The effect of surfactants and their concentration on the liquid exfoliation of graphene†
Abstract
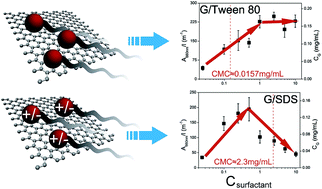
We investigated the effect of surfactants and their concentration on the final graphene concentration via the liquid-phase exfoliation method. Six typical surfactants including ionic and non-ionic ones are explored and the optimized concentration for each surfactant is determined. For ionic surfactants, the graphene concentration increases with surfactant addition and then decreases after reaching its maximum value. In contrast, for non-ionic surfactants, graphene concentration firstly increases with surfactant concentration and then saturates. The different mechanisms of ionic and non-ionic surfactants in stabilizing graphene dispersions are explained by the theory for colloidal stability. Surfactant molecules can adhere to exfoliated graphene sheets and provide an available repulsive force for their stabilization. The as-prepared graphene sheets are verified to be highly exfoliated through transmission electron microscopy and atomic force microscopy studies. The defect level is investigated by Raman spectra and X-ray photoelectron spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...