangdong, China
First published on 22nd July 2016
Abstract
In fetal skin, there is a process of scarless repair that does not exist in adults. The characteristics of the fetal skin extracellular matrix (ECM) are presumed to play a pivotal role for this scarless wound healing. The use of an acellular dermal matrix (ADM) with certain specific characteristics of the skin ECM thus is implied to have impact on skin repair and regeneration and the alteration of ADM properties may allow for the development of an effective intervention for scarless wound healing. In this study, two types of ADM from skin ECM were adopted to explore the potential to achieve scarless wound healing in adult mice. We transplanted this matrix onto the 10-week-old full-thickness cutaneous wound mice model. The ADM derived from the ECM of 1-day-old mouse skin (ADM-1D) was chosen to approximate certain characteristics of fetal skin while the ADM from the ECM of 20-week-old mouse skin (ADM-20W) provided a control with characteristics of mature skin. Second-harmonic generation (SHG) microscopic imaging was performed to dynamically demonstrate the collagen reconstruction process in the new born dermis during primary wound healing. The outcome of healing on day 21 was evaluated. Compared to ADM-20W, ADM-1D provided a more favorable influence on the re-establishment of the epidermis as well as collagen density, orientation and stiffness in the new born dermis, to a degree approaching normal uninjured adult dermal tissue. A remarkable difference in biomechanical stiffness is present between ADM-1D and ADM-20W, which might be one of the crucial determinants for potential adult scarless wound healing.
1 Introduction
Composed of collagen and a series of other secreted functional molecules including glycoproteins, glycosaminoglycans and proteoglycans, the extracellular matrix (ECM) provides cells with a dynamic and complex microenvironment characterized by specific biophysical, biomechanical and biochemical properties, and indeed determines cellular behaviors.1–4Wound healing, a complex physiology process, is orchestrated by multiple cell types, a series of biological factors and the dynamic crosstalk among cells and their surrounding ECM. The aim of wound healing is for a perfect regeneration, including a scarless repair.5 In fetal skin, there is a process of scarless wound healing,6,7 but that hardly occurs in the adult skin. Fetal and adult skin demonstrate multiple distinctions in regard to underlying wound healing processes, including in matrix deposition and epithelialization,8 and ECM in the skin is presumed to play a central role.9,10 The experiment shows that when full-thickness adult sheep skin is transplanted onto the backs of 60 -day-gestation fetal lambs, wounds in the fetal skin heal with scar formation, suggesting the scarless skin healing is intrinsic to fetal skin itself, rather than the fetal environment.11 On the contrary, when a human placenta-derived ECM sheet is transplanted to the full-thickness cutaneous wound on an adult rat, it provides a microenvironment favorable to the growth and differentiation of cells12 and modulates the healing of the wound. Hence characteristic of ECM is implied to be a crucial factor in promoting wound healing for scarless repair, and making a change of the characteristics of ECM might allow for the development of an effective intervention for scarless wound healing.13
Acellular dermal matrix (ADM) is a native decellularized ECM from full-thickness human or animal skin by removing the epidermis, the subcutaneous fat layer and all cells of the dermis,14 which is widely used for skin reconstruction and surgical applications for its favorable biocompatibility and biodegradability.15,16 ADM retains the basic dermal architecture, and collagen type I degrades gradually and synthesizes into collagen type III. Except acting as a pure scaffold for host cellular infiltration, ADM is a regulator which can alter the default wound healing response and promote healing.17 Nevertheless, effects of different sources of ADMs carrying specific features on wound healing have not been concerned. Collagen protein are the dominant component of ADM and only other minor ECM proteins such as fibronectin, laminin are left.18 ADM keeps the collagen matrix structure of ECM thus stiffness could be one of the influential biomechanical properties of ADM as it acts at the wound site. Cells can feel and respond to the stiffness of their substrate,19 the stiffness of the substrate thus exerts defined biological activities influencing a series of cell behaviors such as the morphology, proliferation, differentiation and migration etc.20 Fibroblast and myofibroblast are the main cell types responsible for collagen synthesizing hence play a crucial role for new dermis formation in wound healing.21,22 A great number of research has confirmed that stiffness of the substrate influence fibroblast biology23 and the differentiation of fibroblast into myofibroblast,24 a specialized fibrosis-associated fibroblastic cell that appears transiently during skin wound healing, but its accumulation may become the main source of the excessive deposition of new extracellular matrix components and lead to scars.25 It is noteworthy that in wound healing response it is the stiffness, no other features such as the scaffold architecture, concentration or adhesion ligand density that affects dramatically the fibroblast cell morphologies,26 suggesting the simply modulating the matrix biomechanical properties of a given biomaterial deposited at the wound site could regulate the progression of wound healing. Therefore, it is prospect that the stiffness of ADM may influence the wound healing process especially the collagen deposition and scar remodeling27 and ultimately the quality of skin repair.
Fetal and adult skin ECM themselves show different stiffness. The enhanced stiffness in adult skin is possibly induced by the reorganization of collagen fibrils and the increased covalent cross-linking of collagen molecules caused by enzyme lysyl oxidase.28 The biomechanical characteristics of stiffness in fetal skin itself might be the crucial factor responsible for the scarless wound healing, and it is expected that partly in possession of features of the fetal skin ECM, like stiffness may also be favorable for minimal scar formation. In this study, we adopt two types of ADMs to investigate the different features of ADM on adult full-thickness cutaneous wound healing. ADM-1D, which is derived from the ECM of 1-day-old mice skin, is to approximate the characteristics of fetal skin ECM; ADM-20W derived from the ECM of 20-week-old mice skin possesses the characteristics of ECM of mature skin. These ADMs were applied to the normal adult full-thickness cutaneous wound mouse model (10-week-old). We evaluated the tissue rebuilt process in the new born dermis, including collagen deposition and remodeling, focusing on the variation of density, orientation, and layer thickness.
