Enhancing the thermophysical properties and tribological behaviour of engine oils using nano-lubricant additives
Abstract
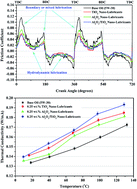
This paper presents the enhancement of the thermophysical properties (thermal conductivity and viscosity) of engine oil using nano-lubricant additives and a characterization of tribological behaviour in terms of sliding contact interfaces (piston ring assembly) in automotive engines. Al2O3, TiO2 and Al2O3/TiO2 hybrid nanoparticles were suspended in commercially available engine oil (5W-30) in a concentration of 0.25 wt% for formulating nano-lubricants. The sizes of Al2O3 nanoparticles were within the range 8–12 nm while the TiO2 nanoparticles used had a size of 10 nm. The tribological experiments were performed using a tribotester to simulate the sliding reciprocating motion of the piston ring/cylinder liner interface in an engine. The performed tribological tests were all carried out under varying speeds, loads and sliding distances. The experimental results showed that nano-lubricant additives enhanced the thermophysical and tribological properties. The thermal conductivity of lube oil was measured by the 3ω-wire method. Nano-lubricants provide low kinematic viscosity and an increase in the viscosity index by 2%. Meanwhile, thermal conductivity was enhanced by a margin of 12–16% for a temperature range of 10–130 °C facilitating the dissipation of frictional heat and maintaining engine oil properties, as compared with commercial lubricants. The tribological tests showed a minimization of the friction coefficient and wear rate of the ring by 40–50% and 20–30%, respectively. According to the results, nano-lubricants can contribute to improving the efficiency of engines and fuel economy in automotive engines.


 Please wait while we load your content...
Please wait while we load your content...