DOI:
10.1039/C6RA10885G
(Paper)
RSC Adv., 2016,
6, 66385-66393
Fabrication of a magnetic nanoparticle embedded NH2-MIL-88B MOF hybrid for highly efficient covalent immobilization of lipase
Received
27th April 2016
, Accepted 28th June 2016
First published on 30th June 2016
Abstract
Metal–organic frameworks (MOFs), a class of porous hybrid materials composed of metal ions and organic ligands, have been studied for a variety of applications. In this work, for the first time, magnetic MOFs are developed for lipase immobilization. A general one-step in situ hydrothermal route is developed for the construction of MOFs encapsulating superparamagnetic Fe3O4 nanoparticles. The integration of Fe3O4 nanoparticles into the MOFs exhibits many interesting inherent properties including a porous nature, easy functionalization as well as strong superparamagnetism. Here Candida rugosa lipase (CRL) is covalently attached to amino-rich magnetic MOFs. The resulting magnetic MOFs are characterized by means of field emission scanning electron microscopy (FESEM), Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA) and vibrating sample magnetometer (VSM) measurements. Then the enzymatic activities of the immobilized CRL are compared with free CRL. The immobilized CRL presented a wider pH tolerance and excellent thermal stability than free CRL. The Michaelis–Menten kinetic constant (Km) and maximum reaction velocity (Vmax) for both free and immobilized lipase are investigated. The loading amount of CRL on the magnetic MOFs was 280 mg per g of support and the immobilized CRL was efficiently recycled for up to nine cycles.
1. Introduction
Biocatalysis has been frequently used to synthesize pure products with high effectiveness and specificity. As one of the most widely used biocatalysts, CRL has drawn much attention because of its use in a broad diversity of reactions such as enantioselective hydrolysis, esterification, chiral resolution, and trans-esterification.1–6 However, for applications on an industrial scale, lipase shows various problems such as high production costs, low stability, and difficulties in recovery and reusability. To overcome the above problems, the immobilization of lipase on a support is considered to be a promising approach to enhance its catalytic stability, feasibility for continuous operation and ease of recycling, and significantly reduce costs and so on. To date, many materials have been developed as an effective support for lipase immobilization.7–11 Covalent binding and physical adsorption are the common methods to immobilize lipase on a support. In the case of adsorption, the adsorbed lipase is leached out from the support material, resulting in lipase loss and showing low operational stability.12,13 On the other hand, covalent binding is a better approach to immobilize lipase which can prevent leaching from the support. The important parameter for covalent binding on a solid support is the number of functional groups available on the surface. Different methods have been explored to synthesize functionalized supports from either polymers or different types of silane derivatives.14–17 However, these methods not only show low loading capacities of enzyme immobilization but also have poor catalytic stabilities. In general, it is not clear whether lipase immobilization by covalent binding can enhance the catalytic activity. Covalent binding can either enhance catalytic performance by stabilization of the active conformation, or decrease catalytic performance due to affecting the active centre.18,19 Therefore, it is a challenge to design a support for lipase immobilization by covalent binding with high activity, loading capacity and stability.
In recent years, metal–organic frameworks (MOFs) have emerged as a new class of multifunctional porous materials composed of metal ions and organic linkers. The exclusive features of MOFs such as ultra-high porosity and tuneable functionality have attracted great attention in various research fields.20–23 More recently, MOFs that can act as supports for enzyme immobilization have received much attention for new applications of MOFs. MOFs have more advantages compared to inorganic porous materials (e.g. mesoporous silica) and polymers as supports for immobilization. MOFs are generally microporous materials with a high surface area compared to these two types of support. MOFs can be easily functionalized by suitably selecting the organic linkers in one step. But the functionalization of inorganic porous materials is composed of a multistep reaction. Due to their high surface area, a huge amount of guest molecules are encapsulated (either electrostatic or covalent binding) inside the MOFs in comparison to in inorganic porous materials. Recently, Zhao et al. have synthesized magnetic MOFs as carriers for trypsin immobilization.24 Chen et al. have developed mesoporous MOFs with hierarchical pore sizes for myoglobin immobilization.25 Lykourinou et al. have demonstrated successful immobilization of microperoxidase-11 into a mesoporous MOF.26 Wu et al. have investigated the facile synthesis of multiple enzyme-containing MOFs.27 In addition, Hou et al. have reported immobilization of glucose oxidase in a magnetic zeolitic imidazolate framework.28 In all of these approaches, enzymes were immobilized into the MOFs by physical adsorption. To the best of our knowledge, the covalent binding of enzymes with MOFs as supports for catalysis has not been explored so far. The main objective of the present work is the functionalization of MOFs for covalent binding of enzymes, to realize a good support for enzyme immobilization. Among the thousands of known MOFs, NH2-MIL-88B(Fe) (MIL standing for Material of Institute Lavoisier) built from dimers of Fe(III) octahedral and 2-amino benzene dicarboxylic acid ligands has particularly attracted a great deal of attention due to its enhanced stability, high porosity, and enormous surface area.29 Several successful demonstrations have been explored using NH2-MIL-88B(Fe) as a naked-eye sensor of organic vapors,30 drug delivery systems31,32 and catalysts for reactions that include photocatalytic CO2 reduction,33 and aerobic oxidation of alcohols.34 More importantly, NH2-MIL-88B(Fe) contains free amine groups on its surface.29 Therefore, the NH2-MIL-88B(Fe) compounds could be a new class of promising supports for enzyme immobilization. However, these supports for enzyme immobilization are difficult to separate from large volumes of reaction solutions. Therefore, the development of NH2-MIL-88B(Fe) as a support that can easily be separated from solution is required. A possible approach is the introduction of magnetic materials to produce magnetically separable NH2-MIL-88B(Fe) MOFs. We have selected Fe3O4 nanoparticles as a magnetic material attached to the surface of the MOFs or embedded inside the MOFs. After introduction of the Fe3O4 nanoparticles into NH2-MIL-88B(Fe), the magnetic MOFs should have superparamagnetic properties, so that the particles can be easily separated from the reaction mixture using an external magnetic field and simultaneously dispersed back into the solution after removal of the external magnetic field.
