Capping agent-free highly dispersed noble metal nanoparticles supported in ordered mesoporous carbon with short channels and their catalytic applications†
Wenjun Gao,
Siwen Li,
Manas Pal,
Yong Liu,
Xiaoyue Wan,
Wei Li,
Shuai Wang,
Changyao Wang,
Gengfeng Zheng* and
Dongyuan Zhao*
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai 200433, China. E-mail: dyzhao@fudan.edu.cn; gfzheng@fudan.edu.cn
First published on 8th June 2016
Abstract
Homogeneously dispersed small noble metal nanoparticles such as Pt and Au supported on ordered mesoporous carbon nanospheres have been successfully synthesized via a facile hydrothermal method without using any capping agent and further post-treated with reducing agent. The average sizes of the Pt and Au nanoparticles are estimated to be ∼1.7 and ∼5 nm. The small size of the nanoparticles and short length channels of mesopores greatly improve their catalytic properties. As proof-of-concept, the catalytic performances of the mesoporous metal/C composites are investigated using the reduction of 4-nitrophenol as a model reaction. Furthermore, the catalytic activity of the obtained catalysts for oxidation of benzyl alcohol to benzoic acid in the presence of O2 at 60 °C is also investigated. The results demonstrate that such mesoporous carbon supported metal nanoparticles can be used as reusable catalysts with high catalytic activity.
Introduction
Noble metal nanoparticles (such as Pt and Au) have been attracting substantial attention due to their unique catalytic properties1 and potential applications in oxygen reduction reaction (ORR),2 fuel oxidation reaction,3 CO oxidation,4 selective cyclohexene oxidation,5 and Suzuki reaction.6 Generally, nanoparticles with small sizes typically enable highly active centers that are closely related to the catalytic activities,7 while they also tend to aggregate due to high surface energy.7b,8 One effective approach for obtaining desired catalysts with a high dispersion and proper particle size is to locate metal nanoparticles on support with large surface areas, excellent electrical conductivity, high chemical stability, and good thermal stability, as well as preventing the nanoparticle aggregation and thus maintaining the catalytic performances.9 Carbon-based materials have been widely used as catalyst supports in acidic or alkaline media,5,7a,10 such as graphene,11 reduced graphene oxide,12 carbon nanotubes,13 carbon nanofibers,14 mesoporous carbon nitride,10c,15 and mesoporous carbon.10c,16 In addition, the synthesized metal nanoparticles are often capped with stabilizer,17 such as, thiol-containing groups,12b,16b,18 polymer,12a,19 inbuilt functional –NH2 or –NH groups,10b,15a citrate,10b,11a,20 etc.Another key factor determining catalytic performance is the accessibility of active sites, which is important for the mass transfer rate of reactants and products from the active sites.7b,10c To further improve the catalytic activity and durability of the metal–carbon catalysts, the catalysts with controlled porosities and architectures have been reported in recent years. For example, metal nanoparticles embedded in mesoporous carbon nitride,15a metal/ordered mesoporous carbon,16a,b mesoporous carbon spheres.10c Compared with the bulk mesoporous carbon, mesoporous carbon nanospheres with a short channel length as catalyst supports are more easily accessible for reactants, thus improving the diffusion rates.
Herein, we report a facile hydrothermal method without the need for surface modification and any external agent to prepare well-defined Pt or/and Au nanoparticles supported on ordered mesoporous carbon nanospheres. The mesoporous structure can effectively prohibit the aggregation of metal nanoparticles. Additionally, the opened mesopores with short channels make it easy for reactants access pores and contact with active sites. The catalytic results reveal that the synthesized ordered mesoporous nanospheres supported Pt or Au nanoparticles show high catalytic performances for the reduction of 4-nitrophenol to 4-aminophenol and aerobic oxidation of benzyl alcohol to benzoic acid.