Collagen is one of the native biomaterials which produces strong second-harmonic generation (SHG) signals.29 SHG is a nonlinear optical phenomenon induced by an intense laser excitation as it passes through non-centrosymmetric molecules, which produces a second-order nonlinear polarization in those molecules and emits light with exactly twice the frequency (half the wavelength) of the excitation light.30 SHG microscopic imaging therefore provides a unique tool for intravital monitoring of collagen characteristics and has a wide range of applications in life science etc.31 As SHG is an intrinsic property of collagen, no extra staining dye is needed, which allows for preservation of normal function of the tissue of interest as it is being observed.
2 Experimental methods
2.1 Animals care
All experimental procedures were conducted in conformity with institutional guidelines for the care and use of laboratory animals in Southern Medical University, Guangzhou, China, and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication no. 85-23, revised 1985). All animal procedures were approved by the Ethical Committee for Animal Experiments of South China Normal University.2.2 Preparation of ADM
Full-thickness skin was resected from the backs of 1-day-old and 20-week-old BALB/c male mice with sterilized surgical scissors and immersed into Phosphate Buffered Saline (PBS) for 5 minutes. After cleaning in PBS, skin was transferred into 0.25% Dispase at 4 °C for 24 h, ensuring complete immersion including the hair. Tissue was then immersed in a new 0.25% Dispase bath at 4 °C for 24 h to remove the epidermis including the hair. The remaining skin tissues were washed in PBS 3 times to remove the Dispase, then soaked in 0.3% Triton X-100 for 48 h (changed every 24 h), before being treated with 50%, 75%, 100% ethanol respectively for 30 min, 30 min, 40 min, and then CCl4 for 10 min. Finally, the material was treated with 100%, 75%, 50% ethanol for 30 min each. The resulting ADM-1D and ADM-20W were then cut into segments of 7 × 7 mm2 and stored in 75% ethanol at 4 °C until use.2.3 Full-thickness cutaneous wound mice models
Ten-week-old BALB/c male mice were used as the normal adult recipient mice for full-thickness cutaneous wound models. The dorsal skin hair was shaved and sterilized with 70% ethanol and iodine prior to surgery. The mice were anesthetized with an intraperitoneal injection of chloral hydrate (400 mg kg−1, Fuchen, Tianjin, China). Once the mice were anesthetized, a 7 mm biopsy punch was used to make an impression on the dorsum, and then the circular region of tissue was grabbed and pulled with forceps and excised with scissors to create a full-thickness cutaneous wound. Immediately thereafter, the two types of ADM, ADM-1D and ADM-20W, were individually transplanted onto the wound site. Comfeel transparent dressings (Coloplast, Beijing, China), cut into pieces of 10 × 10 mm2, were attached to the wound site and the surrounding normal skin, to prevent the animal from biting, and to help prevent infection.2.4 Atomic force microscopy (AFM) measurement of elastic modulus (E)
Stiffness refers to a material's ability to resist elastic deformation under external force, which can be characterized by the parameter called the elastic modulus (E). The elastic modulus (E) of an object is defined as the slope of its stress–strain curve in the elastic deformation region: a stiffer material will have a higher modulus (stress is the force causing the deformation divided by the area to which the force is applied; strain is the ratio of the change in some length parameter caused by the deformation to the original value of the length parameter). Atomic force microscopy (AFM) (Dimension FastScan, Bruker, Germany) is used to measure E for the evaluation of stiffness of tissues and is effective doing so in ADM-1D and ADM-20W, normal adult recipient mice skin, and the new born wound tissues.According to Herze's model, a model dealing with the relationship of stress and strain, the loading force F and the elastic modulus E have the following relationship:
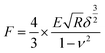 | (1) |
Here, the indentation depth δ = (z − z0) − (d − d0) can be obtained under peak force tapping scanning mode from the withdrawing force curve (Fig. 1B), a reflection of the relationship of the sample piezo displacement (z − z0, height sensor) with the cantilever deflection (d − d0, deflection error) as the tip of the probe gradually withdraws from the sample after touching. z is the piezo displacement, d is the cantilever deflection and z0 and d0 are the initial values. The approaching force curve (“Approaching”) in Fig. 1B is the one when the tip of the probe gradually approaches and touches the sample. While based on Hooke's law (F = kx), the loading force induced by the deflection of cantilevers can be represented by F = k(d − d0), where k is the cantilever spring constant.
Therefore, E is given by
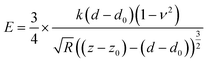 | (2) |
A non-conductive silicon nitride tip with a radius (R) of 20 nm and a rectangular cantilever (MLCT, Bruker, Germany) with resonant frequency of 15 kHz and the spring constant k of 0.02 N m−1 respectively were used in this study. The elastic modulus E was then analyzed by AFM NanoScope Analysis software (Bruker, Germany).
