Cross-linkable polymers containing a triple bond backbone and their application in photovoltaic devices
Abstract
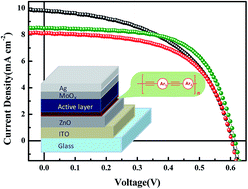
Two novel polymers containing triple bonds in the backbone with different conjugation types (acceptor–acceptor and acceptor–donor structures) were synthesized and investigated for their crosslinking characteristics under UV irradiation. The crosslink formation was proven via UV-vis and IR spectroscopy, and it was found that the crosslinked polymers have solvent resistance properties during subsequent solvent washing with a similar solvent for the active layer. The two triple bond polymers were used as buffer layer materials to modify the interface properties of electron-selective ZnO in inverted PSCs via a spin-coating process. The effects of the buffer layer on the surface of the ZnO were studied via atomic force microscopy and contact angle measurements. The increased hydrophobic nature of the ZnO surface resulted in better contact with the active layer, and led to an improvement in the performance of the photovoltaic devices with increased fill factor (FF).

 Please wait while we load your content...
Please wait while we load your content...