In the present study, we have designed an amine-functionalized magnetic MOF for lipase immobilization. Herein, in the first step of synthesis, magnetic Fe3O4 nanoparticles were synthesized. Then, magnetic MOFs were synthesized by a one-step in situ hydrothermal method, which is a facile process with a capability for large-scale production. The magnetic MOFs revealed good distribution of Fe3O4 nanoparticles with huge numbers of amine groups on the surface of the MOFs and a significant saturation magnetization. Then the lipase was immobilized on the magnetic embedded MOFs. For the confirmation of lipase immobilization, a hydrolysis reaction of p-nitrophenylbutyrate (pNPB) was used. The released p-nitro phenol was measured by spectrophotometric assay at 410 nm for testing the lipase activity. The lipase immobilization was well characterized by physical characterization such as the effect of time, pH and temperature. Most importantly, these magnetic MOFs possess a high loading capacity, immobilization efficiency, catalytic activity, stability and reusability of immobilized lipase, which can be extended to the immobilization of other enzymes.
2. Materials and methods
2.1. Materials
Ferric chloride hexahydrate (FeCl3·6H2O), ferrous sulphate heptahydrate (FeSO4·7H2O), and dimethylformamide (DMF) solution were purchased from Merck India. Candida rugosa lipase (CRL) was purchased from Sigma Aldrich Chemicals, USA. 2-Aminoterepthalic acid (NH2-BDC) was purchased from Alfa Aesar, and N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC) was purchased from Spectrochem, India. The substrate solution was prepared using p-nitrophenylbutyrate (pNPB), purchased from Sigma Aldrich Chemicals, USA and absolute ethanol, purchased from Merck Germany. Phosphate buffered saline (PBS) was prepared using disodium hydrogen phosphate (Na2HPO4), potassium dihydrogen ortho phosphate (KH2PO4), sodium chloride (NaCl) and potassium chloride (KCl), which were purchased from Merck India. Millipore water was used for all reactions.
2.2. Synthesis of superparamagnetic Fe3O4 nanoparticles
Superparamagnetic Fe3O4 nanoparticles were synthesized using a previously reported co-precipitation method with some modification.35 Ferric chloride (FeCl3) and ferrous sulphate (FeSO4) were used as the sources of the Fe(III) ion and Fe(II) ion respectively. In brief, 0.324 g of FeCl3 and 0.274 g of FeSO4·7H2O were dissolved in 50 mL of water under an argon atmosphere. The solution mixture was heated to 50 °C, and then 5 mL of 25% ammonia solution was injected dropwise into the above mixture with continuous stirring and the temperature was raised to 80 °C for proper growth of the nanoparticles. After 45 min, the solution was cooled to room temperature. The nanoparticles were collected by magnetic decantation and washed with water until the ammonia was totally removed. Finally the nanoparticles were allowed to dry at 60 °C for further use.
2.3. Synthesis of magnetic nanoparticle embedded NH2-MIL-88B (NH2-MIL-88B/Fe3O4)
Magnetic Fe3O4 nanoparticle embedded NH2-MIL-88B was prepared using a reported method by a hydrothermal process with slight modification.36 At first, separate solutions of (1) 0.05 g of Fe3O4 dispersed in 15 mL DMF, (2) 0.04 g of NH2-BDC in 7.5 mL DMF, and (3) 0.0716 g of FeCl3 in 7.5 mL DMF were prepared. Thereafter, the solution of NH2-BDC followed by FeCl3 was added to the solution of Fe3O4 and the whole solution mixture was allowed to sonicate for 15 min. Then the mixture was loaded in a 100 mL Teflon lined autoclave and heated at 170 °C for 24 h. After that, the autoclave was cooled to room temperature and the product was collected by magnetic separation, washed with water and ethanol each for three times to remove the solvent DMF properly. Finally the sample was dried under vacuum at 55 °C for further application.