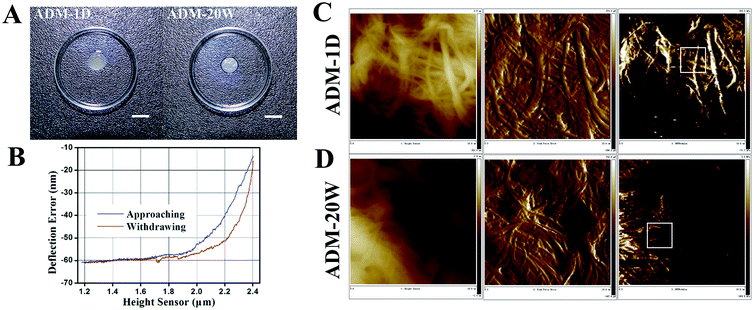
Normal adult skin tissues were harvested from the skin removed during the establishment of the wound model in the adult recipient mice. The new born wound tissues were harvested from the repaired wound site. Tissue was immersed into 0.25% Dispase for 48 h to remove the epidermis for AFM measurement. Resulting material was flattened and extended on glass slides by double faced adhesive tapes and immersed in PBS during measurement to simulate the in vivo environment. Three different modes, height sensor, peak force error and DMTModulus were chosen to present the characteristics of two ADMs. The height sensor, namely surface shape appearance, demonstrates the collagen morphology. The peak force error reflects the rolling of the collagen surface. While each pixel in DMTModulus represents the elastic modulus (E) at the corresponding point of ADM samples, E was taken as the average of measurements of three selected fields (2 × 2 μm2) (white square boxes) in each random field (10 × 10 μm2). At least three random fields were chosen for each piece of ADM (n = 3).
2.5 Nonlinear optical imaging of new born wound tissues
An intense laser light (femtosecond laser) can cause a polarization in the medium as it passes through,| P = χ(1) ⊗ E + χ(2) ⊗ E ⊗ E + χ(3) ⊗ E ⊗ E ⊗ E + … | (3) |
The multi-photon microscopic imaging system in this study consists of a commercial LSM 710 NLO confocal microscope (Zeiss, Jane, Germany) coupled with a femtosecond Ti: sapphire laser (Chameleon Vision II, Coherent, Santa Clara, CA, USA). Ti: sapphire laser offers an excitation pulse laser with wavelengths tunable from 680 nm to 1080 nm. The optical emission signals are collected in a backward geometry. This imaging system can realize high-contrast multi-mode imaging, the SHG imaging of collagen and the auto-fluorescence imaging of living cells simultaneously.
New born wound tissue resected from wound sites of anesthetized living mice, and the side near fascia layer were laid on coverslips and immediately imaged. The excitation wavelength was set to 820 nm for SHG imaging of collagen and 850 nm for auto-fluorescence imaging of living cells. Two independentchannels were selected to receive the SHG (400–420 nm) and auto-fluorescence (500–600 nm) signals. A water-immersion 63× C-Apochromat objective (NA = 1.2) was used to delineate the morphology of collagen with a field of view 135 × 135 μm2 and a 20× Plan-Apochromat objective (NA = 0.8) with a field of view of 425 × 425 μm2 was used for the layer thickness measurement. The dwell time of scanning in each pixel was 3.15 μs and the step size along z axis was 1 μm for 3D imaging. Three random fields were selected for imaging from each mouse (n = 3) and all imaging procedures were completed within 30 minutes in PBS to ensure that the skin tissue specimens remained vital.
2.6 Histological and immunofluorescent analysis
The wound models in animals were built in descending order on designated days so that the wound tissue regeneration could be assessed at various durations by harvesting them all on the same day. The tissues were collected via a 7 mm biopsy punch. Tissue specimens were fixed in 4% paraformaldehyde at 4 °C for 24 h, embedded in paraffin and sectioned in 4 μm increments perpendicular to the long axis of the skin surface. The sections were deparaffinized, rehydrated, washed in distilled H2O and stained with hematoxylin and eosin (H&E). For immunohistochemistry, sections were autoclaved in citrate buffer (pH = 6.0) for antigen retrieval and then blocked with peroxide block and normal goat serum (Beyotime, Shanghai, China) for 30 min at room temperature. The sections were incubated overnight at 4 °C with primary antibodies against collagen I and collagen III (1:100, 1:200, respectively, Abcam, London, UK). After being washed in PBS, the sections were incubated with IgG secondary antibody conjugated to Alexa Fluor 488 and Alexa Fluor 594 (1:200, Abcam, London, UK) for 2 h at 37 °C. Fluorescence signals were visualized on the single-photon microscopic imaging system (the same LSM 710 NLO confocal microscope but excited by an Ar/Kr gas laser operated at 488 nm and a LASOS He/Ne gas laser operated at 594 nm respectively). Five random fields for each specimen (n = 5) were imaged.2.7 Evaluation of the characteristics of new born tissues
