A new catalyst for the production of furfural from bagasse
M. Yazdizadeh,
M. R. Jafari Nasr* and
A. Safekordi
Department of Chemical Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran. E-mail: nasrmrj@ripi.ir; yazdizadeh.m@gmail.com; safekordi.a@sharif.ac.ir
First published on 27th May 2016
Abstract
Furfural is a poisonous, flammable compound that is widely used in the chemical industry. The main raw materials for producing furfural are “pentosan-rich plant” components such as bagasse. In this study, furfural was produced in a pilot plant using sulfuric acid as a catalyst and an inorganic salt (NaHSO4) as a promoter. The resulting furfural product was experimentally measured and reported in the presence of NaCl + H2SO4 or H2SO4, which were used as reaction catalysts. The obtained results show that H2SO4 plus NaHSO4 is more effective for producing furfural than the other catalysts investigated. Furthermore, a three-layer feed-forward neural network analysis was applied to predict furfural production from the reactor. It was observed that the neural network systems could effectively predict the experimental results. The model for 385 experimental data under various conditions resulted in a squared correlation coefficient of 0.959 and a mean square error of approximately 10% for this analysis.
1. Introduction
Furfural is a commercial, poisonous, and flammable chemical substance. It is water soluble and has a boiling point of 161.8 °C. On the industrial scale, furfural can be produced by chemical hydrolysis performed on farm production residue, such as rice bran and corncob with bagasse, which is rich in pentosan. Furfural can also be produced from hydrolysis in an acidic environment. Just before immersion into the hydrolysis conditions, the prepared bagasse is usually blended with a dilute sulfuric acid solution. In these reactions, sulfuric acid acts as a catalyst. Pentosan-rich plant resources can also be utilized to produce furfural. Initially, pentosan is converted into a monosaccharide. Later, the monosaccharide is dehydrated to form furfural.One of the issues with this process is that the rate of pentose hydrolysis can be several times higher than the rate of monosaccharide dehydration. In both reactions, furfural, as an effective monomer, reacts strongly with the chemical compounds of plant materials. Moreover, the reverse reaction occurs simultaneously. As a result, the generated furfural should immediately be removed from the reaction medium. Fig. 1 shows the overall diagram of furfural production from the bagasse. In 1821, furfural was first produced by Döbereiner, who aimed to produce formic acid from sugar and manganese dioxide.1,2 In 1922, the primary industrial-scale production of furfural was achieved by Quaker Oats, who utilized oats and corncob as raw materials.3 Monomeric sugars, such as pentoses and hexoses, could be obtained from hydrolyzing wood and agricultural waste, which is credited to Henri Branconnot in 1810.4
The method of generation includes three sections, namely, raw material preparation, acid hydrolysis and the separation of the resulting furfural from residual pentosan. Pentosan is available in agricultural residue, which can be converted to xylose by simply hydrolysis. Xylose can be converted into furfural with the loss of approximately three molecules of water. Synthesized furfural may be purified through distillation and dehydration and it can be mixed within a vacuum.5,6 Yang7 and Root8 conducted a comprehensive study on the kinetics of the furfural production reaction. Lamminpaa and coworkers also studied the kinetics of furfural decomposition in an acidic environment.9 They used formic acid as the reaction catalyst. They assumed that the reaction rate, with a reaction constant, was a function of the hydrogen ion concentration and temperature. In 2014, Danon and colleagues validated this procedure, as well as the kinetics of furfural production by evaluating pentose in acidic surroundings.10 They studied the production of furfural from bagasse with the combination of various inorganic salts and diluted H2SO4, which was used as a catalyst. Extensive studies were conducted to evaluate the kinetics of pentosan hydrolysis. Rong and coworkers utilized sulfuric acid plus NaCl or FeCl3 as a catalyst. They showed that the combination of sulfuric acid plus FeCl3 could be more effective than sulfuric acid plus NaCl.11 In 2014, Hongsiri and coworkers studied the kinetics of xylose dehydration when generating furfural in a dilute acidic environment in the presence of salt. Salts could increase the production of furfural and decrease the formation of byproducts.12 Liu and colleagues investigated the conversion of xylose to furfural in the presence of various inorganic salts without using an acid catalyst. In their study, FeCl3 generated the largest increase in conversion rate compared to the other salts.13
Hosaka investigated furfural production conditions without a loss and carbonization of cellulose and observed considerable efficiency. According to Hosaka's studies, a temperature between 140 and 200 °C is appropriate for preventing cellulose loss. The utilization of hydrochloric acid, sulfuric acid, nitric acid, and other acids as catalysts in the furfural production process has been reported.3 Singh et al. reported the production of furfural and fermentable sugars using hydrolyzed bagasse and rice bran with dilute sulfuric acid in the presence of high-pressure water. The experiments were conducted using 0.4% sulfuric acid at 200 °C.14 The maximum efficiencies of furfural production from bagasse and rice bran were 11.5% and 10.9%, respectively.
As a result, from the viewpoint of chemical reaction engineering, the optimum production of furfural depends on various factors such as the initial concentration of pentosan in the feed, the added catalyst level, time of hydrolysis, hydrolysis temperature, and the retention time of the produced furfural in the reaction medium. Many studies have demonstrated the promising efficiency of furfural production per pentose unit in terms of the hydrolysis process, optimum time of hydrolysis, required catalyst, and steam consumption.15 Theoretically, furfural production from pentosan follows eqn (1):16
 | (1) |
Several methods for efficient furfural production from pentosan in large quantities have been published. Some side reactions or the formation of intermediate compounds can also be effective in producing furfural. Likewise, the obtained results from Schonemann's investigations in multistage continuous reactors with a solvent extraction process demonstrated acceptable results with an increase of production efficiency from approximately 60% to 65%.3
In the current study, sulfuric acid plus sodium hydrogen sulfate (NaHSO4 + H2SO4) were used as the catalyst. To predict the output furfural percent from reactors, a three-layer feed-forward neural network employing temperature, reactor pressure, reaction time, sulfuric acid level, and bagasse humidity was introduced. The application of the model for 385 experimental data under various conditions obtained a squared correlation coefficient of 0.959 and a mean square error of approximately 10%.
2. Experimental section
2.1. Materials
Bagasse with a moisture context of 45–70% was obtained from Karun Argo Industry Inc. All other materials used in this study were analytical grade and were purchased from Merck Co.2.2. Experimental procedure
The experimental tests were performed in a stainless steel reactor with a diameter of 400 mm, a height of 1750 mm, and a thickness of 5 mm. Diluted sulfuric acid + aqueous solutions of different salts and 40 kg of bagasse were mixed in the reactor and then the temperature and pressure were increased to the set point. The reactor was heated to 160 °C for 7 minutes. After a suitable time, the produced furfural left the reactor from the top as a vapor. At the end of the hydrolysis process, the residue was removed from the reactor under proper pressure conditions. Subsequently, the furfural that was removed from the reactor was sent to the separator to separate the solid particles from any possible condensation. In the next step, aldehyde vapors were cooled and condensed in a condenser of stainless steel 316 (100 cm in length, 20 cm in diameter, and 5 mm in thickness) with 21 tubes (diameter of 1 inch). The remaining condensate was discharged to the tank, which comprised aldehyde. Subsequently, hydroxylamine (50 mL) and bromophenol (1 mL) were reacted with 1.5 g of the sample. The mixture was then treated with NaOH (1 N). Fig. 2 shows the pilot plant used in this study.3. Furfural production reactions
Furfural is generally obtained from pentosan-rich agricultural products. In this case, pentosan was hydrolyzed to pentose using an acid catalyst. Then, furfural was produced by pentose dehydration. The stoichiometry of the aforementioned reactions is expressed in the following equations:161. Pentosan hydrolysis
| (C5H8O4)n + nH2O → nC5H10O5 |
2. Pentose dehydration
| C5H10O5 − 3H2O → C5H4O2 |
The overall reaction is as follows:
| (C5H8O4)n − 2H2O → C5H4O2 |
Therefore, from a reaction kinetics point of view, furfural production can be explained in a two-step process. In the first step, pentosan (xylan) was hydrolyzed to xylose (pentose) and then xylose was converted to furfural. Some water, according to the number and type of monosaccharides in pentosan, is consumed to dissolve the pentosan (polysaccharide) and convert it into monosaccharides such as xylose and arabinose. In the next stage, each xylose molecule loses three water molecules and is converted into furfural. According to the explained cases, the acid catalyst seems to be involved in both of the above steps. Therefore, both the reaction rate in the two stages and the intensity of furfural production dramatically increase with increasing catalyst consumption. It is worth noting that excess catalyst in the furfural production process reactions results in the generation of unwanted byproducts. These byproducts are produced according to reactions (1) and (2). Thus, the amount of catalyst should be optimized to prevent excess hydrolysis, ultimately leading to a more efficient furfural production.17–25
4. Artificial neural networks (ANNs)
One of the most well-liked simulation resources involves artificial neural networks, which incorporate a mixture of straightforward features and parallel computational tactic functionality. Through considering these features in the setting of biological nervous systems, these elements could be called neurons.26 Many strategies for ANNs have been applied.27,28 An ANN system comprises neurons and the weights associated with them. There are at least three layers of neurons in any model, including the input, hidden and output layers. The three-layer feed-forward neural network (3FFNN) is one of the best techniques with the shift purpose of a sigmoid (hyperbolic) type. This set of ANN can be used to generate nonlinear correlations with input and output parameters. The 3FFNN strategy is shown in Fig. 3. With this network, the input layer, the hidden layer (which contains ‘n’ neurons) and the output layer can be visualized. One of the most important parameters within the 3FFNN technique is ‘n’, which should be specified through the optimization system. This may occur immediately after generating the normal design connected with network. A mathematical representation of this course is as follows:
output(i) = W2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tanh[W1input(i) + b1] + b2 tanh[W1input(i) + b1] + b2
| (2) |
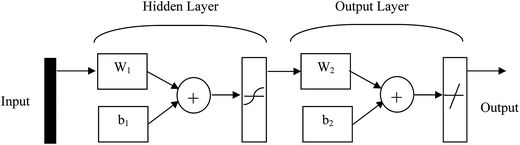 | ||
| Fig. 3 Schematic of the Artificial Feed-Forward Neural Network.27 | ||
5. Results and discussion
The percentage of produced furfural was calculated in the presence of NaHSO4 + H2SO4. The acid percentage, bagasse humidity, temperature, and pressure are important parameters in furfural production. The results of produced furfural in dilute sulfuric acid solutions with different concentrations of NaHSO4 are shown in Fig. 4. From Fig. 4, it can be observed that by increasing the amount of NaHSO4, more furfural will be produced in the reactor. The results of furfural production in the presence of diluted sulfuric acid, including (NaHSO4 + H2SO4), (NaCl + H2SO4) and H2SO4, are shown in Fig. 5. As can be observed in Fig. 5, a significant increase in furfural production is observed in the presence of NaHSO4 + H2SO4. NaHSO4 causes an interaction or complex formation with xylose, which improves the stabilization of the xylose transition states during dehydration and promotes furfural production. It seems that NaHSO4 in dilute aqueous sulfuric acid can increase the rate of xylose reaction. In addition, NaHSO4 increases the xylose reaction rate even at relatively low concentrations in acidic solutions. Therefore, this process increases the acid activity, boiling temperature and salting-out effect. In general, NaHSO4 has a double positive effect on furfural production. First, it helps salt out the reaction product. Second, it increases the furfural selectivity and formation rate. | ||
| Fig. 4 The results of produced furfural in dilute sulfuric acid solutions with different concentrations of NaHSO4. | ||
 | ||
| Fig. 5 The results of produced furfural with various catalysts (bagasse humidity: 53%, H2SO4: 10%, NaHSO4: 23%, NaCl: 23%). | ||
Furthermore, the percentage of produced furfural versus the time in different conditions of bagasse and at various sulfuric acid in NaHSO4 + H2SO4 solution concentrations is tabulated in Table 1. Table 1 summarizes the various bagasse humidities and acid percentages, ranging from 46% to 68% and from 7 to 13%, respectively. In addition, in Table 1, the temperature, pressure and percentage of sodium hydrogen sulfate in solution were fixed at 160 °C, 8 bar and 23% according to the optimum conditions. Then, the percentage of furfural was calculated at various time points from 10 min up to 110 min after the start of the reaction. The furfural level varied with the bagasse humidity or acid concentration under constant time conditions. This may be due to the presence of pentosane in bagasse, which decreases with increasing bagasse humidity. In fact, the humidity can alter the fermentation of pentosane. In other words, fermentation is an important parameter in furfural production and might change the acid percentage in the reactor. For instance, the acid percentage increases with increasing humidity or fermentation and decreases with decreasing humidity or fermentation. Table 2 compares the percentage of furfural formed in the reactor at various conditions and with various catalysts, including (NaHSO4 + H2SO4), (NaCl + H2SO4) and H2SO4. The results are shown in Table 2 and demonstrate that the combination of sulfuric acid + sodium hydrogen sulfate can be an efficient catalyst in the furfural production process. Furthermore, the percentage of furfural leaving the reactor in the vapor phase in the presence of NaHSO4 + H2SO4 (as a catalyst) is higher than for the systems using other catalysts. As mentioned earlier, an artificial neural network (ANN) was developed to calculate and predict the furfural percentage during the process. The temperature, reactor pressure, reaction time, sulfuric acid level, and bagasse humidity are input parameters in the artificial neural network (ANN). The results of the ANN for furfural production are listed in Table 1. Moreover, Fig. 6 shows the comparison between the ANN model results and experimental data, wherein the model results coincide well with the produced data.