2.4. CRL immobilization on NH2-MIL-88B/Fe3O4
At first a stock solution of lipase was prepared by dissolving 50 mg of lipase in 100 mL of PBS buffer of pH 7.4. Synthesized magnetic MIL-88B (4 mg) was dispersed in 10 mL of phosphate buffer solution (PBS) (pH = 7.4) and then 2 mL of the lipase solution was added to it. After 10 min sonication, 1.5 mg of EDC was added to activate the carboxylic functional groups (–COOH) of lipase. Then the solution was stirred for 4 h in an orbital shaker at 150 rpm at 25 °C for immobilization. The immobilized lipase was separated by magnetic separation and washed 5 times with PBS buffer. Then the immobilised lipase was stored at 4 °C for further study.
2.5. CRL activity assay and enzyme immobilization efficiency determination
The immobilization efficiency of an enzyme is stated by the amount of the enzyme bound on a support of unit mass divided by the amount of enzyme taken using p-nitrophenyl butyrate as a substrate. p-Nitro phenol which was released from pNPB as a result of a lipase-catalysed hydrolysis reaction was measured by spectrophotometric assay at 410 nm for testing the lipase activity. The efficiency of immobilization was evaluated in terms of the activity yield and immobilisation yield using the following equations:| |
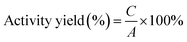 | (i) |
| |
 | (ii) |
where, A is the initial activity of lipase, B is the total activity of the residual lipase, and C is the activity of the immobilized lipase, respectively.37 The relative activity (%) is the ratio between the activity of every sample and the maximum activity of the sample.
2.6. Study of the effect of pH and temperature
The effects of pH on the activities of the free and immobilized lipase were studied by keeping each solution at 30 °C under a variety of pH values ranging from 6 to 10. Each of the free and immobilized lipase solutions was prepared in PBS at different pH values and left for 30 min. Then the enzymatic activities were compared using a lipase assay i.e. by hydrolysis of pNPB.
The effects of temperature on the activities of the free and immobilized lipase were studied at various temperatures, ranging from 30 °C to 80 °C. Each of the free and immobilized lipase solutions was kept in PBS at pH 7.4 and strength 0.1 M for 30 min at each temperature. Then the enzymatic activity was calculated by hydrolysis of pNPB.
2.7. Study of thermal stabilities
The thermal stabilities of the free and immobilized lipase were studied by evaluating the residual activities of lipase. Both the free and immobilized lipase were incubated at 50 °C in PBS (0.1 M) at pH 7.4 and the activity of both of the solutions was measured at one hour intervals for 10 hours using hydrolysis of pNPB.
2.8. Study of kinetic parameters (Km and Vmax)
The kinetic parameters i.e. the Michaelis constant (Km) and the maximum rate of reaction (Vmax) of the free and immobilized lipase were studied by measuring the rates of reaction at different concentrations of substrate pNPB from 0.02 mM to 0.06 mM in PBS (0.1 M) at pH 7.4 and 35 °C. Km and Vmax values for both the free and immobilized lipase are calculated using the Lineweaver–Burk plot derived from the Michaelis–Menten equation:| |
 | (iii) |
where, [S] is the concentration of the substrate. A linear line was obtained by plotting the graph 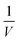 vs.
vs.  . From the intercept, the maximum reaction velocity Vmax was obtained, and the Michaelis constant Km value was calculated from the value of the slope.38
. From the intercept, the maximum reaction velocity Vmax was obtained, and the Michaelis constant Km value was calculated from the value of the slope.38
2.9. Study of reusability
The reusability of the immobilized lipase was studied by measuring the activities of lipase using hydrolysis of pNPB. The immobilized lipase was magnetically separated and washed well with PBS (0.1 M) at pH 7.4 after each use, and the activities were compared with the activity of the first run (activity defined as 100%) using the same lipase assay, hydrolysis of pNPB.
2.10. Characterization
The morphologies of the Fe3O4 nanoparticles and the magnetic MOF were investigated by field emission scanning electron microscopy (FESEM) analysis which was achieved using a Supra 55 with an airlock chamber for scientific research. The surface functional groups were investigated by Fourier transform infrared spectroscopy (FTIR) (Thermo Nicolet Nexus FTIR (model 870)) at room temperature with the KBr pellet technique, and a scanning range from 400 to 4000 cm−1. The magnetic properties were evaluated using vibration sample magnetometry (VSM) at 25 °C by a Lake Shore magnetometer (Model-7410). The phase structure and purity were determined by powder X-ray diffraction (XRD) with an Expert Pro (Philips) X-ray diffractometer using Cu Kα radiation. Thermogravimetric analysis (TGA) was executed by a TGA 2850 thermogravimetric analyser (TA instruments) under N2 atmosphere with temperatures ranging from 50 °C to 900 °C. The lipase activity was observed by UV-Vis spectroscopy on a Shimadzu UV-1700 spectrophotometer.
3. Results and discussion
The present approach for improving the immobilization of lipase by covalent bonding on magnetic MOFs involves two steps: (i) synthesis of Fe3O4 nanoparticles by a co-precipitation method; and (ii) controlled synthesis of the magnetic MOFs, in order to grow the MOFs with deposited Fe3O4 nanoparticles. The synthesis protocol of the magnetic MOFs has been designed in view of two key points (i) to get maximal immobilization of lipase on the surface of the magnetic MOFs and (ii) easy separation by a permanent magnet. The characteristics of both the Fe3O4 nanoparticles and NH2-MIL-88B/Fe3O4 have been investigated.
3.1. Surface morphology analysis
Here the morphology of the Fe3O4 nanoparticles, NH2-MIL-88B, NH2-MIL-88B/Fe3O4 and NH2-MIL-88B/Fe3O4-CRL was analysed by FESEM as shown in Fig. 1. It can be seen that NH2-MIL-88B exhibited a bipyramidal hexagonal prism like structure with a smooth surface shown in Fig. 1a. Fe3O4 exhibited a spherical particle like morphology with average diameters of 80 ± 5 nm as shown in Fig. 1b. Fig. 1c shows direct evidence of the deposition of Fe3O4 nanoparticles onto the MOFs. NH2-MIL-88B/Fe3O4 is synthesized under the same synthetic conditions applied for the pure MOFs, however the Fe3O4 nanoparticles are well dispersed on the surface of the MOFs, showing superior magnetic properties. Aggregation of the Fe3O4 nanoparticles is observed on the surface of the MOFs due to the magnetic nature of the particles. After immobilization of CRL on the magnetic MOFs, the morphology was observed as shown in Fig. 1d. From this figure it is observed that there are no changes in the morphology of the magnetic MOFs after immobilization.
 |
| | Fig. 1 FESEM image of (a) NH2-MIL-88B, (b) pure Fe3O4, (c) NH2-MIL-88B/Fe3O4 and (d) NH2-MIL-88B/Fe3O4-CRL. | |
3.2. FTIR analysis
The FTIR spectra of NH2-MIL-88B, NH2-MIL-88B/Fe3O4, CRL and CRL immobilized NH2-MIL-88B/Fe3O4 are depicted in Fig. 2. The FTIR spectrum of NH2-MIL-88B contains amino groups as evidenced by bands at 3490, 3370, and 1628 cm−1 due to symmetric stretching, asymmetric stretching and a bending vibration respectively.29,39 A doublet with one band at 575 cm−1 and another at 479 cm−1 was attributed to the Fe–O vibration of the metal–organic framework.40 However after compositing with Fe3O4, the adjacent bands at 3490 and 3370 cm−1 overlapped with the –OH band and produced a broad band at 3420 cm−1 (Fig. 2(ii) and (iv)) while the other bands appeared at nearly the same positions with different intensities. The FTIR spectrum of free lipase shows the most prominent band at 1658 cm−1 which corresponds to N–H bending and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of an amide I band. Other bands at 1029, 1082 and 1152 cm−1 are attributed to the carbohydrate moiety of free lipase.41
O stretching of an amide I band. Other bands at 1029, 1082 and 1152 cm−1 are attributed to the carbohydrate moiety of free lipase.41
 |
| | Fig. 2 FTIR spectrum of (a) (i) NH2-MIL-88B, (ii) NH2-MIL-88B/Fe3O4, (iii) CRL and (iv) NH2-MIL-88B/Fe3O4-CRL. (b) Magnified spectra of free and immobilized lipase. | |
Fig. 2(iv) depicts the immobilization of CRL on NH2-MIL-88B/Fe3O4. Here merging of the bands centred at 1029, 1082, and 1152 cm−1 leads to broadening of the peak centred at 1069 cm−1 as shown in Fig. 2b. The broadening of the bands appearing in the spectrum of NH2-MIL-88B/Fe3O4-CRL confirmed the immobilization of lipase onto NH2-MIL-88B/Fe3O4.
3.3. XRD analysis
The phase structure and purity of Fe3O4, NH2-MIL-88B and NH2-MIL-88B/Fe3O4 were determined by the powder X-ray diffraction method and the obtained patterns are shown in Fig. 3a. In the case of the Fe3O4 nanoparticles, the typical and as usual diffraction pattern was observed.42 For pure NH2-MIL-88B, the diffraction pattern is found to be consistent with the earlier data of ref. 43. The prominent diffraction peaks are located at 2θ = 9°, 16.5°, 18°, 20.5°, 22.50°, 25° and 27.38°. It is observed that NH2-MIL-88B/Fe3O4 exhibits a similar pattern to that of NH2-MIL-88B, including the peaks at 30.01°, 35.5°, 43.19°, 53.7°, 57.33°, 62.9° and 74.52°. This confirms the conjugation of Fe3O4 nanoparticles with no change in phase of NH2-MIL-88B. However it should be noted that the intensity of the different peaks related to NH2-MIL-88B as well as Fe3O4 decreased.
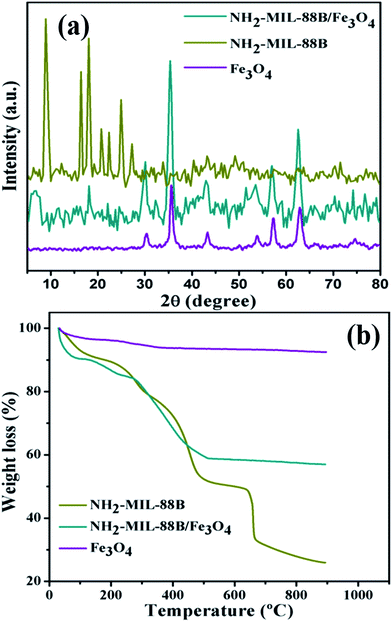 |
| | Fig. 3 (a) X-ray diffraction pattern for NH2-MIL-88B, magnetic NH2-MIL-88B/Fe3O4 and pure Fe3O4. (b) TGA thermogram of pure Fe3O4, NH2-MIL-88B and magnetic NH2-MIL-88B/Fe3O4. | |
3.4. TGA analysis
The formation of the magnetic MOFs (NH2-MIL-88B/Fe3O4) was also assessed by thermogravimetric analysis. The TGA curves of the pure Fe3O4 nanoparticles, NH2-MIL-88B and NH2-MIL-88B/Fe3O4 are shown in Fig. 3b. The TGA curve of Fe3O4 shows a first small weight loss of 3.63%, when the temperature increases to 99 °C, which corresponds to the loss of physically adsorbed water.42 The second small weight loss (2.47%) is detected up to the temperature range of 230 °C, which could be attributed to the loss of surface hydroxyl groups. In the case of NH2-MIL-88B, the TGA curve shows that the weight loss occurs via three stages. In the first stage, a weight loss of almost 8.49% takes place when the temperature rises to around 125 °C. When the temperature is increased from 221 °C to 515 °C, a 36.76% weight loss is observed. Around 16.42% weight loss is detected when the temperature increases from 635 °C to 670 °C. However, as for NH2-MIL-88B/Fe3O4, the weight loss occurred via three stages. The first stage weight loss (9.46%) at 95 °C is due to the loss of physically adsorbed water. The second stage weight loss (4.72%) between 130 °C and 224 °C is ascribed to the loss of surface hydroxyl groups and adsorbed solvent molecules, while the weight loss (25.47%) detected in between 273 °C and 516 °C corresponds to the decomposition of the organic ligands.44 This result further confirms that the Fe3O4 nanoparticles are successfully deposited on the surface of NH2-MIL-88B. From the TGA analysis, the amount of Fe3O4 nanoparticles can be calculated in NH2-MIL-88B/Fe3O4. It is observed that approximately 24% Fe3O4 nanoparticles are deposited in the magnetic MOFs.
3.5. Magnetization study
The magnetic behaviour of pure Fe3O4 and NH2-MIL-88B/Fe3O4 was characterised by a VSM study. VSM plots for both the particles are shown in Fig. 4. From the figure it is seen that the saturation magnetization value for pure Fe3O4 and NH2-MIL-88B/Fe3O4 is 82 emu g−1 and 60 emu g−1 respectively. From the hysteresis loop it is shown that the coercivity and remanence values are zero. It recognised that both the magnetic nanoparticles and NH2-MIL-88B/Fe3O4 are superparamagnetic. So the magnetic MOFs can be easily separated from the reaction mixture by magnetic separation. This magnetically active property of NH2-MIL-88B/Fe3O4 renders it very susceptible to magnetic fields and easily separated from the reaction mixture by magnetic separation.
 |
| | Fig. 4 Magnetization versus magnetic field plot at 300 K for pure Fe3O4 and magnetic NH2-MIL-88B/Fe3O4. | |
3.6. Lipase immobilization
The amount of lipase immobilized on magnetic NH2-MIL-88B was calculated from enzyme immobilization efficiency. This was calculated by varying the concentration of lipase from 0.02 to 0.15 mg mL−1 during immobilization. The activity of both the free and immobilized lipase was analysed using a pNPB assay. The relative activity of lipase is plotted against the enzyme concentration as shown in Fig. 5a. It is observed that the activity increases with different concentrations of lipase and the supported enzyme is slightly less efficient than the free enzyme. But if the free enzyme is used, it is not possible to recover it from the reaction. So it can only be used in a reaction one time. But after immobilization on a solid support, the enzyme can be used several times. Fig. 5a shows that at high concentrations the supported enzyme has constant activity, while for the free enzyme the activity increased with increasing concentration. To calculate the immobilization efficiency a fixed amount of NH2-MIL-88B/Fe3O4 was used. So, after the addition of almost 0.10 mg mL−1 lipase, NH2-MIL-88B/Fe3O4 is saturated with lipase, i.e. after increasing the concentration of lipase, the relative activity remains constant. From this experiment the immobilization efficiency and catalytic activity of lipase are calculated using eqn (i). The loading amount of lipase on NH2-MIL-88B/Fe3O4 was almost 280 mg of protein per unit g of magnetic MOF and the activity of the immobilized lipase was retained at almost 86% of that of the free lipase.
 |
| | Fig. 5 Effect of (a) concentration of lipase, (b) pH and (c) temperature on the relative activity of free lipase and lipase immobilized on NH2-MIL-88B/Fe3O4. (d) Thermal stabilities of the free and immobilized lipase at 55 °C. | |
3.7. Effect of pH
The activity of free and immobilized lipase was determined in PBS with different pH values from 6.0 to 10.0 using pNPB as a substrate at 30 °C to study the influence of pH and the relative activity is shown in Fig. 5b. It is observed that the free and immobilised lipase show highest activity at pH 8.0. But the activity of immobilized lipase is greater in the acid and alkali regions compared to free lipase.
3.8. Effect of temperature
The relative activity of free and immobilized lipase was also determined at different temperatures from 30 °C to 60 °C in PBS (0.1 M) at pH 7.4 to study the effect of temperature using pNPB as a substrate. As the temperature was increased, the relative activity of lipase decreased for both free and immobilized lipase. The influence of temperature is shown in Fig. 5c. The decreasing rate of relative activity for free lipase is much faster than that for the immobilized lipase. So it can be concluded that after immobilization, the rate of transformation of lipase is reduced during the temperature and pH changes. So the immobilized lipase has greater stability than free lipase at higher temperature.
3.9. Thermal stability
The thermal stability of immobilized and free lipase was studied by keeping two different solutions at 55 °C in an oil bath for 10 h using pNPB as a substrate in PBS (0.1 M) of pH 7.4. The relative activity of both of the enzymes was measured at one hour intervals and it was observed that the activity is lowered with increasing observance time. The variation of the relative activity at different time intervals at 55 °C for free and immobilized lipase is shown in Fig. 5d. The residual activity of free lipase was maintained at about 46% after 6 h, whereas for immobilized lipase almost 68% relative activity was retained after 6 h incubation. So the immobilized lipase has good thermal stability due to covalent bond formation between the carrier molecule functional group (–NH2) and the functional group of the lipase molecule (–COOH), which is an important characteristic for different applications.
3.10. Kinetic studies
The kinetic parameters, Km and Vmax values, of immobilized and free lipase were measured using substrate pNPB at different concentrations (0.02–0.06 mM) in PBS (0.1 M) at pH 7.4 and 30 °C. The Michaelis–Menten and Lineweaver–Burk plots for both the immobilized and free lipase are shown in Fig. 6a and b respectively. The Km and Vmax values are calculated and summarized in Table 1. The Km value of immobilized lipase is lower than that of free lipase. So after immobilization lipase has a greater substrate affinity than free lipase.38,45 The Vmax value for immobilised lipase is also lower as usual than that for free lipase. This indicates that after immobilization of lipase in NH2-MIL-88B/Fe3O4, the activity of the enzyme is lowered.38
 |
| | Fig. 6 (a) Michaelis–Menten plots and (b) Lineweaver–Burk plots of free lipase and immobilized lipase. | |
Table 1 Kinetic parameters for free lipase and immobilized lipase
| |
Km (mM mL−1) |
Vmax (mM min−1) |
| Free lipase |
0.56 |
0.04488 |
| Immobilized lipase |
0.20 |
0.0189 |
3.11. Reusability study
The reusability of immobilized lipase is the most important plus point compared to free lipase for practical applications. Reusability was studied using the hydrolysis reaction of pNPB and after each cycle the magnetic particles were separated using a permanent magnet and washed well with PBS (0.1 M) at pH 7.4 and 30 °C. The activity of immobilized lipase slowly decreased after several cycles as shown in Fig. 7a. It is observed that the immobilized lipase has good stability after multiple uses and retains an activity of almost 84% after 5 cycles. But the activity subsequently decreases to 66% after 9 cycles. The reduction in activity is considered to be due to the denaturation of lipase.10,46 Again we have checked whether the Fe3O4 nanoparticles exist on the surface of NH2-MIL-88B after 9 cycles. To verify this, we used FESEM as shown in Fig. 7b. This confirms that Fe3O4 nanoparticles are available on the surface of NH2-MIL-88B after successive uses. Again, the support (NH2-MIL-88B/Fe3O4) can be easily separated from the solvent by a permanent magnet. From these observations it is easy to conclude that after recycling, the magnetization remains intact.
 |
| | Fig. 7 (a) Reusability plot of immobilized lipase and (b) FESEM image of NH2-MIL-88B/Fe3O4 after 9 cycles of use. | |
4. Conclusion
In conclusion, we demonstrated an approach to synthesize amine-functionalized magnetic MOFs for lipase immobilization. FESEM observations reveal that the magnetic nanoparticles with an average diameter of ∼80 nm are deposited on the surface of the MOFs. FTIR analysis indicated that lipase was successfully immobilized on the surface of magnetic NH2-MIL-88B/Fe3O4. The amounts of the Fe3O4 nanoparticles and enzyme are calculated for NH2-MIL-88B/Fe3O4-CRL from TGA analysis and UV absorption spectroscopy using an enzyme assay, respectively. In 10 mg of NH2-MIL-88B/Fe3O4-CRL, 1.7 mg of Fe3O4 and 2.8 mg of CRL were found. The catalytic efficiency of immobilized lipase enhanced the enzymatic activity and storage stability, opening up a new perspective in enzyme based catalytic reactions. Compared to the free lipase, the activity of immobilized lipase is considerably enhanced by different parameters like pH and temperature and immobilized lipase has higher thermal stability. The NH2-MIL-88B/Fe3O4 possessed high magnetic responsibility, which means that rapid separation from the reaction mixture can be achieved. Additionally, the immobilized lipase is reused more than 9 times whilst retaining more than 65% of its initial activity. This magnetic based system may be a promising material for the immobilization of other industrially important enzymes. We believe that this study may bring great extendibility to design other MOFs/magnetic nanoparticles for diverse applications.
References
- I. Venditti, C. Palocci, L. Chronopoulou, I. Fratoddi, L. Fontana, M. Diociaiuti and M. V. Russo, Colloids Surf., B, 2015, 131, 93–101 CrossRef CAS PubMed.
- F. Bellezza, A. Cipiciani, G. Cruciani and F. Fringuelli, J. Chem. Soc., Perkin Trans. 1, 2000, 4439–4444 RSC.
- E. G. Lee, H. S. Won and B. H. Chung, Process Biochem., 2001, 37, 293–298 CrossRef CAS.
- S. Sabbani and E. Hedenström, J. Mol. Catal. B: Enzym., 2009, 58, 6–9 CrossRef CAS.
- M. C. R. Franssen, H. Jongejan, H. Kooijman, A. L. Spek, N. L. F. L. Camacho Mondril, P. M. A. C. Boavida dos Santos and A. de Groot, Tetrahedron: Asymmetry, 1996, 7(2), 497–510 CrossRef CAS.
- P. Beier and D. O’Hagan, Chem. Commun., 2002, 1680–1681 RSC.
- Z. Chen, M. Wang, C. Zhao, Y. Lin, R. Yang and Z. Wang, CrystEngComm, 2015, 17, 2536–2543 RSC.
- K. Solanki and M. N. Gupta, New J. Chem., 2011, 35, 2551–2556 RSC.
- Z. Ali, L. Tian, P. Zhao, B. Zhang, A. Nisar, X. Li, H. Zhang and Q. Zhang, RSC Adv., 2015, 5, 92449–92455 RSC.
- Z. Chen, W. Xu, L. Jin, J. Zha, T. Tao, Y. Lin and Z. Wang, J. Mater. Chem. A, 2014, 2, 18339–18344 CAS.
- D. H. Zhanga, L. X. Yuwen, Y. L. Xie, W. Li and X. B. Li, Colloids Surf., B, 2012, 89, 73–78 CrossRef PubMed.
- K. M. Ponvel, D. G. Lee, E. J. Woo, I. S. Ahn and C. H. Lee, Korean J. Chem. Eng., 2009, 26(1), 127–130 CrossRef CAS.
- Y. Li, G. Zhou, C. Li, D. Qin, W. Qiao and B. Chu, Colloids Surf., A, 2009, 341, 79–85 CrossRef CAS.
- W. Zhu, Y. Li, F. Zeng, H. Yin, L. Wang and H. Zhu, RSC Adv., 2015, 5, 23039–23045 RSC.
- M. Z. Borowska, T. Siódmiak, D. Chełminiak, A. Cyganiuk and M. P. Marsza, Appl. Surf. Sci., 2014, 288, 641–648 CrossRef.
- N. Balistreri, D. Gaboriau, C. Jolivalt and F. Launaya, J. Mol. Catal. B: Enzym., 2016, 127, 26–33 CrossRef CAS.
- C. Y. Shang, W. X. Li and R. F. Zhang, Mater. Res. Bull., 2015, 68, 336–342 CrossRef CAS.
- L. Chronopoulou, G. Kamel, C. Sparago, F. Bordi, S. Lupi, M. Diociaiutic and C. Palocci, Soft Matter, 2011, 7, 2653–2662 RSC.
- C. H. Kuo, Y. C. Liu, C. M. J. Chang, J. H. Chen, C. Chang and C. J. Shieh, Carbohydr. Polym., 2012, 87, 2538–2545 CrossRef CAS.
- Z. Hasan and S. H. Jhung, ACS Appl. Mater. Interfaces, 2015, 7, 10429–10435 CAS.
- A. H. Chughtai, N. Ahmad, H. A. Younus, A. Laypkov and F. Verpoort, Chem. Soc.
Rev., 2015, 44, 6804–6849 RSC.
- A. Morozan and F. Jaouen, Energy Environ. Sci., 2012, 5, 9269–9290 CAS.
- C. Sanchez, P. Belleville, M. Popall and L. Nicole, Chem. Soc. Rev., 2011, 40, 696–753 RSC.
- M. Zhao, X. Zhang and C. Deng, Chem. Commun., 2015, 51, 8116–8119 RSC.
- Y. Chen, V. Lykourinou, T. Hoang, L. J. Ming and S. Ma, Inorg. Chem., 2012, 51, 9156–9158 CrossRef CAS PubMed.
- V. Lykourinou, Y. Chen, X. S. Wang, L. Meng, T. Hoang, L. J. Ming, R. L. Musselman and S. Ma, J. Am. Chem. Soc., 2011, 133, 10382–10385 CrossRef CAS PubMed.
- X. Wu, J. Ge, C. Yang, M. Hou and Z. Liu, Chem. Commun., 2015, 51, 13408–13411 RSC.
- C. Hou, Y. Wang, Q. Ding, L. Jiang, M. Li, W. Zhu, D. Pan, H. Zhu and M. Liu, Nanoscale, 2015, 7, 18770–18779 RSC.
- M. H. Pham, G. T. Vuong, A. T. Vu and T. O. Do, Langmuir, 2011, 27, 15261–15267 CrossRef CAS PubMed.
- Z. Hu, C. a. Tao, H. Liu, X. Zou, H. Zhu and J. Wang, J. Mater. Chem. A, 2014, 2, 14222–14227 CAS.
- L. Paseta, G. Potier, S. Abbott and J. Coronas, Org. Biomol. Chem., 2015, 13, 1724–1731 CAS.
- N. Liédana, P. Lozano, A. Galve, C. Téllez and J. Coronas, J. Mater. Chem. B, 2014, 2, 1144–1151 RSC.
- D. Wang, R. Huang, W. Liu, D. Sun and Z. Li, ACS Catal., 2014, 4, 4254–4260 CrossRef CAS.
- V. Pascanu, A. B. Gómez, C. Ayats, A. E. P. Prats, F. Carson, J. Su, Q. Yao, M. À. Pericás, X. Zou and B. M. Matute, ACS Catal., 2015, 5, 472–479 CrossRef CAS.
- B. Sahoo, S. K. Sahu, D. Bhattacharya, D. Dhara and P. Pramanik, Colloids Surf., B, 2013, 101, 280–289 CrossRef CAS PubMed.
- S. Bauer, C. Serre, T. Devic, P. Horcajada, J. Marrot, G. Férey and N. Stock, Inorg. Chem., 2008, 47, 7568–7576 CrossRef CAS PubMed.
- X. Liu, Y. Li, W. Zhu and P. Fu, CrystEngComm, 2013, 15, 4937–4947 RSC.
- C. Hou, Y. Wang, Y. Li and H. Zhu, J. Mater. Chem. B, 2015, 3, 2883–2891 RSC.
- H. Guo, D. Wang, J. Chen, W. Weng, M. Huang and Z. Zheng, Chem. Eng. J., 2016, 289, 479–485 CrossRef CAS.
- T. K. Mahto, A. Ray Chowdhuri, B. Sahoo and S. K. Sahu, Polym. Compos., 2016, 37(4), 1152–1160 CrossRef CAS.
- C. Zhou, W. Jiang, B. K. Via, O. Fasina and G. Han, Carbohydr. Polym., 2015, 121, 336–341 CrossRef CAS PubMed.
- A. Ray Chowdhuri, D. Bhattacharya and S. K. Sahu, Dalton Trans., 2016, 45, 2963–2973 RSC.
- M. Ma, A. Bétard, I. Weber, N. S. Al-Hokbany, R. A. Fischer and N. Metzler-Nolte, Cryst. Growth Des., 2013, 13, 2286–2291 CAS.
- Z. Hu, C. Tao, H. Liu, X. Zou, H. Zhu and J. Wang, J. Mater. Chem. A, 2014, 2, 14222–14227 CAS.
- M. Kalantari, M. Kazemeini, F. Tabandeh and A. Arpanaei, J. Mater. Chem., 2012, 22, 8385–8393 RSC.
- J. N. Talbert, L. S. Wang, B. Duncan, Y. Jeong, S. M. Andler, V. M. Rotello and J. M. Goddard, Biomacromolecules, 2014, 15(11), 3915–3922 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 


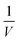 vs.
vs.  . From the intercept, the maximum reaction velocity Vmax was obtained, and the Michaelis constant Km value was calculated from the value of the slope.38
. From the intercept, the maximum reaction velocity Vmax was obtained, and the Michaelis constant Km value was calculated from the value of the slope.38

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of an amide I band. Other bands at 1029, 1082 and 1152 cm−1 are attributed to the carbohydrate moiety of free lipase.41
O stretching of an amide I band. Other bands at 1029, 1082 and 1152 cm−1 are attributed to the carbohydrate moiety of free lipase.41