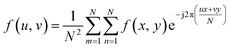 | (4) |
3 Results
3.1 Characterization of ADM by AFM
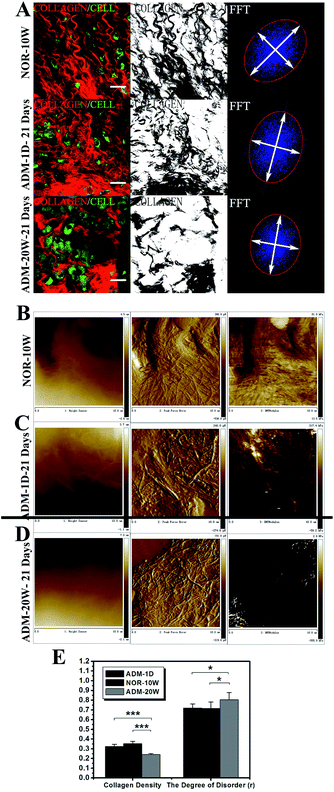
The appearance of ADM-1D and ADM-20W flattened in Petri dish are shown in Fig. 1A. AFM images of height sensor (left), peak force error (middle) and DMTModulus (right) of ADM-1D and ADM-20W are showed in Fig. 1C and D, respectively. From the height sensor images, a difference of the surface shape appearance is noted between the ADM-1D and ADM-20W. The rolling of the collagen surface in ADM-1D and ADM-20W is 3.91 ± 1.51 μm and 4.62 ± 2.14 μm respectively (Table 1), which shows no statistical difference. It's found the average elastic modulus (E) of ADM-1D and ADM-20W has substantially statistical difference (28.3 ± 8.5 kPa vs. 236.1 ± 36.6 kPa) (one-way ANOVA, p < 0.001) (Table 1). The stiffness of ADM-20W is approximately eight times of that of ADM-1D, which illustrates that ADM-20W is a much stiffer biomaterial than ADM-1D.| ADM type | Elastic modulus (E) (kPa) (t test) | p (one-way ANOVA) | Surface rolling (μm) (t test) | p (one-way ANOVA) |
|---|---|---|---|---|
| ADM-1D | 28.3 ± 8.5 (p < 0.001) | <0.001 | 3.91 ± 1.51 (p < 0.05) | >0.05 |
| ADM-20W | 236.1 ± 36.6 (p < 0.001) | 4.62 ± 2.14 (p < 0.05) |
3.2 ADM-1D promoting restoration of epidermis in wound healing
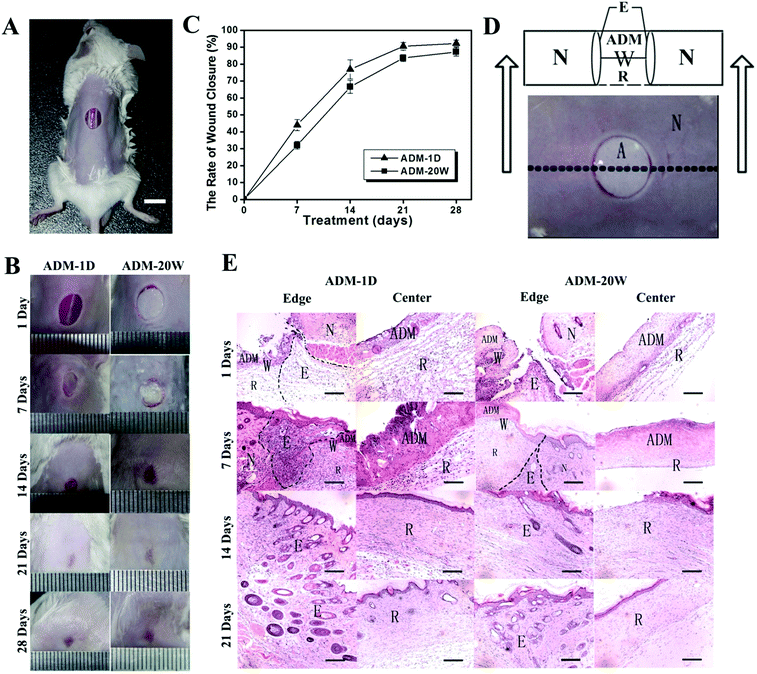
The full-thickness cutaneous wound model is created by punch biopsy and sterilized surgical scissors in the back of the mouse (after being anesthetized; Fig. 2A). The digital photographs of the wound surface size as shown in Fig. 2B and C reveal the trend of wound closing under the treatment of ADM-1D and ADM-20W at days 0, 7, 14, 21, and 28 respectively. Fig. 2D (top) is the cross-sectional schematic of the wound cut made in the wound site (W, bottom), which is used to delineate the relative position of the new born tissue and the surrounding tissue. The ADM scaffold (ADM) covers the top of the wound site (W), and the new born tissue (R) gradually grows from the bottom of the wound site (W). Around the wound site (W) is the normal tissue (N) while the edge of wound (E) refers to the transition part of wound site (W) connecting to the normal tissue (N).The H&E staining analyses of the tissues in the wound site are shown in Fig. 2E. These reveal that rich new born tissue (R) has already appeared in the center of the wound site (center) at day 1 after being treated by ADM-1D and shows a smooth connection (edge) to the surrounding normal skin tissue. However, when treated with ADM-20W, there remains a gap between the new born tissue (R) and the surrounding normal tissue (edge). Moreover, the new born tissue (R) is thin and the cells within it are relatively more sparse (center). At 7 days, more cellularity is found in the edge of the wound (E) (edge). The new born tissue (R) (center) is more obvious in ADM-1D than in ADM-20W. At 14 days, the intact epidermis has formed and linked to the surrounding normal skin in ADM-1D group, while the epidermis is not intact in the wound center in the ADM-20W group. By the 21st days of repair, hair follicles appear in both wound center and wound edge in ADM-1D group, but only in the wound edge in the ADM-20W group. In short, the wound treated by ADM-1D reveals better healing performance than that by ADM-20W in regard to epidermal restoration.
