Selective enhancement of human stem cell proliferation by mussel inspired surface coating†
Abstract
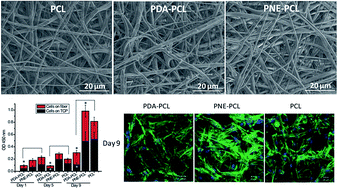
In tissue engineering, promoted cell adhesion and proliferation on a 3D scaffold is desired. Surface functionalization of the scaffolds is often utilized to realize this goal. Polydopamine (PDA) has been extensively studied for promoted cell adhesion and proliferation. Norepinephrine, sharing a similar structure with dopamine, polymerizes slower and forms an even and ultrasmooth surface coating on almost all substrates. However, the effects of polynorepinephrine (PNE) on stem cell adhesion and proliferation have barely been studied. In this study, we compared the biocompatibility and cell adhesion properties of mussel inspired PDA and PNE surface coatings on poly(ε-caprolactone) (PCL) fibers for human mesenchymal stem cells (hMSCs) and human induced pluripotent stem cell derived mesenchymal stem cells (hiPS-MSCs). The surface modification of PDA and PNE on PCL fibers was characterized by environmental scanning electron microscopy (ESME), X-ray photoelectron spectroscopy (XPS) and contact angle measurement. The biocompatibility of the PDA and PNE coatings with hMSCs and hiPS-MSCs was measured using lactase dehydrogenase (LDH) activity, live dead cell staining and cell counting kit 8 (CCK-8) assays. The results show that both PDA and PNE successfully formed a surface coating on the PCL fiber, which dramatically increased the hydrophilicity. The PNE coating showed a much thinner and smoother surface while the PDA coating formed uneven aggregates among the fibers. The biocompatibility analysis and cell proliferation results suggest that the PNE coating is more biocompatible with both hiPS-MSCs and hMSCs than the PDA coating. The PNE coating preferentially promoted cell proliferation for hiPS-MSCs but not for hMSCs, while PDA decreased the cell proliferation of hiPS-MSCs on the PCL fibers. These results suggest that the effect of the PDA and PNE surface coatings on cell proliferation can be cell-dependent. The surface roughness of the PDA coating can negatively affect cell adhesion and proliferation. Different mechanisms of interaction of the PDA and PNE coatings might affect cell adhesion and proliferation, and this needs to be carefully investigated before their application in specific cell type based tissue engineering.

 Please wait while we load your content...
Please wait while we load your content...