Novel nanostructural contrast for magnetic resonance imaging of endothelial inflammation: targeting SPIONs to vascular endothelium†
Abstract
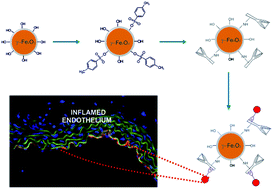
This study aimed to develop superparamagnetic iron oxide nanoparticles (SPIONs) targeted to the areas of vascular endothelium changed in the initial inflammation process, a first step of numerous cardiovascular diseases. Iron oxide nanoparticles coated with a cationic derivative of chitosan (CCh) and having attached monoclonal antibodies (anti VCAM-1 and anti P-selectin) were successfully prepared. Owing to electrostatic stabilization, they form a stable colloidal dispersion in aqueous media. The superparamagnetic properties of the resulting SPION-CCh-anti-VCAM-1 maghemite nanoparticles were proved by magnetometric and Mössbauer measurements. In vitro studies confirmed the specific interaction of anti-VCAM-1 antibodies bound to the surface of SPIONs with endothelial cells of aorta of db/db mice, known to display endothelial inflammation associated with diabetes. The nanoparticles obtained were also visualized using MRI in the aortic arch of ApoE/LDLR−/− mice displaying endothelial inflammation associated with atherosclerosis.

 Please wait while we load your content...
Please wait while we load your content...