3.3 3D imaging of collagen morphology and layer thickness in new born dermis
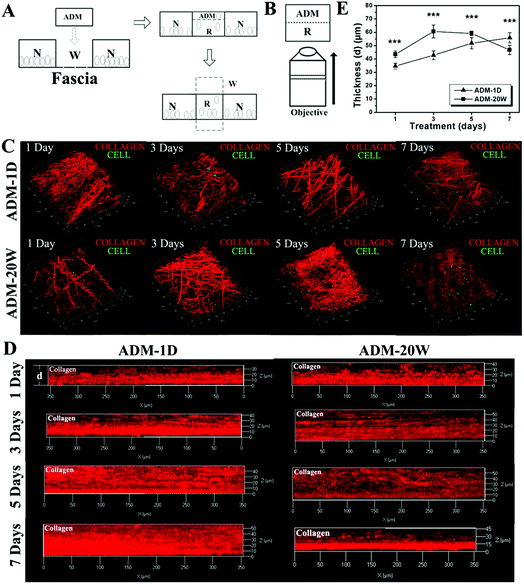
Fig. 3A shows our experimental scheme of using ADM for skin wound repair and Fig. 3B is the protocol for 3D imaging of regenerative tissue, that is, the new born tissue adjoined to the fascia is faced to the excitation laser light for imaging. All the images acquired are from intravital new born tissues. The morphological changes of the new born collagen at the 30 μm focusing plane treated by ADM-1D and ADM-20W respectively are demonstrated in Fig. 3C. Collagen imaged by SHG is indicated by the color red. The repair process on days 1, 3, 5 and 7 are monitored. The morphology of the newly synthesized and deposited collagens varies with the repair time and demonstrates different patterns under the treatment with different ADM types. In the ADM-1D group, collagen presents a helical bending morphology on day 1 and this continues to day 5, then the collagen straightens and thickens. However, the collagen morphology later returns to helical bending and becomes slender again on day 7. On day 1 collagen morphology in the ADM-20W group is similar to that in the ADM-1D group. However, the straight, thick morphological characteristics of collagen appear earlier (day 3) in the ADM-20W group than in the ADM-1D group, and gain a helical bending morphology on day 5.Fig. 3D demonstrates the representative SHG images of the thickness variation of the new born dermal layer treated by ADM-1D and ADM-20W respectively, and Fig. 3E is the corresponding statistical results in two groups. It reveals that there is a significant difference of the new born dermal layer thickness between two groups within the first 7 days of repair (two-way ANOVA, p < 0.001). In ADM-1D group, the thickness of the new born collagen layer gradually increases from 34.7 ± 2.2 μm to 55.8 ± 3.8 μm. In contrast, the thickness of the new born collagen layer in ADM-20W group starts to decrease after an initial increase to 60.6 ± 4.8 μm on day 3.
3.4 The density and orientation of collagen in new born dermis
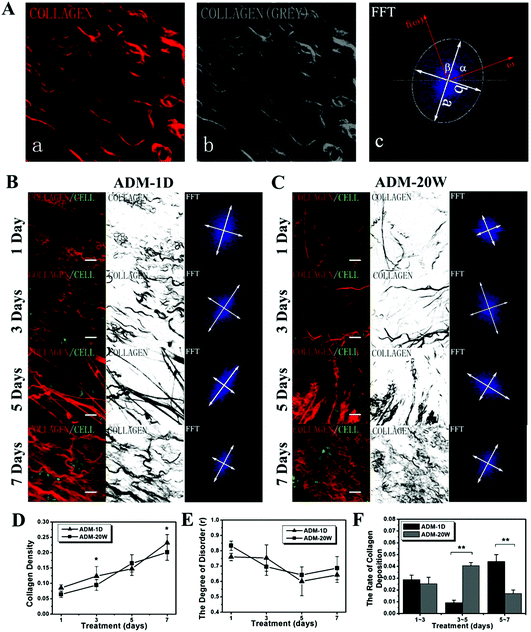
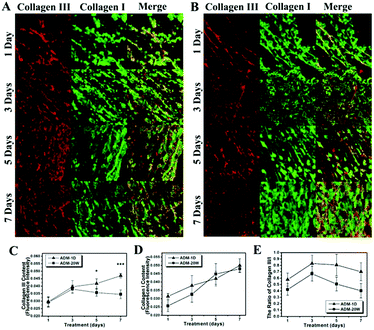
The original SHG images, grey-scale images and corresponding FFT images of collagen in new born dermis on wound healing days 1, 3, 5 and 7 treated by ADM-1D and ADM-20W are demonstrated in Fig. 4B and C respectively. Analyses of collagen density, collagen disorder (r), and the rate of collagen deposition are presented in Fig. 4D–F respectively. The density of collagen in both ADM-1D (one-way ANOVA, p < 0.05) and ADM-20W (one-way ANOVA, p < 0.001) groups during the first 7 days of repair is increasing (Fig. 4D). Also, the difference between two groups as a whole is verified (two-way ANOVA, p < 0.05). The density of collagen on day 3 and 7 in ADM-1D group shows higher than that in ADM-20W group (two-way ANOVA, p < 0.05), and there is no statistical significant difference between two groups on day 1 and 5, indicating a different increase trend of collagen density in two groups, where in ADM-1D it is quicker.Right images in Fig. 4B and C are FFT images demonstrating collagen orientation. Fig. 4E accordingly demonstrates the variation of collagen disorder degree (r). It shows that the newly produced collagen in both ADM-1D (one-way ANOVA, p < 0.01) and ADM-20W groups (one-way ANOVA, p < 0.001) goes through a variation within 7 days which decreases during the first 5 days and then increases, suggesting an assembling process of collagen fibrils from random to ordered alignment. There is no statistical significant difference of collagen orientation between two groups within the first 7 days (two-way ANOVA, p > 0.05). The rate of collagen deposition within several periods in Fig. 4F demonstrates that collagen has a similar deposition rate in ADM-1D and ADM-20W within the first period (day 1–3), then there is a very quick deposition rate on day 3–5 in the ADM-20W group than that in ADM-1D group (one-way ANOVA, p < 0.01), while on day 5–7 the rate of collagen deposition in ADM-1D group is significant higher than that in ADM-20W group (one-way ANOVA, p < 0.01).