Experimental section
Synthesis of ordered mesoporous Pt/C, Au/C and Pt–Au/C composites
The metallic nanoparticles incorporated ordered mesoporous carbon were prepared by using phenol/formaldehyde resols, triblock copolymer F127, H2PtCl6 and/or HAuCl4 co-assembly under a hydrothermal condition.21 In a typical synthesis, phenol (0.6 g) and NaOH aqueous solution (0.1 M, 15 mL) were added to the round-bottom flask with continuous stirring at 69 °C. Then, 2.2 mL of formaldehyde solution (37 wt%) was added. After stirring for 20–30 min, an aqueous solution of F127 (6.0 wt%, 15 mL) was added and stirred at 69 °C for another 2 h. After that, 50 mL of H2O was added to mixture and stirred at 69 °C for 13 h. Subsequently, 20 mL of the above mixture was dispersed in H2O (55 mL), and mixed with a H2PtCl6 aqueous solution (0.20 mL, 38.6 mmol L−1) or a HAuCl4 aqueous solution (0.50, 48.6 mmol L−1), or a mixed solution containing aqueous H2PtCl6 (0.20 mL, 38.6 mmol L−1) and HAuCl4 (0.30 mL, 48.6 mmol L−1) under ultrasound for 30 min. Afterward, the mixture was transferred into a Teflon-lined stainless-steel autoclave (100 mL) and heated at 130 °C for 24 h. After cooling down to room temperature, the products were separated by centrifugation, followed by washing with deionized water for 4 times and dried at 60 °C. Finally, the obtained powders were calcined at 600 °C in N2 for 3 h.Catalysis performance measurements
Results and discussion
Ordered mesoporous carbon nanospheres with embedded noble metal nanoparticles (Pt, Au) have been synthesized through a hydrothermal route. The mesostructure and metal content in the final composites can be controlled by adjusting the initial amount of the metal precursors. Small-angle X-ray spectroscopy (SAXS) patterns (Fig. 1A) of the mesoporous Pt/C, Au/C and Pt–Au/C composites show three well-resolved scattering peaks, assigned to the 110, 200, and 211 reflections of a cubic Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesostructure. Wide-angle XRD patterns (Fig. 1B) of the Pt/C, Au/C composites display several well-resolved diffraction peaks, which can be assigned to the 111, 200, 220, and 311 reflections of the metallic Pt (JCPDS: 04-0802), and 111, 200, 220, 311, 222 reflections of the metallic Au (JCPDS: 04-0784) without diffraction assigned to platinum oxides or gold oxides. These results clearly indicate that the mesoporous Pt/C, Au/C composites possess well-crystallized Pt, Au nanoparticles. The Pt and Au contents are about 1.5% and 5.4%, respectively, determined by the ICP-AES analysis. While compared with the mesoporous Au/C composite, the Pt–Au/C composite sample exhibits four well-resolved diffraction peaks with a little right-shift due to some overlap range, suggesting bimetallic crystalline structure. This result can be further supported by XPS spectra. As shown in Fig. S1c and d,† the typical XPS peaks of Au (4f7/2), Au (4f5/2) and Pt (4f7/2), Pt (4f5/2) can be observed at 84.2, 87.9 eV and 71.1, 74.5 eV, respectively, indicating that the gold and platinum in this composite are present in metallic state.22 Additionally, XPS spectra of Pt (4f) in the Pt/C composites and Au (4f) in the Au/C samples (Fig. S1a and b†) show the two resolved peaks of metallic state at 71.7, 75.1 eV and 84.3, 88.0 eV, respectively. N2 adsorption–desorption isotherms (Fig. S2†) of all the metal/C composites exhibit type I curves with H1 hysteresis loop at the high relative pressure. The pore distribution curves show uniform mesopores centered at ∼2.2 nm (Table 1).
m mesostructure. Wide-angle XRD patterns (Fig. 1B) of the Pt/C, Au/C composites display several well-resolved diffraction peaks, which can be assigned to the 111, 200, 220, and 311 reflections of the metallic Pt (JCPDS: 04-0802), and 111, 200, 220, 311, 222 reflections of the metallic Au (JCPDS: 04-0784) without diffraction assigned to platinum oxides or gold oxides. These results clearly indicate that the mesoporous Pt/C, Au/C composites possess well-crystallized Pt, Au nanoparticles. The Pt and Au contents are about 1.5% and 5.4%, respectively, determined by the ICP-AES analysis. While compared with the mesoporous Au/C composite, the Pt–Au/C composite sample exhibits four well-resolved diffraction peaks with a little right-shift due to some overlap range, suggesting bimetallic crystalline structure. This result can be further supported by XPS spectra. As shown in Fig. S1c and d,† the typical XPS peaks of Au (4f7/2), Au (4f5/2) and Pt (4f7/2), Pt (4f5/2) can be observed at 84.2, 87.9 eV and 71.1, 74.5 eV, respectively, indicating that the gold and platinum in this composite are present in metallic state.22 Additionally, XPS spectra of Pt (4f) in the Pt/C composites and Au (4f) in the Au/C samples (Fig. S1a and b†) show the two resolved peaks of metallic state at 71.7, 75.1 eV and 84.3, 88.0 eV, respectively. N2 adsorption–desorption isotherms (Fig. S2†) of all the metal/C composites exhibit type I curves with H1 hysteresis loop at the high relative pressure. The pore distribution curves show uniform mesopores centered at ∼2.2 nm (Table 1).
 | ||
| Fig. 1 SAXS (A) and wide-angle XRD patterns (B) of the mesoporous noble metal/C catalysts prepared via a hydrothermal method: (a) Pt/C, (b) Au/C, (c) Pt–Au/C, respectively. | ||
| Sample | Metal content | SBET (m2 g−1) | DP (nm) | V (cm3 g−1) | ka (min−1) | Conversionb (%) |
|---|---|---|---|---|---|---|
| a Rate constant for the catalytic reduction of 4-NP to 4-AP with NaBH4 at 25 °C.b The conversion of benzyl alcohol for the oxidation of benzyl alcohol with O2 in water at 60 °C and a reaction time of 12 h.c Pt% = 1.3%, Au% = 2.9%. | ||||||
| Pt/C | 1.5% | 569 | 2.2 | 0.47 | 0.19 | 46 |
| Au/C | 5.4% | 624 | 2.2 | 0.53 | 0.31 | 100 |
| Pt–Au/C | 1.3%/2.9%c | 521 | 2.2 | 0.37 | 0.39 | 88 |
Field-emission scanning electron microscopy (FESEM) images (Fig. 2a and c) show that all the Pt/C, Au/C composites have the similar uniform nanospherical morphology in a large domain. The high-resolution SEM (HRSEM) images (Fig. 2b and d) further confirm that uniform nanospheres contain ordered and opened mesopores, the average diameter of the Pt/C and Au/C composite nanospheres is observed to be 200–300 nm. Nevertheless, the mesoporous Pt–Au/C composite sample possesses rhombic dodecahedral morphology with average size of 300–400 nm (Fig. 2e and f), larger than Pt/C and Au/C composite nanospheres. As displayed in Fig. S3,† the morphologies of the resulting products are significantly altered with a high amount of metal precursors added. This result is probably due to the pH change as initial amount of metal precursor solution increases, thus leading to form single crystal, pie-like, even interconnected nanospheres during the hydrothermal process (Fig. S3b, d and f†). The above results imply that the pH value of the reactant solution plays an important role in this synthesis process. Moreover, another phenomenon observed is that a small number of metal nanoparticles are visible on the nanospheres surface, while at the same time no obvious aggregated metal nanoparticles are observed (Fig. 2d and f).
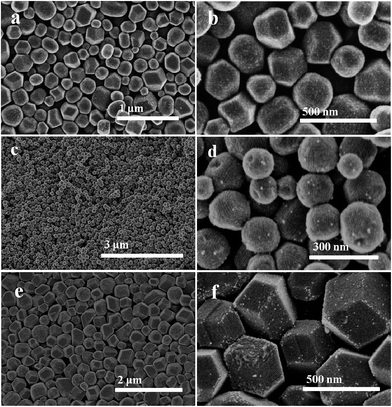 | ||
| Fig. 2 FESEM images of the mesoporous noble metal/C catalysts prepared via a hydrothermal method: (a and b) Pt/C, (c and d) Au/C, (e and f) Pt–Au/C, respectively. | ||
Transmission electron microscopy (TEM) images of all the mesoporous metal/C composites further demonstrate that the mesopores are opened and ordered (Fig. 3). The average channel length of the Pt/C and Au/C composite nanospheres is observed to be 200–300 nm, smaller than Pt–Au/C composites channel length, which is in agreement with the SEM observations. Uniform Pt, Au and Pt–Au bimetallic nanoparticles are well dispersed in the mesoporous carbon matrix and no noticeable aggregation of nanoparticles can be detected. The high-resolution TEM (HRTEM) images of the mesoporous Pt/C sample show that the nanoparticles with an average size of 1.7 nm have a d-spacing of 0.23 nm, which corresponds to the (111) plane of Pt (Fig. 3b inset, Fig. S4a†). The size of Au nanoparticles in the mesoporous Au/C catalysts based on the corresponding HRTEM images (Fig. 3f) is about 5 nm larger than Pt particles. This observation is in agreement with the result from the XRD. Meanwhile, the Au nanoparticles show well-resolved lattices of 0.24 nm identified as Au (111) (Fig. 3f). Similarly, highly dispersed and uniform Pt and Au nanoparticles of the Pt–Au/C composite sample are observed in the mesoporous carbon frameworks (Fig. 3g–i). Pt and Au contents of the mesoporous Pt–Au/C sample are estimated to be ∼1.3 wt% and ∼2.9 wt% from ICP-AES analysis, respectively. Fig. 3e, h and i with the enlargement of the nanoparticle size show that these nanoparticles can penetrate the carbon walls and confined in the carbon nanospheres. It is worth to note that the nanoparticles are also highly dispersed in the amorphous mesoporous carbon matrix, even if obtained from that the higher metal precursor added into this synthesis (Fig. S4b–d†).
 | ||
| Fig. 3 TEM images of the mesoporous noble metal/C catalysts prepared via a hydrothermal method: (a–c) Pt/C, (d–f) Au/C, (g–i) Pt–Au/C, respectively. | ||
To investigate the transformation of the precursor to metal nanoparticles in the hydrothermal process, XRD patterns for the as-made mesoporous Au/C samples without calcination were measured (Fig. S5†). The characteristic diffraction peaks for Au nanoparticles are observed, suggesting that the formation of Au nanoparticles due to the reduction of formaldehyde in this process. TEM images of the as-made mesostructured Pt/C samples without calcination further confirm that Pt nanoparticles are formed in the presence of formaldehyde in this hydrothermal process (Fig. S5†).
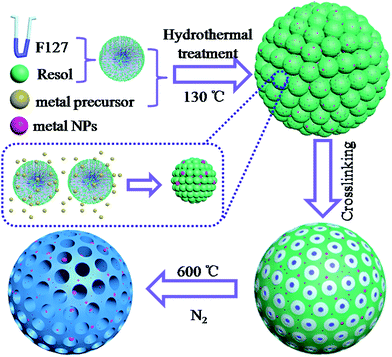
On the basis of the results described above, we propose the following mechanization (Scheme 1). First, spherical resol–F127 monomicelles are obtained from the assembly between Pluronic triblock copolymer (F127) and phenolic resols. With addition of HAuCl4, small metal nanoparticles are formed in the presence of formaldehyde in this mixed solution.23 Subsequently, metal nanoparticles are embedded in the resol–F127 monomicelles, forming metal–resol–F127 monomicelles. These spherical monomicelles further assemble to an ordered array by a close-packing structure. The formation of metal nanoparticles embedded in the ordered mesoporous carbon is mainly attributed to the co-assembly of resol–F127 monomicelles with metal nanoparticles, while preventing the aggregation during pyrolysis. As the content of metal precursor solution increases, although the metal nanoparticles show no significant change in the size, the morphology has apparently changed because of different pH caused by adding the metal precursor solution. Therefore, with an appropriate amount of metal precursor solution, the metal nanoparticles are highly dispersed in the ordered mesoporous carbon without using any capping agent.
The catalytic performance of the samples was evaluated by employing the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) with NaBH4 at 25 °C as a model reaction (Fig. 4). The conversion of (4-NP) was in situ monitored by UV-vis absorption spectrophotometer, recording the change of its characteristic absorbance at λ = 400 nm. In the absence of catalyst, the peak intensity at 400 nm is remained almost unchanged for about 24 h, indicating that the reduction does not take place (Fig. S7†). Fig. 4 shows that all the mesoporous composite nanosphere catalysts exhibit good activity for reduction of 4-NP. The complete (>95%) conversion of 4-NP can be reached within ∼18, ∼13, and ∼9 min for the mesoporous Pt/C, Au/C and Pt–Au/C nanosphere catalysts, respectively. The rate constants (k) of the reduction reaction are evaluated and summarized in Fig. 4 and Table 1. We calculated the TOF (turnover frequency), the corresponding results are summarized in Table 2. The TOF values of these catalysts are comparable or higher than that reported previously.22c,24 In addition, with the increase of temperature, the catalytic activity of the mesoporous metal/C catalysts improves remarkably (Fig. 5, Table S1†).
| Catalyst | kwa (s−1 g−1) | TOFb (mmol s−1 g−1) | Reference |
|---|---|---|---|
| a The rate constant k was obtained from the slope of the fit line for the first cycle of the reduction of 4-NP to 4-AP. kw was obtained from the ratio of rate constant k (25 °C) over the weight of catalyst Pt, Au, or Pt + Au: kw = k/total metal weight of catalyst.b TOF (turn over frequency) was estimated from the equation: (mmol of 4-NP) × kw. | |||
| Pt–C | 111.1 | 2.2 × 10−3 | This work |
| Au/C | 50.4 | 1.0 × 10−3 | This work |
| Pt–Au/C | 81.5 | 1.6 × 10−3 | This work |
| Pt–P2VP | 8.57 | 8.6 × 10−4 | 24a |
| Ag–Au–carbon sphere | 9.75 | 1.4 × 10−3 | 24b |
| Spongy Au nanocrystals | 0.35 | 1.1 × 10−4 | 24c |
| Au–Pd/CS | 42.6 | 6.0 × 10−3 | 24d |
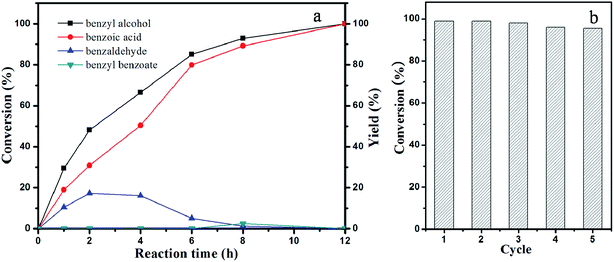
Furthermore, the catalytic performance was also investigated by employing the selective oxidation of benzyl alcohol with O2 in water at 60 °C as a model reaction. The product concentrations were analyzed by high-performance gas chromatography, and the results are summarized in Table 1. The mesoporous Au/C nanosphere catalyst gives complete conversion (∼100%) in 12 h (Fig. 6a, Table 1), whereas within the same reaction time, the Pt/C and Pt–Au/C samples have conversions of only 46% and 88%, respectively. Remarkably, the Au/C composites can serve as efficient catalysts for the complete and selective oxidation of benzyl alcohol to benzoic acid under atmospheric pressure (Fig. 6a). As shown in Fig. 6a, the conversion of benzyl alcohol on the mesoporous Au/C (Au: ∼5.4 wt%) catalyst is ∼29.5% initially, and then almost increases to ∼100% after 12 h under atmospheric pressure. In addition, benzaldehyde and benzyl benzoate are formed as byproducts during reaction process. The yield of benzaldehyde can reach ∼17% at 2 h and then gradual decrease to ∼0 when the reaction time is extended to 12 h. Compared with benzaldehyde, only a tiny small amount of benzyl benzoate is detected and can be neglected. The catalytic activity of the Au/C (Au: ∼5.4 wt%) catalyst is superior to that for the Au–mesoporous carbon materials with a long mesopore channel reported previously.16b To our knowledge, the aforementioned result is the best one among the Au-based catalysts reported previously (Table 3). The high catalytic performance can be attributed to the efficient contact of reactants with metal nanoparticles, which resulting from the improvement of infiltration and diffusion of reactants in these short length mesopore channels during the reaction.
| Catalyst | Au content | Reaction condition | t (h) | Conv. (%) | Sel.% (acid) | TOF (h−1) | Ref. |
|---|---|---|---|---|---|---|---|
| a TOF (turn over frequency: mmol of product/mmol of total Au of catalyst)25b was measured after 1.0 h of reaction.b The selectivity to benzaldehyde.c n.p: not provided. | |||||||
| Au/C | 5.4 wt% | T = 60 °C | 12 | >99 | >99 | 517a | This work |
| P = 1 atm | |||||||
| Au/SBA-15 | 4 wt% | T = 80 °C | 8 | 6.42 | ∼97b | 308 | 25a |
| P = 1 atm | |||||||
| Au–C | 5.9 wt% | T = 60 °C | 12 | >99 | >99 | 384 | 16b |
| P = 1 atm | |||||||
| Au/TiO2 | 1 wt% | T = 160 °C | 6 | 55 | 15.1 | 9780 | 22b |
| P = 10 atm | |||||||
| Au/P0PD | 3 mol% | T = RT | 24 | 99 | 99 | n.pc | 3a |
| P = 1 atm | |||||||
Reusability is also important merit of robust heterogeneous catalysts. In order to evaluate the reusability of the mesoporous Pt–Au, Au/C composites, the recovered catalyst is re-dispersed in the previous mixture maintaining the same reaction condition as described above. After five cycles of catalytic hydrogenation (Fig. 5d) and oxidation of benzyl alcohol (Fig. 6b), respectively, the reused catalyst gives almost the same activity as that of first run. TEM images of the reused Pt–Au/C and Au/C catalysts still present highly dispersed metal nanoparticles without obvious aggregation (Fig. S8†). The reaction solutions for the oxidation of benzyl alcohol were collected by using hot filtration and analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES) to determine the amount of the metal leached. No metals were detected in the filtered reaction mixtures, implying the negligible metal leaching in the process of the reaction. These results demonstrate the excellent stability of the mesoporous Pt–Au/C and Au/C nanosphere catalysts, suggesting that these catalysts have potential applications in hydrogenation and selective oxidation.
Conclusions
Ordered mesoporous carbon nanospheres with highly dispersed noble metal nanoparticles have been designed and synthesized through a hydrothermal method without addition of any capping agent or further post-treatment with reducing agent. In these mesoporous Pt/C, Au/C and Pt–Au/C composites, the small-sized metal nanoparticles are homogeneously dispersed and easily accessible in these mesopore channels with a short length of 200–400 nm. Meanwhile, the sintering of metal nanoparticles can be avoided, even under harsh reaction conditions, because of the confinement effect and chemical inertness of mesoporous carbon. Compared to the mesoporous carbon with a long length pore channel supported Au catalysts, the resultant ordered mesoporous Pt/C, Au/C and Pt–Au/C catalysts exhibit smaller metal particle size, more uniform metal dispersion, and higher catalytic performance for the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) and oxidation of benzyl alcohol to benzoic acid. This facile synthetic route is expected to open new routes for designing highly efficient catalysts.Acknowledgements
We thank the following funding agencies for supporting this work: the National Key Basic Research Program of China (2013CB934104, 2012CB224805), the Natural Science Foundation of China (21210004, 21322311, 21473038, U1463206), the Science and Technology Commission of Shanghai Municipality (14JC1400700, 14JC1490500), and the Collaborative Innovation Center of Chemistry for Energy Materials (2011-iChem). The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the Prolific Research group (PRG-1436-14).References
- (a) M. Stratakis and H. Garcia, Chem. Rev., 2012, 112, 4469 CrossRef CAS PubMed; (b) M. Turner, V. B. Golovko, O. P. H. Vaughan, P. Abdulkin, A. Berenguer-Murcia, M. S. Tikhov, B. F. G. Johnson and R. M. Lambert, Nature, 2008, 454, 981 CrossRef CAS PubMed; (c) R. Wang, C. Wang, W.-B. Cai and Y. Ding, Adv. Mater., 2010, 22, 1845 CrossRef CAS PubMed; (d) Z. Wen, J. Liu and J. Li, Adv. Mater., 2008, 20, 743 CrossRef CAS.
- (a) Y. Nie, L. Li and Z. Wei, Chem. Soc. Rev., 2015, 44, 2168 RSC; (b) K. Miyabayashi, H. Nishihara and M. Miyake, Langmuir, 2014, 30, 2936 CrossRef CAS PubMed; (c) J. Zhang, K. Sasaki, E. Sutter and R. R. Adzic, Science, 2007, 315, 220 CrossRef CAS PubMed.
- (a) J. Han, Y. Liu and R. Guo, Adv. Funct. Mater., 2009, 19, 1112 CrossRef CAS; (b) C.-R. Chang, X.-F. Yang, B. Long and J. Li, ACS Catal., 2013, 3, 1693 CrossRef CAS; (c) J. Chen, B. Lim, E. P. Lee and Y. Xia, Nano Today, 2009, 4, 81 CrossRef CAS.
- (a) M. Comotti, W.-C. Li, B. Spliethoff and F. Schüth, J. Am. Chem. Soc., 2006, 128, 917 CrossRef CAS PubMed; (b) N. Lopez, T. V. W. Janssens, B. S. Clausen, Y. Xu, M. Mavrikakis, T. Bligaard and J. K. Nørskov, J. Catal., 2004, 223, 232 CrossRef CAS; (c) K. Ding, A. Gulec, A. M. Johnson, N. M. Schweitzer, G. D. Stucky, L. D. Marks and P. C. Stair, Science, 2015, 350, 189 CrossRef CAS PubMed.
- M. D. Hughes, Y.-J. Xu, P. Jenkins, P. McMorn, P. Landon, D. I. Enache, A. F. Carley, G. A. Attard, G. J. Hutchings, F. King, E. H. Stitt, P. Johnston, K. Griffin and C. J. Kiely, Nature, 2005, 437, 1132 CrossRef CAS PubMed.
- Y. Li, X. Fan, J. Qi, J. Ji, S. Wang, G. Zhang and F. Zhang, Mater. Res. Bull., 2010, 45, 1413 CrossRef CAS.
- (a) Q.-L. Zhu, N. Tsumori and Q. Xu, J. Am. Chem. Soc., 2015, 137, 11743 CrossRef CAS PubMed; (b) P. Munnik, P. E. de Jongh and K. P. de Jong, Chem. Rev., 2015, 115, 6687 CrossRef CAS PubMed; (c) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS PubMed.
- X. Yan, X. Wang, Y. Tang, G. Ma, S. Zou, R. Li, X. Peng, S. Dai and J. Fan, Chem. Mater., 2013, 25, 1556 CrossRef CAS.
- (a) F.-Z. Song, Q.-L. Zhu, N. Tsumori and Q. Xu, ACS Catal., 2015, 5, 5141 CrossRef CAS; (b) Y. Chen, Q.-L. Zhu, N. Tsumori and Q. Xu, J. Am. Chem. Soc., 2015, 137, 106 CrossRef CAS PubMed.
- (a) B. N. Zope, D. D. Hibbitts, M. Neurock and R. J. Davis, Science, 2010, 330, 74 CrossRef CAS PubMed; (b) R. Cui, C. Liu, J. Shen, D. Gao, J.-J. Zhu and H.-Y. Chen, Adv. Funct. Mater., 2008, 18, 2197 CrossRef CAS; (c) C. Zhang, L. Xu, N. Shan, T. Sun, J. Chen and Y. Yan, ACS Catal., 2014, 4, 1926 CrossRef CAS.
- (a) Y. Fang, S. Guo, C. Zhu, Y. Zhai and E. Wang, Langmuir, 2010, 26, 11277 CrossRef CAS PubMed; (b) B.-S. Kong, J. Geng and H.-T. Jung, Chem. Commun., 2009, 2174 RSC.
- (a) S.-S. Kim, Y.-R. Kim, T. D. Chung and B.-H. Sohn, Adv. Funct. Mater., 2014, 24, 2764 CrossRef CAS; (b) J. Huang, L. Zhang, B. Chen, N. Ji, F. Chen, Y. Zhang and Z. Zhang, Nanoscale, 2010, 2, 2733 RSC.
- (a) G. G. Wildgoose, C. E. Banks and R. G. Compton, Small, 2006, 2, 182 CrossRef CAS PubMed; (b) B. Yoon and C. M. Wai, J. Am. Chem. Soc., 2005, 127, 17174 CrossRef CAS PubMed; (c) J. John, E. Gravel, A. Hagège, H. Li, T. Gacoin and E. Doris, Angew. Chem., 2011, 123, 7675 CrossRef; (d) B. Xue, P. Chen, Q. Hong, J. Lin and K. L. Tan, J. Mater. Chem., 2001, 11, 2378 RSC.
- (a) H. S. Qian, M. Antonietti and S. H. Yu, Adv. Funct. Mater., 2007, 17, 637 CrossRef CAS; (b) P. Serp, M. Corrias and P. Kalck, Appl. Catal., A, 2003, 253, 337 CrossRef CAS; (c) D. Wang, Y. Liu, J. Huang and T. You, J. Colloid Interface Sci., 2012, 367, 199 CrossRef CAS PubMed.
- (a) K. K. R. Datta, B. V. S. Reddy, K. Ariga and A. Vinu, Angew. Chem., Int. Ed., 2010, 49, 5961 CrossRef CAS PubMed; (b) X.-H. Li and M. Antonietti, Chem. Soc. Rev., 2013, 42, 6593 RSC; (c) B. Dai, X. Li, J. Zhang, F. Yu and M. Zhu, Chem. Eng. Sci., 2015, 135, 472 CrossRef CAS; (d) Y. Zhou, K. Neyerlin, T. S. Olson, S. Pylypenko, J. Bult, H. N. Dinh, T. Gennett, Z. Shao and R. O'Hayre, Energy Environ. Sci., 2010, 3, 1437 RSC.
- (a) J. Kang, O. L. Li and N. Saito, Nanoscale, 2013, 5, 6874 RSC; (b) S. Wang, Q. Zhao, H. Wei, J.-Q. Wang, M. Cho, H. S. Cho, O. Terasaki and Y. Wan, J. Am. Chem. Soc., 2013, 135, 11849 CrossRef CAS PubMed; (c) S. H. Joo, S. J. Choi, I. Oh, J. Kwak, Z. Liu, O. Terasaki and R. Ryoo, Nature, 2001, 412, 169 CrossRef CAS PubMed.
- (a) S. Zhang, Y. Shao, H.-g. Liao, J. Liu, I. A. Aksay, G. Yin and Y. Lin, Chem. Mater., 2011, 23, 1079 CrossRef CAS; (b) L. Shang, T. Bian, B. Zhang, D. Zhang, L.-Z. Wu, C.-H. Tung, Y. Yin and T. Zhang, Angew. Chem., 2014, 126, 254 CrossRef.
- Y.-T. Kim and T. Mitani, J. Catal., 2006, 238, 394 CrossRef CAS.
- M. Chen and Y. Xing, Langmuir, 2005, 21, 9334 CrossRef CAS PubMed.
- Y. Qian, C. Wang and Z.-G. Le, Appl. Surf. Sci., 2011, 257, 10758 CrossRef CAS.
- (a) D. Gu, H. Bongard, Y. Meng, K. Miyasaka, O. Terasaki, F. Zhang, Y. Deng, Z. Wu, D. Feng, Y. Fang, B. Tu, F. Schüth and D. Zhao, Chem. Mater., 2010, 22, 4828 CrossRef CAS; (b) Y. Fang, D. Gu, Y. Zou, Z. Wu, F. Li, R. Che, Y. Deng, B. Tu and D. Zhao, Angew. Chem., Int. Ed., 2010, 49, 7987 CrossRef CAS PubMed; (c) R. Liu, L. Wan, S. Liu, L. Pan, D. Wu and D. Zhao, Adv. Funct. Mater., 2015, 25, 526 CrossRef CAS.
- (a) J. Zhang, Y. Jin, C. Li, Y. Shen, L. Han, Z. Hu, X. Di and Z. Liu, Appl. Catal., B, 2009, 91, 11 CrossRef CAS; (b) N. Dimitratos, J. A. Lopez-Sanchez, D. Morgan, A. Carley, L. Prati and G. J. Hutchings, Catal. Today, 2007, 122, 317 CrossRef CAS; (c) J. Li, C.-y. Liu and Y. Liu, J. Mater. Chem., 2012, 22, 8426 RSC; (d) A. Bisht, P. Zhang, C. Shivakumara and S. Sharma, J. Phys. Chem. C, 2015, 119, 14126 CrossRef CAS.
- (a) J. Chen, R. Zhang, B. Tu and D. Zhao, Nano Res., 2013, 6, 871 CrossRef CAS; (b) L. Han, H. Wei, B. Tu and D. Zhao, Chem. Commun., 2011, 47, 8536 RSC.
- (a) H. Ma, Y. Geng, Y.-I. Lee, J. Hao and H.-G. Liu, Colloids Surf., A, 2013, 419, 201 CrossRef CAS; (b) S. Tang, S. Vongehr and X. Meng, J. Mater. Chem., 2010, 20, 5436 RSC; (c) M. H. Rashid, R. R. Bhattacharjee, A. Kotal and T. K. Mandal, Langmuir, 2006, 22, 7141 CrossRef CAS PubMed; (d) S. Tang, S. Vongehr, G. He, L. Chen and X. Meng, J. Colloid Interface Sci., 2012, 375, 125 CrossRef CAS PubMed.
- (a) C. Y. Ma, B. J. Dou, J. J. Li, J. Cheng, Q. Hu, Z. P. Hao and S. Z. Qiao, Appl. Catal., B, 2009, 92, 202 CrossRef CAS; (b) B. Hvolbæk, T. V. W. Janssens, B. S. Clausen, H. Falsig, C. H. Christensen and J. K. Nørskov, Nano Today, 2007, 2, 14 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10636f |
| This journal is © The Royal Society of Chemistry 2016 |