| T (°C) | P (bar) | Bagasse humidity (%) | Dilute sulfuric acid (%) | Sodium hydrogen sulfate solution (%) | Time (min) | Furfural (%) | AAD% | |
|---|---|---|---|---|---|---|---|---|
| Exp. | Calc. | |||||||
a  |
||||||||
| 165 | 8 | 58 | 11 | 23 | 10 | 4.2 | 4.2434 | 4.95 |
| 165 | 8 | 58 | 11 | 23 | 20 | 5.1 | 5.0803 | |
| 165 | 8 | 58 | 11 | 23 | 30 | 5.8 | 6.1816 | |
| 165 | 8 | 58 | 11 | 23 | 40 | 6.5 | 7.2263 | |
| 165 | 8 | 58 | 11 | 23 | 50 | 8.02 | 7.7416 | |
| 165 | 8 | 58 | 11 | 23 | 60 | 7.8 | 7.1726 | |
| 165 | 8 | 58 | 11 | 23 | 70 | 6.65 | 6.8002 | |
| 165 | 8 | 58 | 11 | 23 | 80 | 6.64 | 6.5917 | |
| 165 | 8 | 58 | 11 | 23 | 90 | 6.63 | 6.2374 | |
| 165 | 8 | 58 | 11 | 23 | 100 | 5.4 | 5.706 | |
| 165 | 8 | 58 | 11 | 23 | 110 | 4.6 | 5.0231 | |
| 165 | 8 | 53 | 10 | 23 | 10 | 4.8 | 4.799 | 4.19 |
| 165 | 8 | 53 | 10 | 23 | 20 | 5.2 | 5.4978 | |
| 165 | 8 | 53 | 10 | 23 | 30 | 6.4 | 6.4757 | |
| 165 | 8 | 53 | 10 | 23 | 40 | 7.2 | 7.671 | |
| 165 | 8 | 53 | 10 | 23 | 50 | 8.8 | 8.6199 | |
| 165 | 8 | 53 | 10 | 23 | 60 | 7.3 | 7.3855 | |
| 165 | 8 | 53 | 10 | 23 | 70 | 7 | 6.7489 | |
| 165 | 8 | 53 | 10 | 23 | 80 | 6.7 | 6.5657 | |
| 165 | 8 | 53 | 10 | 23 | 90 | 6.6 | 6.1589 | |
| 165 | 8 | 53 | 10 | 23 | 100 | 5.6 | 5.5753 | |
| 165 | 8 | 53 | 10 | 23 | 110 | 4.2 | 4.9012 | |
| 165 | 8 | 46 | 7 | 23 | 10 | 4.1 | 4.3037 | 2.52 |
| 165 | 8 | 46 | 7 | 23 | 20 | 5.5 | 5.6113 | |
| 165 | 8 | 46 | 7 | 23 | 30 | 7 | 6.9342 | |
| 165 | 8 | 46 | 7 | 23 | 40 | 8.4 | 8.0289 | |
| 165 | 8 | 46 | 7 | 23 | 50 | 8.8 | 8.8432 | |
| 165 | 8 | 46 | 7 | 23 | 60 | 7.5 | 7.2592 | |
| 165 | 8 | 46 | 7 | 23 | 70 | 6.4 | 6.4284 | |
| 165 | 8 | 46 | 7 | 23 | 80 | 5.6 | 5.7943 | |
| 165 | 8 | 46 | 7 | 23 | 90 | 5.1 | 5.1471 | |
| 165 | 8 | 46 | 7 | 23 | 100 | 4.7 | 4.5703 | |
| 165 | 8 | 46 | 7 | 23 | 110 | 4 | 4.1635 | |
| 165 | 8 | 67 | 13 | 23 | 10 | 4.2 | 4.566 | 4.28 |
| 165 | 8 | 67 | 13 | 23 | 20 | 5.3 | 5.3542 | |
| 165 | 8 | 67 | 13 | 23 | 30 | 6.7 | 6.3023 | |
| 165 | 8 | 67 | 13 | 23 | 40 | 7.7 | 7.2393 | |
| 165 | 8 | 67 | 13 | 23 | 50 | 8.5 | 7.7801 | |
| 165 | 8 | 67 | 13 | 23 | 60 | 7.4 | 6.9918 | |
| 165 | 8 | 67 | 13 | 23 | 70 | 6.8 | 6.3199 | |
| 165 | 8 | 67 | 13 | 23 | 80 | 6 | 6.03 | |
| 165 | 8 | 67 | 13 | 23 | 90 | 5.6 | 5.6513 | |
| 165 | 8 | 67 | 13 | 23 | 100 | 5.1 | 5.1743 | |
| 165 | 8 | 67 | 13 | 23 | 110 | 4.7 | 4.6284 | |
| 165 | 8 | 49 | 11 | 23 | 10 | 4.4 | 4.4999 | 2.58 |
| 165 | 8 | 49 | 11 | 23 | 20 | 5.8 | 5.4972 | |
| 165 | 8 | 49 | 11 | 23 | 30 | 6.3 | 6.6533 | |
| 165 | 8 | 49 | 11 | 23 | 40 | 7.5 | 7.7765 | |
| 165 | 8 | 49 | 11 | 23 | 50 | 8.6 | 8.5435 | |
| 165 | 8 | 49 | 11 | 23 | 60 | 8.5 | 8.4347 | |
| 165 | 8 | 49 | 11 | 23 | 70 | 7.8 | 7.7764 | |
| 165 | 8 | 49 | 11 | 23 | 80 | 7.3 | 7.3819 | |
| 165 | 8 | 49 | 11 | 23 | 90 | 6.7 | 6.6807 | |
| 165 | 8 | 49 | 11 | 23 | 100 | 5.6 | 5.6838 | |
| 165 | 8 | 49 | 11 | 23 | 110 | 4.8 | 4.4656 | |
| 165 | 8 | 60 | 12 | 23 | 10 | 5.1 | 5.2312 | 3.68 |
| 165 | 8 | 60 | 12 | 23 | 20 | 5.8 | 6.3681 | |
| 165 | 8 | 60 | 12 | 23 | 30 | 7.2 | 7.5948 | |
| 165 | 8 | 60 | 12 | 23 | 40 | 8.8 | 8.6141 | |
| 165 | 8 | 60 | 12 | 23 | 50 | 9.2 | 9.1138 | |
| 165 | 8 | 60 | 12 | 23 | 60 | 8.1 | 8.2799 | |
| 165 | 8 | 60 | 12 | 23 | 70 | 7.6 | 7.5196 | |
| 165 | 8 | 60 | 12 | 23 | 80 | 7.3 | 6.964 | |
| 165 | 8 | 60 | 12 | 23 | 90 | 6.4 | 6.2911 | |
| 165 | 8 | 60 | 12 | 23 | 100 | 5.2 | 5.5724 | |
| 165 | 8 | 60 | 12 | 23 | 110 | 4.7 | 4.832 | |
| 165 | 8 | 58 | 13 | 23 | 10 | 4.1 | 4.3127 | 3.65 |
| 165 | 8 | 58 | 13 | 23 | 20 | 5.4 | 5.3576 | |
| 165 | 8 | 58 | 13 | 23 | 30 | 6.9 | 6.6472 | |
| 165 | 8 | 58 | 13 | 23 | 40 | 7.6 | 7.8937 | |
| 165 | 8 | 58 | 13 | 23 | 50 | 8.4 | 8.6432 | |
| 165 | 8 | 58 | 13 | 23 | 60 | 8.1 | 7.9289 | |
| 165 | 8 | 58 | 13 | 23 | 70 | 7.2 | 6.9446 | |
| 165 | 8 | 58 | 13 | 23 | 80 | 6.4 | 6.3329 | |
| 165 | 8 | 58 | 13 | 23 | 90 | 5.1 | 5.6434 | |
| 165 | 8 | 58 | 13 | 23 | 100 | 4.9 | 4.9171 | |
| 165 | 8 | 58 | 13 | 23 | 110 | 4.5 | 4.2294 | |
| 165 | 8 | 61 | 12 | 23 | 10 | 5.6 | 5.1921 | 6.75 |
| 165 | 8 | 61 | 12 | 23 | 20 | 6.3 | 6.1506 | |
| 165 | 8 | 61 | 12 | 23 | 30 | 6.9 | 7.1365 | |
| 165 | 8 | 61 | 12 | 23 | 40 | 7.5 | 7.8979 | |
| 165 | 8 | 61 | 12 | 23 | 50 | 8.7 | 8.1834 | |
| 165 | 8 | 61 | 12 | 23 | 60 | 7.4 | 7.2115 | |
| 165 | 8 | 61 | 12 | 23 | 70 | 7 | 6.513 | |
| 165 | 8 | 61 | 12 | 23 | 80 | 6.8 | 6.0947 | |
| 165 | 8 | 61 | 12 | 23 | 90 | 6.1 | 5.5586 | |
| 165 | 8 | 61 | 12 | 23 | 100 | 5.9 | 4.9494 | |
| 165 | 8 | 61 | 12 | 23 | 110 | 4.1 | 4.3097 | |
| 165 | 8 | 68 | 13 | 23 | 10 | 5.8 | 5.4877 | 3.83 |
| 165 | 8 | 68 | 13 | 23 | 20 | 6.3 | 6.3513 | |
| 165 | 8 | 68 | 13 | 23 | 30 | 6.9 | 7.3239 | |
| 165 | 8 | 68 | 13 | 23 | 40 | 7.5 | 8.2273 | |
| 165 | 8 | 68 | 13 | 23 | 50 | 8.8 | 8.6855 | |
| 165 | 8 | 68 | 13 | 23 | 60 | 7.2 | 7.7666 | |
| 165 | 8 | 68 | 13 | 23 | 70 | 6.9 | 6.9922 | |
| 165 | 8 | 68 | 13 | 23 | 80 | 6.4 | 6.6124 | |
| 165 | 8 | 68 | 13 | 23 | 90 | 6.1 | 6.1401 | |
| 165 | 8 | 68 | 13 | 23 | 100 | 5.7 | 5.5689 | |
| 165 | 8 | 68 | 13 | 23 | 110 | 5.1 | 4.9332 | |
| T (°C) | P (bar) | Bagasse humidity (%) | Dilute sulfuric acid (%) | Salinity% | Time (min) | Furfural (%) | ||
|---|---|---|---|---|---|---|---|---|
| Calc. H2SO4 | Calc. NaCl + H2SO4 | Calc. NaHSO4 + H2SO4 | ||||||
| 165 | 8 | 53 | 10 | 23 | 10 | 6 | 7.2 | 4.8 |
| 165 | 8 | 53 | 10 | 23 | 20 | 4.5 | 8.02 | 5.2 |
| 165 | 8 | 53 | 10 | 23 | 30 | 3.51 | 7.8 | 6.4 |
| 165 | 8 | 53 | 10 | 23 | 40 | 2.5 | 6.3 | 7.2 |
| 165 | 8 | 53 | 10 | 23 | 50 | 1.75 | 5.7 | 8.8 |
| 165 | 8 | 53 | 10 | 23 | 60 | 1.25 | 5 | 7.3 |
| 165 | 8 | 53 | 10 | 23 | 70 | 1.2 | 4.1 | 7 |
| 165 | 8 | 53 | 10 | 23 | 80 | 1.2 | 3.2 | 6.7 |
| 165 | 8 | 53 | 10 | 23 | 90 | 1.1 | 2.4 | 6.6 |
| 165 | 8 | 53 | 10 | 23 | 100 | 1 | 1.9 | 5.6 |
| 165 | 8 | 53 | 10 | 23 | 110 | 0.96 | 1.6 | 4.2 |
| 165 | 8 | 67 | 13 | 23 | 10 | 3.7 | 4.7 | 4.2 |
| 165 | 8 | 67 | 13 | 23 | 20 | 3.9 | 5.6 | 5.3 |
| 165 | 8 | 67 | 13 | 23 | 30 | 4.5 | 6.4 | 6.7 |
| 165 | 8 | 67 | 13 | 23 | 40 | 3.1 | 4.3 | 7.7 |
| 165 | 8 | 67 | 13 | 23 | 50 | 2.2 | 3.8 | 8.5 |
| 165 | 8 | 67 | 13 | 23 | 60 | 2 | 3.1 | 7.4 |
| 165 | 8 | 67 | 13 | 23 | 70 | 1.9 | 2.9 | 6.8 |
| 165 | 8 | 67 | 13 | 23 | 80 | 1.8 | 2.3 | 6 |
| 165 | 8 | 67 | 13 | 23 | 90 | 1.5 | 2 | 5.6 |
| 165 | 8 | 67 | 13 | 23 | 100 | 0.8 | 1.9 | 5.1 |
| 165 | 8 | 67 | 13 | 23 | 110 | 0.5 | 1.2 | 4.7 |
| 165 | 8 | 60 | 12 | 23 | 10 | 3.2 | 2.8 | 5.1 |
| 165 | 8 | 60 | 12 | 23 | 20 | 4.1 | 4.2 | 5.8 |
| 165 | 8 | 60 | 12 | 23 | 30 | 5.7 | 4.7 | 7.2 |
| 165 | 8 | 60 | 12 | 23 | 40 | 3.9 | 5.3 | 8.8 |
| 165 | 8 | 60 | 12 | 23 | 50 | 2.6 | 5.9 | 9.2 |
| 165 | 8 | 60 | 12 | 23 | 60 | 2.2 | 6.3 | 8.1 |
| 165 | 8 | 60 | 12 | 23 | 70 | 1.9 | 5.8 | 7.6 |
| 165 | 8 | 60 | 12 | 23 | 80 | 1.6 | 4.6 | 7.3 |
| 165 | 8 | 60 | 12 | 23 | 90 | 1 | 3.7 | 6.4 |
| 165 | 8 | 60 | 12 | 23 | 100 | 0.7 | 2.9 | 5.2 |
| 165 | 8 | 60 | 12 | 23 | 110 | 0.3 | 2.1 | 4.7 |
| 165 | 8 | 61 | 12 | 23 | 10 | 1.8 | 5.9 | 5.6 |
| 165 | 8 | 61 | 12 | 23 | 20 | 2.4 | 6.2 | 6.3 |
| 165 | 8 | 61 | 12 | 23 | 30 | 4.2 | 7.3 | 6.9 |
| 165 | 8 | 61 | 12 | 23 | 40 | 3.5 | 7.4 | 7.5 |
| 165 | 8 | 61 | 12 | 23 | 50 | 3 | 7.5 | 8.7 |
| 165 | 8 | 61 | 12 | 23 | 60 | 2.8 | 7 | 7.4 |
| 165 | 8 | 61 | 12 | 23 | 70 | 2.6 | 6.6 | 7 |
| 165 | 8 | 61 | 12 | 23 | 80 | 2.1 | 6 | 6.8 |
| 165 | 8 | 61 | 12 | 23 | 90 | 1.8 | 5.4 | 6.1 |
| 165 | 8 | 61 | 12 | 23 | 100 | 1.5 | 5 | 5.9 |
| 165 | 8 | 61 | 12 | 23 | 110 | 1.2 | 4.5 | 4.1 |
| 165 | 8 | 68 | 13 | 23 | 10 | 2.1 | 3.1 | 5.8 |
| 165 | 8 | 68 | 13 | 23 | 20 | 2.9 | 4.7 | 6.3 |
| 165 | 8 | 68 | 13 | 23 | 30 | 4.6 | 4 | 6.9 |
| 165 | 8 | 68 | 13 | 23 | 40 | 4 | 3.7 | 7.5 |
| 165 | 8 | 68 | 13 | 23 | 50 | 3.7 | 3 | 8.8 |
| 165 | 8 | 68 | 13 | 23 | 60 | 3.1 | 2.8 | 7.2 |
| 165 | 8 | 68 | 13 | 23 | 70 | 2.8 | 2.1 | 6.9 |
| 165 | 8 | 68 | 13 | 23 | 80 | 1.5 | 2 | 6.4 |
| 165 | 8 | 68 | 13 | 23 | 90 | 0.95 | 1.8 | 6.1 |
| 165 | 8 | 68 | 13 | 23 | 100 | 0.83 | 1.3 | 5.7 |
| 165 | 8 | 68 | 13 | 23 | 110 | 0.4 | 1 | 5.1 |
6. Conclusions
In this study, furfural production in the presence of sulfuric acid and an inorganic salt (NaHSO4 and H2SO4) in a pilot scale were studied experimentally. It is concluded that:- The addition of NaHSO4 to sulfuric acid, used as a furfural production reaction catalyst, increases the furfural yield in the system.
- The percentage of furfural and the energy consumption in the presence of NaHSO4 + H2SO4 (as a catalyst) is higher than that for the systems using other catalysts.
- The bagasse humidity, percentage of sulfuric acid, reaction time, temperature, and pressure were the strongest, most influential parameters in the furfural production process.
- Temperature, pressure and the percentage of sodium hydrogen sulfate in solution were fixed at 160 °C, 8 bar and 23% according to optimum conditions.
- A three-layer feed-forward ANN was successfully developed to represent and predict the furfural concentration in the reactor.
- The results demonstrate that the model was suitable to estimate the furfural percentage with a mean square error of about 10%.
Acknowledgements
The authors wish to thank managers of Behran Oil Company of Furfural Factory in Iran for their financial support and also from Islamic Azad University, Science and Research Branch for granting this research project.References
- W. Jaggle, Escher Wyss Mitteilungen, 1975, 2, 38–51 Search PubMed
.
- J. W. Dunning and E. C. Lathrop, J. Ind. Eng. Chem., 1945, 37, 24–29 CrossRef CAS
.