3.5 Alteration of collagen composition in new born dermis
Fig. 5A and B demonstrate the variation of collagen composition of collagen I and III in wounds treated with ADM-1D and ADM-20W respectively at 7 days, by immunohistochemical staining. Red represents collagen III and green represents collagen I. The content over time of collagen III (Fig. 5C) and collagen I (Fig. 5D) in the ADM-1D and ADM-20W treatment groups is shown. Collagen III gradually increases in ADM-1D group (one-way ANOVA, p < 0.001), while in ADM-20W group it increases within the first 3 days but then declines (one-way ANOVA, p < 0.001). There is no statistical significant difference in content of collagen III between two groups within the first 3 days. Content of collagen III in new born dermis treated by ADM-1D is significantly higher than that treated by ADM-20W at day 5 (two-way ANOVA, p < 0.05) and 7 (two-way ANOVA, p < 0.001). Collagen I exhibits a obvious increasing trend over seven days in both groups (one-way ANOVA, p < 0.001 and p < 0.001 respectively), and the content of collagen I in both groups do not have statistically significant difference. The ratio of collagen III/I (Fig. 5E) is substantially higher in ADM-1D group than that in ADM-20W group at day 5 and 7 (two-way ANOVA, p < 0.01) but has no statistical difference within first 3 days. In both treatment groups, collagen I is still the dominant composition in the new born dermis.3.6 Evaluation of the repaired dermis
New born dermal tissue on the 21st day of repair undergoes quality evaluation, including assessment of collagen density, the degree of disorder (r), and the elastic modulus (E) of the new born collagen. Controls are corresponding indicators in normal skin dermal tissues from 10-week-old BALB/c mice (NOR-10W). Compared ADM-20W, the new born dermis treated by ADM-1D is more alike to the dermis of NOR-10W in Fig. 6A and E (statistical analysis shows no significant difference between these two groups). It shows that the collagen density in both the ADM-1D and ADM-20W groups are less and prominently less than that in the NOR-10W respectively. On the contrary, the density of collagen in ADM-20W group is greatly lower than that in NOR-10W (one-way ANOVA, p < 0.001) and the difference of collagen density between group ADM-1D and group ADM-20W is significant (one-way ANOVA, p < 0.001). The collagen alignment in the new born dermis in ADM-1D (0.721 ± 0.040) also has no statistical difference with that of the NOW-10W (0.717 ± 0.065), indicating a similar trend of uniform orientation, while the collagen disorder degree in ADM-20W group was 0.805 ± 0.073, a more disorganized orientation than that of NOR-10W and has a significant distinction with that of NOR-10W (one-way ANVOA, p < 0.01).Comparing the AFM images of the dermal tissue in NOR-10W (Fig. 6B) with those of repaired dermal tissues treated by ADM-1D and ADM-20W, respectively (Fig. 6C and D), we find the elastic modulus (E) (denoting the stiffness) of the constituted collagen in new born dermis after 21 days of repair in ADM-1D group has no statistical difference with the control NOR-10W dermis, while E in the ADM-20W group only achieves 50% (one-way ANOVA, p < 0.05) of the value of the controls (Table 2).
| Elastic modulus (E) (kPa) | p (one-way ANOVA) | ||
|---|---|---|---|
| ADM-1D-21 days | 35.53 ± 8.72 | >0.05 | — |
| NOR-10W | 47.92 ± 3.23 | <0.05 | |
| ADM-20W-21 days | 23.98 ± 4.77 | — | |
4 Discussion
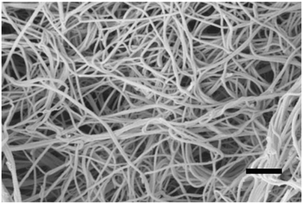
Biomaterial scaffolds derived from ECM of mammalian tissues have been successfully used in a variety of tissue engineering and regenerative medicine applications, both preclinical and clinical.32 Inspiration for this study came from the clues that ECM with fetal skin characteristics could induce scarless healing. ADM of ECM from the skin of 1-day-old mice were applied for the first time to compare with that from 20-week-old mature mice to investigate the effect on the scarless full-thickness cutaneous wound healing in normal 10-week-old adult mice. The skin of 1-day-old mice approaches the characteristics of the fetal skin and has the advantage of being more obtainable for experimentation. Our study demonstrates that ADM from 1-day-old mouse skin does promote regeneration of the epidermis. By means of SHG microscopic imaging, dynamic monitoring of the collagen in new born dermis during the healing process could be undertaken. Compared to the ADM-20W treated animals, SHG images reveal that the new born collagen of the ADM-1D treated animals possessed closer characteristics of density, orientation to that of normal uninjured adult skin.SEM images (Fig. 7) of the ADM processed by our experimental method shows that collagen is the main component and ADM retains the natural dermal structure of skin ECM, providing an analogous biomechanical micro-environment for cell adherence and expansion.16 The study shows that the behavior of dermal fibroblasts involved in the wound healing response is exclusively regulated by the stiffness of biomaterial deposited at the wound site. Enhancing the number of binding sites to which the fibroblasts can adhere does not affect the effects of biomechanics on cell spreading and contraction.26 Also, study has proved that the substrate stiffness regulate the myofibroblast differentiation process.33 Fibroblasts and myofibroblasts are tightly associated with the collagen deposition in cutaneous wound healing.34 Therefore, the biomechanical stiffness characterizing the constitutive collagen matrix appears to be the primary relevant feature of ADM that affects healing in collagen generation.35
Our studies demonstrate that the morphology, density, orientation and type, as well as the layer thickness of the reconstructed collagen in the new born dermis undergo different patterns of alteration under the impact of different ADM. ADM-20W and ADM-1D themselves show remarkable difference (8 times) in elastic modulus (E) (stiffness). We can infer that the stiffness in ADM triggers a signal pathway for collagen production and could be the dominant factor that causes the wound repair differences we have observed. How the stiffness in ADM ultimately affects the regeneration of collagen through regulating cellular signal pathways in fibroblasts and/or myofibroblasts requires further exploration. However, the net effect of these signaling processes and the cellular activity that results is that the softer ADM—with characteristics of ECM from 1-day-old mice skin—is capable of providing support for dermal repair that, essentially, is less scarring.
By immunofluorescence analysis, we learned that during the first seven days of repair, collagen I and collagen III coexist, with the content of collagen I higher than that of collagen III, a finding consistent with results in wound healing studies in the absence of an ADM.36,37 The use of ADM-1D increased collagen III relatively more than it increased collagen I (a higher collagen III/I ratio) during the primary period, and this may be benefit for subsequent scarless wound healing.
In this study, we dynamically demonstrate the morphologic, orientation and thickness alterations of collagen in new born dermis during wound healing by SHG microscopic imaging. Within the first 7 days of repair, the deposited collagen undergoes morphological alteration from slender helical bending to thick/straight and then back to helical bending. This transition occurs at day 5 and day 3 in the ADM-1D and ADM-20W groups respectively (Fig. 3C). Also, the newly deposited collagen in the new born dermis undergoes an alignment alteration (Fig. 4E). Through 21 days of repair, collagen in ADM-1D forms more organized alignment which is closer to that in normal skin, while collagen in ADM-20W becomes much disordered. Also, SHG microscopic images show that the thickness of collagen in new born dermis undergoes two different alteration patterns under the impact of ADM-1D and ADM-20W respectively. The dynamics of dermal collagen fibers is closely related to the skin wound healing as dermal collagen fibers contribute to the morphology and mechanical properties of the repaired skin. A detailed characterization of the structural modification of the collagen fibrillar matrix thus is particularly important. With special non-centrosymmetric molecular organization, collagen becomes an excellent native biomaterial for SHG evaluation. Collagen-sensitive SHG microscopic imaging applied in this study thus provides an effective tool for cutaneous wound healing investigation by revealing collagen-related alterations in new born dermis.38,39
Previous research has demonstrated that there is a lower risk of scar formation later if the wound is primarily healed within 21 days.40 Therefore, 21 days of repair was chosen as the standard to assess final performance of healing. At 21 days of repair, collagen density, orientation, and stiffness in the ADM-1D group was closer to normal skin than that in the ADM-20W group. The ADM-1D new born collagen has no statistical significant difference with that of the stiffness of normal skin, while the ADM-20W new born collagen achieved about 50% of the stiffness of normal skin. Both induce substantially higher stiffness than when no ADM is used.41 These results indicate that the use ADM and especially the use of the softer ADM-1D can promote wound healing performance.
3D inkjet bioprinting technology could provide a feasible way to create a biomimicked ADM with specific characteristics of stiffness, by controlling the collagen type, content and the structure of the fibrillar matrix. This technology promises delivery of biomimicked tissues with high throughput and digital control, and has shown great potential in regenerative medicine and transplant applications.42,43 Bioprinted ADM scaffolding should be designed to leverage the advantages of the fetal and very young skin, by particular arrangement of the collagen fibrillar matrix. The resulting biomaterials should have value for transplant applications in regenerative medicine.
5 Conclusions
Compared to the ADM derived from ECM of 20-week-old mice skin, the use of ADM derived from ECM of 1-day-old mice skin favorably influences scarless adult cutaneous wound repair. The characteristics of the new born dermal tissue treated by ADM-1D are closer to the normal adult dermal tissue in regard to collagen density, orientation, and stiffness. The regeneration of the epidermis is enhanced by an ADM-1D. The characteristics of ADM play a key role in adult scarless cutaneous wound healing and the stiffness of the biomechanical features might be the most crucial determinant.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81171379) and (No. 30940020).References
- R. O. Hynes, Science, 2009, 326, 1216–1219 CrossRef CAS PubMed
.
- F. M. Watt and B. L. Hogan, Science, 2000, 287, 1427–1430 CrossRef CAS PubMed
.
- S. H. Kim, J. Turnbull and S. Guimond, J. Endocrinol., 2011, 09, 139–151 CrossRef PubMed
.
- T. Kriega and M. Aumiailley, Exp. Dermatol., 2011, 209, 689–695 CrossRef PubMed
.
- P. Martin, Science, 1997, 276, 75–81 CrossRef CAS PubMed
.
- D. D. Lo, A. S. Zimmermann, A. Nauta, M. T. Longaker and H. P. Lorenz, Birth Defects Res., Part C, 2012, 96, 237–247 CrossRef CAS PubMed
.
- K. J. Rolfe and A. O. Grobbelaar, ISRN Dermatol., 2012, 2012, 698034 CAS
.
- C. C. Yates, P. Hebda and A. Wells, Birth Defects Res., Part C, 2012, 96, 325–333 CrossRef CAS PubMed
.
- A. Leung, T. M. Crombleholme and S. G. Keswani, Curr. Opin. Pediatr., 2012, 24, 371–378 CrossRef PubMed
.
- P. Samuels and A. K. Tan, J. Otolaryngol, 1999, 28, 296–302 CAS
.
- M. Longaker, D. J. Whitby, M. W. Ferguson, H. P. Lorenz, M. R. Harrison and N. S. Adzick, Ann. Surg., 1994, 219, 65–72 CrossRef CAS PubMed
.
- J. S. Choi, J. D. Kim, H. S. Yoon and Y. W. Cho, J. Tissue Eng., 2013, 19, 329–339 CrossRef CAS PubMed
.
- C. C. Yates, R. Bodnar and A. Wells, Cell. Mol. Life Sci., 2011, 68, 1871–1881 CrossRef CAS PubMed
.
- R. N. Chen, H. O. Ho, Y. T. Tsai and M. T. Sheu, Biomaterials, 2004, 25, 2679–2686 CrossRef CAS PubMed
.
- X. Zhang, Z. Deng, H. Wang, Z. Yang, W. Guo, Y. Li, D. Ma, C. Yu, Y. Zhang and Y. Jin, Biomaterials, 2009, 30, 2666–2674 CrossRef CAS PubMed
.
- G. P. Maxwell and A. Gabriel, Aesthetic Surg. J., 2009, 29, 485–493 CrossRef PubMed
.
- Q. Wang, Y. Jin, X. Deng, H. Liu, H. Pang, P. Shi and Z. Zhan, Biomaterials, 2015, 53, 659–668 CrossRef CAS PubMed
.
- D. M. Hoganson, E. M. O. Doherty, G. E. Owens, D. O. Harilal, S. M. Goldman, C. M. Bowley, C. M. Neville, R. T. Kronengold and J. P. Vacanti, Biomaterials, 2010, 31, 6730–6737 CrossRef CAS PubMed
.
- D. E. Discher, P. Janmey and Y. L. Wang, Science, 2005, 310, 1139–1143 CrossRef CAS PubMed
.
- N. D. Leipzig and M. S. Shoichet, Biomaterials, 2009, 30, 6867–6878 CrossRef CAS PubMed
.
- M. Abe, Y. Yokoyama and O. Ishikawa, Eur. J. Dermatol., 2012, 22, 46–53 CAS
.
- C. G. Elliott, J. Wang, X. Guo, S. W. Xu, M. Eastwood, J. Guan, A. Leask, S. J. Conway and D. W. Hamilton, J. Cell Sci., 2012, 125, 121–132 CrossRef CAS PubMed
.
- B. Hinz, Curr. Rheumatol. Rep., 2009, 11, 120–126 CrossRef CAS PubMed
.
- P. J. Wipff and B. Hinz, J. Bodyw. Mov. Ther., 2009, 13, 121–127 CrossRef PubMed
.
- C. Helary, L. Ovtracht, B. Coulomb, G. Godeau and M. M. Giraud-Guille, Biomaterials, 2006, 27, 4443–4452 CrossRef CAS PubMed
.
- C. Branco da Cunha, D. D. Klumpers, W. A. Li, S. T. Koshy, J. C. Weaver, O. Chaudhuri, P. L. Granja and D. J. Moonery, Biomaterials, 2014, 35, 8927–8936 CrossRef CAS PubMed
.
- B. Eckes, R. Nischt and T. Krieg, Fibrog. Tissue Repair, 2010, 3, 1–11 CrossRef PubMed
.
- A. K. Langton, C. E. Grifiths, M. J. Sherratt and R. E. Watson, Biogerontology, 2013, 14, 89–97 CrossRef CAS PubMed
.
- L. Tian, J. Qu, Z. Guo, Y. Jin, Y. Meng and X. Deng, J. Appl. Phys., 2010, 108, 054701 CrossRef
.
- E. Brown, T. Mckee, E. di Tomaso, A. Pluen, B. Seed, Y. Boucher and R. K. Jain, Nat. Med., 2003, 9, 796–800 CrossRef CAS PubMed
.
- W. R. Zipfel, R. M. Williams and W. W. Webb, Nat. Biotechnol., 2003, 21, 1369–1377 CrossRef CAS PubMed
.
- S. F. Badylak, D. O. Freytes and T. W. Gilbert, Acta Biomater., 2015, 23, 17–26 CrossRef PubMed
.
- A. M. Kloxin, J. A. Benton and K. S. Anseth, Biomaterials, 2010, 31, 1–8 CrossRef CAS PubMed
.
- M. C. Choi, K. K. Cheung, X. Li and G. L. Y. Cheing, Arch. Dermatol. Res., 2016, 308, 21–29 CrossRef PubMed
.
- R. G. Wells, Hepatology, 2008, 47, 1394–1400 CrossRef CAS PubMed
.
- K. Haukipuro, J. Melkko, L. Risteli, M. Kairaluoma and J. Ristel, Ann. Surg., 1991, 213, 75–80 CrossRef CAS PubMed
.
- J. Zhang, J. Guan, X. Niu, G. Hu, S. Guo, Q. Li and Z. Xie, J. Transl. Med., 2015, 13, 49 CrossRef PubMed
.
- J. A. Palero, H. S. deBruijn, A. vanderPloeg-vanden Heuvel, H. J. Sterenborg and H. C. Gerritsen, Opt. Express, 2006, 14, 4395–4402 Search PubMed
.
- M. J. Koehler, K. König, P. Elsner, R. Bückle and M. Kaatz, Opt. Lett., 2006, 31, 2879–2881 CrossRef PubMed
.
- T. C. Cubison, S. A. Pape and N. Parkhouse, Burns, 2006, 32, 992–999 CrossRef PubMed
.
- S. M. Levenson, E. F. Geever, L. V. Crowly, J. F. Oates, C. W. Berard and H. Rosen, Ann. Surg., 1965, 161, 293–308 CrossRef CAS PubMed
.
- S. V. Murphyf and A. Atala, Nat. Biotechnol., 2014, 32, 773–785 CrossRef PubMed
.
- G. Rasperini, S. P. Pilipchuk, C. L. Flanagan, C. H. Park, G. Pagni, S. J. Hollister and W. V. Giannobile, J. Dent. Res., 2015, 94, 153S–157S CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |