Nickel sulfide counter electrode modified with polypyrrole nanoparticles to enhance catalytic ability for flexible dye-sensitized solar cells
Abstract
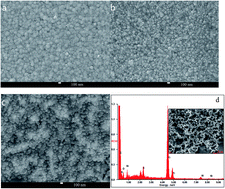
An efficient counter electrode (CE) of nickel sulfide/polypyrrole/titanium foil (NiS/PPy/Ti) with excellent electrochemical catalytic ability was prepared and used as a platinum (Pt)-free CE for flexible dye-sensitized solar cells (FDSSCs). The surface morphology of the NiS/PPy/Ti CE was characterized by scanning electron microscopy. The electrochemical performance was also characterized according to a series of electrochemical tests which indicated that the NiS/PPy/Ti CE showed great electrocatalytic ability and low charge transfer resistance compared to the Pt/Ti electrode in an iodide/triiodide electrolyte. As a result, the FDSSC based on the NiS/PPy/Ti CE exhibited a high light–electric conversion efficiency of 7.66% under 100 mW cm−2 illumination, comparable to that of the Pt/Ti-based FDSSC (7.31%). Furthermore, the FDSSC based on the NiS/PPy/Ti counter electrode had increased short-circuit current density and is very stable.

 Please wait while we load your content...
Please wait while we load your content...