- E. Hagglund, Chemistry of Wood, Academic Press Inc, New York, 1951, p. 235 Search PubMed
.
- E. Lin, An experimental investigation of Furfural production process, M. S. thesis, Mississippi State University, 1990
.
- J. Mcketta, Encyclopedia of chemical processing and design, Taylor & Francis, 1986, vol. 24, p. 40 Search PubMed
.
- J. A. Horwath, R. Mutharason and E. D. Grossmann, Biotechnol. Bioeng., 1983, 25, 19–32 CrossRef CAS PubMed
.
- W. Yang, P. Li, D. Bo and H. Chang, Carbohydr. Res., 2012, 357, 53–61 CrossRef CAS PubMed
.
- D. Root, J. Seaman, J. Harris and W. Neil, For. Prod. J., 1959, 9, 158–165 CAS
.
- K. Lamminpaa, J. Ahola and J. Tanskanen, Ind. Eng. Chem. Res., 2010, 12, 6297–6303 Search PubMed
.
- B. Danon, G. Marcotullio and W. De Jong, Green Chem., 2014, 16, 39 RSC
.
- C. Rong, X. Ding, Y. Zhu, Y. Li, L. Wang, Y. Qu, X. Ma and Z. Wang, Carbohydr. Res., 2012, 350, 77–80 CrossRef CAS PubMed
.
- W. Hongsiri, B. Danon and W. De Jong, Ind. Eng. Chem. Res., 2014, 53, 5455–5463 CrossRef CAS
.
- C. Liu and C. Wyman, Carbohydr. Res., 2006, 341, 2550–2556 CrossRef CAS PubMed
.
- A. Singh, K. Das and D. K. Sharma, J. Chem. Technol. Biotechnol., 1984, 34, 51–61 CrossRef
.
- R. D. Sproull, P. R. Bienkowski and G. T. Tsao, Biotechnol. Bioeng. Symp., 1985, 15, 561–577 Search PubMed
.
- K. J. Zeitsch, The chemistry and technology of Furfural and its many by-products, (Sugar series; 13), Elsevier Science, 1st edn, 2000 Search PubMed
.
- Y. Wandian, L. Pingli, B. Dechen and C. Heying, Carbohydr. Res., 2012, 357, 53–61 CrossRef PubMed
.
- R. Cunguang, D. Xuefeng, Z. Yanvhao, L. Ying, W. Lili, Q. Yuning, M. Xiaoyu and W. Zichen, Carbohydr. Res., 2012, 350, 77–80 CrossRef PubMed
.
- T. Qiliang, F. Shiyu, Z. Huaiyu, C. Xinsheng and A. Lucian, J. Agric. Food Chem., 2008, 56, 3097–3101 CrossRef PubMed
.
- M. T. Garcia-Dominguez, J. C. Garcia-Dominguez, D. M. Feria, M. J. Gomez-Lozano, F. Lopez and M. J. Diaz, J. Chem. Eng., 2013, 221, 185–192 CrossRef CAS
.
- Y. Wandian, L. Pingli, B. Dechen, C. Heying, W. Xiaowei and Z. Tao, Bioresour. Technol., 2013, 133, 361–369 CrossRef PubMed
.
- C. Branca, C. Di Blasi and A. Galgano, Ind. Eng. Chem. Res., 2010, 49, 9743–9752 CrossRef CAS
.
- V. Choudhary, I. Sandler and D. G. Vlachos, ACS Catal., 2012, 2, 2022–2028 CrossRef CAS
.
- E. Lam, J. H. Chong, E. Majid, Y. Liu, S. Hrapovic, A. C. W. Leung and J. H. T. Luong, Carbon, 2012, 50, 1033–1043 CrossRef CAS
.
- F. Lopez, M. T. Garcia, M. J. Feria, J. C. Garcia, C. M. D. Diego, M. A. M. Zamudio and M. J. Diaz, J. Chem. Eng., 2014, 240, 195–201 CrossRef CAS
.
- M. T. Hagan, H. B. Demuth and M. H. Beale, Neural Network Design, Martin Haugen, 2002 Search PubMed
.
- F. Gharagheizi, A. Eslamimanesh, A. Mohammadi and D. Richon, Ind. Eng. Chem. Res., 2011, 50, 221–226 CrossRef CAS
.
- A. Chouai, S. Laugier and D. Richon, Fluid Phase Equilib., 2002, 199, 53–62 CrossRef CAS
.
- L. Piazza, G. Scalabrin, P. Marchi and D. Richon, Int. J. Refrig., 2006, 29, 1182–1194 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |



