Facile controlled synthesis of a hierarchical porous nanocoral-like Co3S4 electrode for high-performance supercapacitors†
Guijing Liu,
Bo Wang*,
Lei Wang,
Tiefeng Liu,
Tiantian Gao and
Dianlong Wang*
MIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, No. 92 Xidazhi Street, 150001 Harbin, Heilongjiang Province, China. E-mail: wangdianlonghit@163.com; wangbo19880804@163.com; Fax: +86 451 86413721; Tel: +86 451 86413751
First published on 20th May 2016
Abstract
A facile one-step hydrothermal process is developed to synthesize a porous nanocoral-like Co3S4, which directly grows on a three dimensional (3D) macroporous nickel (Ni) foam. The scanning electron microscopy (SEM) images reveal the uniform formation of a Co3S4 nanocoral cluster on Ni foam with a hierarchical porous structure. The crystal growth mechanism and factors that influence the formation of the coral-like Co3S4 directly on Ni foam have been further studied. The subsequent electrochemical measurements demonstrate that the nanocoral-like Co3S4 as a binder-free electrode for supercapacitors possesses a large specific capacitance as high as 1559 F g−1 at a current density of 0.5 A g−1 in 2.0 M KOH aqueous electrolyte. Moreover, to confirm its practical application, an asymmetric supercapacitor is assembled with the nanocoral-like Co3S4 electrode as the positive electrode and activated carbon (AC) as the negative electrode. Such a device achieves a high energy density of 60.1 W h kg−1 at a power density of 418.2 W kg−1 and maintains 38.5 W h kg−1 at a high power density of 3812.5 W kg−1, suggesting that the presented nanocoral-like Co3S4 electrode not only has potential for applying in high energy density fields, but also in high power density applications.
Introduction
Recently, owing to the rapidly increasing global energy consumption and environmental pollution, there is a need for a green and renewable energy source to maintain harmonious, healthy and rapid economic development. Lithium-ion batteries and supercapacitors, which are two of the most promising electrical energy storage and delivery systems, have attracted considerable attention.1,2 Particularly, supercapacitors, as one of the novel and significant energy storage devices, are of huge interest because of their outstanding properties including high power density, fast recharge ability, long lifespan, environmental compatibility and low maintenance cost.3–5 Herein, supercapacitors are considered to be one of the most crucial next-generation energy storage devices, which allow applications in many important areas such as uninterrupted power supplies, memory back-up systems, mobile electronic devices and hybrid electric vehicles and so forth.6,7In general, compared to lithium ion cells, the relatively low energy density is the main barrier for practical applications of supercapacitors. Developing asymmetric supercapacitors, also called electrochemical hybrid supercapacitors, has been considered an attractive approach to deliver substantially higher energy density, which is attributed to a wider voltage window by combining complementary potential windows of the two electrodes. As is known, asymmetric supercapacitors have also drawn increasing attention due to their potential applications in hand-held electronics, hybrid electric vehicles, microelectro-mechanical systems and sensors.8–10 Over the past few years, great progress has been made on the fabrication of asymmetric supercapacitors such as AC//graphite,11 AC//MnO2 (ref. 12) and AC//polyaniline.13 However, the properties of current asymmetric supercapacitors need further improvement to satisfy the practical applications, which are mainly attributed to the properties of the electrode materials. Hence, designing and fabricating electrode materials with ideal structure and performance is still urgent for next-generation (asymmetric) supercapacitors with both high energy and power density.
Generally speaking, through millions of years of natural selection the fittest and most efficient creatures and their structures survive. Coral in nature has a peculiar large-size porous structure with good mechanical strength and stability, which not only ensures that marine organisms can get across the coral structure unblocked, but also provides a large surface area in favor of exchange of substances. Such a unique morphology inspired us to develop a similar structure to make supercapacitor materials with large effective contact areas and stable cycling performances.
Moreover, nanostructured metal chalcogenides have become emerging materials for hydrogenations,14 lithium-ion batteries,15–17 dye-sensitized solar cells18 and supercapacitor19 applications owing to their eminent features. In recent years, considerable research efforts have been devoted to prepare excellent metal sulfides including CuS,19 WS2,20 CoS,21 MoS2 (ref. 22) and Ni3S2 (ref. 23) to fabricate superior capacity and high energy supercapacitors. Cobalt sulfide has been investigated as a low-cost electroactive material with high electrochemical performance.24–29 For instance, Jiao and coworkers fabricated Co3S4 hollow nanospheres grown on reduced graphene oxide by an efficient multi-step transformation route and the electrode exhibited a high specific discharge capacitance.26 However, the electrode was plagued with great capacity loss with nearly 24% from 0.5 A g−1 to 5 A g−1. Moreover, Lin et al. successfully synthesized an interlaced nanosheet-like CoS directly grown on Ni foam with a high specific capacitance of 1471 F g−1 at 4 A g−1.29 Unfortunately, the mass loading of the electrode was relatively low (only 0.4 mg), which will restrict their practical application. From the above discussion, it can be seen that although cobalt sulfide has been widely investigated as an excellent electrode material for supercapacitors, the controllable fabrication of cobalt sulfide with desirable micro-/nanostructures remains a difficult task. In addition, the rate performance and long-term stability for these cobalt sulfides still need to be further improved. Therefore, more efforts are still urgently needed for exploring and developing their properties for supercapacitor applications.
Here, we successfully obtain a well-assembled hierarchical coral-like architecture for Co3S4, which can be easily controlled by tuning the reaction temperature, the solvent polarity and solubility, as well as pH value through a facile and effective hydrothermal process. Meanwhile, it is expected that it will have the following advantages: (1) improves the electrolyte ion diffusion due to the coral-like hierarchical porous nanostructure; (2) speeds up the electron transport due to the close contact between Co3S4 and the conductive Ni foam current collector; and (3) enhances the stability of the integrated electrode by the in situ growth of Co3S4 on Ni foam with good adhesion. Through a synergetic combination of (1), (2), and (3), the as-prepared Ni foam supported the coral-like Co3S4 material showing improved supercapacitive performance with a capacity as high as 1559 F g−1 (0.5 A g−1), as well as excellent stability. To further improve the practical application of the as-prepared material, a unique asymmetric supercapacitor system is also fabricated and studied. Finally, the designed coral-like Co3S4 with good electrical conductivity for the battery-like faradic positive electrode and AC for the double layered capacitive negative electrode are chosen to assemble an asymmetric supercapacitor. Such a device presents eminent rate capability, good cycling stability and enhanced energy density without sacrificing high power density, which suggests that the Co3S4 has great potential as an electroactive material for supercapacitors.
Experimental
Preparation of Co3S4 on Ni foam
All materials and reagents employed were of analytical grade and used without further purification. The nanostructured Co3S4 grown directly on Ni foam has been prepared via a facile one-pot hydrothermal route. In a typical experiment, the Ni foam substrate was cleaned and etched with 10 wt% HCl solution in an ultrasound bath for 0.5 h to remove the surface NiO layer, and then rinsed with acetone, deionized (DI) water and ethanol three times, respectively.23,26 For the hydrothermal process, 0.3 mM CoCl2·6H2O and 0.6 mM thiourea were firstly dispersed in 40 mL deionized water at room temperature. Then the mixture was stirred for 30 min to obtain a transparent pink solution. Subsequently, the resulting solution was transferred to a 50 mL Teflon-lined autoclave for hydrothermal treatment at 160 °C for 4 h. In order to further demonstrate if there is a thin layer of Ni-based compound (NiO or Ni(OH)2 etc.) at the Ni foam surface, a control experiment is performed whereby the hydrothermal reaction of bare Ni foam is carried out without adding the reagents for the Co3S4 precursor. Moreover, in order to gain insight into the growth process of Co3S4, time dependent experiments at different reaction stages (0, 1, 2, 3 and 4 h) were carefully carried out, at the same time the other experimental conditions including the reactant concentration and temperature were kept unchanged. Meanwhile, parallel experiments with different temperatures (140, 180 °C), different volume ratios of ethanol/water (R) and different pH values (pH = 7, 8, 8.5) were also carried out. After the autoclave cooled down to room temperature, the resulting Ni foam product was washed several times with ethanol and water, respectively, and finally dried in an oven at 60 °C for 12 h. The mass loading of Co3S4 on Ni foam was calculated by weighing the Ni foam before and after the hydrothermal treatment, which was about 4.0 mg.23,29,37Preparation of AC electrode and assembly of the asymmetric supercapacitor
To fabricate the asymmetric supercapacitor, the nanostructured Co3S4 electrode and activated carbon (AC) electrode were used as the positive and negative electrodes, respectively. The negative electrode was prepared as follows: first, the electrode material consisted of the active material–AC, acetylene black and PTFE aqueous solution in a mass ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.30–32 A small amount of distilled water was added to the electrode material to produce a homogeneous paste at room temperature. Then, a certain area of the paste was pressed onto 3D Ni foam current collectors. Finally, the fabricated electrode was pressed and dried under vacuum at 60 °C for 12 h to make the AC electrode. Prior to the fabrication of the asymmetric supercapacitor, the mass ratio of the negative electrode (AC) to the positive electrode (porous coral-like Co3S4 electrode) was decided according to the charge balance theory (q+ = q−). The charge balance is shown in the following equation:33–35
5.30–32 A small amount of distilled water was added to the electrode material to produce a homogeneous paste at room temperature. Then, a certain area of the paste was pressed onto 3D Ni foam current collectors. Finally, the fabricated electrode was pressed and dried under vacuum at 60 °C for 12 h to make the AC electrode. Prior to the fabrication of the asymmetric supercapacitor, the mass ratio of the negative electrode (AC) to the positive electrode (porous coral-like Co3S4 electrode) was decided according to the charge balance theory (q+ = q−). The charge balance is shown in the following equation:33–35| q = Cs × ΔE × mac | (1) |
Based on the specific capacitance of the porous coral-like Co3S4 electrode and AC electrode from the CV curves at a scan rate of 5 mV s−1, the optimal mass ratio between the two electrodes was calculated to be about m+/m− = 0.12. Thus, on the basis of the above calculation, 1 mg of Co3S4 and 8 mg of AC was chosen to balance the capacitance of the two electrodes in the asymmetric supercapacitor cells.
Characterization
X-Ray diffraction (XRD) analyses were collected on a D/max-γB X-ray diffractometer (Rigaku, Japan) with Cu Kα radiation (λ = 1.54178 Å). The morphology and microstructure of the as-prepared samples were characterized by field-emission scanning electron microscopy (SEM, SU8000 Series), transmission electron microscopy (TEM, S-7650) and high resolution transmission electron microscopy (HRTEM, JEM-2100). Elemental composition and chemical status of the samples were measured by X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, America). XPS was performed using monochromatic Al Kα radiation (hν = 1486.6 eV) with a power of 150 W and beam spot of 500 μm. The XPS data were calibrated by C 1s (284.8 eV) and fitting using the XPSPEAK 4.0 software. The nitrogen adsorption–desorption was analyzed by the Brunauer–Emmett–Teller (BET) using an ASAP 2020 Surface Area and Porosity Analyzer (Micromeritics, Norcross, GA, USA).Electrochemical measurements
The electrochemical measurements of supercapacitive performance were carried out in a three-electrode cell, in which the porous coral-like Co3S4 electrode was used as the working electrode and a saturated Ag/AgCl and a platinum plate electrode acted as a reference and counter electrode, respectively. The electrolyte was a 2 M KOH aqueous solution for all electrochemical measurements. The cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) tests were performed on an electrochemical workstation system (CHI660E, Shanghai ChenHua Co., Ltd, China). The EIS measurement was obtained in the frequency range of 100 kHz to 10 mHz at open circuit potential with an ac perturbation of 5 mV. The parameters of the equivalent circuit were calculated and fitting based on computer simulations by the ZSimpWin software.31 The cycle life test was measured by repeating the GCD test at a relatively high current density. An asymmetric supercapacitor with the porous coral-like Co3S4 electrode as a positive electrode and AC as negative electrode was assembled in a two-electrode cell, and was also investigated in 2 M KOH electrolyte.Results and discussion
Characterization of electrode materials
The sample was prepared by a one-step process using a hydrothermal reaction to form Co3S4 coral-like nanosheets directly on Ni foam. Fig. S1† shows digital photographs of bare Ni foam (a) hydrothermal treated Ni foam (b) and Co3S4 electrode grown on Ni foam (c). It can be seen that the color of the bare Ni foam and Ni foam treated with the same hydrothermal conditions without Co3S4 reagents (hydrothermal treated Ni foam) is similar to the same Ni metallic luster (the color of Ni-based compound is usually green or dark green, which is much different from the color of Ni metal), suggesting that there is no existence of Ni-based compounds at the surface of the hydrothermally treated Ni foam, which also demonstrates that Ni foam is relatively stable in our designed reaction system. In addition, Fig. S1† also clearly shows that the color of the bare Ni foam changed to black color after the hydrothermal reaction with Co3S4 reagents, which is indicative of the sulfidation reaction during this process. It also shown that the as-prepared particles are fully deposited on the surface of the Ni foam, which suggested that the hydrothermal treatment system was beneficial for growing the particles on the conductive substrates.23,36,37 Besides, cobalt sulfide has various forms and phases with chemical formulas including CoS, CoS2, Co3S4, Co9S8, and so on, which highly depends on the role of the starting precursors, reaction process, temperature, etc. Thus, in order to confirm the phase of the obtained cobalt sulfide, X-ray diffraction (XRD), a well-known technique usually used for the analysis of phases, was carried out.Fig. 1 presented the XRD pattern of Co3S4 grown on Ni foam obtained at 160 °C for 4 h. The XRD pattern revealed seven significant diffraction peaks. Peaks at 44.5°, 51.9° and 76.4° correspond to the (111), (200) and (220) reflections of Ni, and the other peaks at 31.2, 37.8, 49.8 and 55.2° were fully attributed to the reflections from (311), (400), (511) and (440) crystal planes of well-crystallized Co3S4 (JCPDS card no. 73-1703). There was no presence of any peaks of impurities corresponding to any other phases of cobalt sulfide or cobalt oxide observed in the XRD pattern, evidently demonstrating that the porous coral-like Co3S4 electrode was successfully prepared by the facile one-step hydrothermal method. The possible chemical reactions during the hydrothermal reaction conditions are given below:
| NH2CSNH2 + 2H2O → 2NH3 + H2S + CO2 |
| H2S + 2H2O → 2H3O+ + S2− |
| CoCl2 → Co2+ + 2Cl− |
| 6Co2+ + 8S2− + O2 + 2H2O → 2Co3S4 + 4OH− |
 | ||
| Fig. 1 (a) XRD pattern of Co3S4 electrode, (b) crystal structure of Co3S4, (c, d) the high resolution XPS spectrum of Co 2p and S 2p spectrum for the Co3S4 electrode. | ||
Fig. 1b displays the unit cell including S2− (yellow) and cations (Co) with the mixed valence sulfides of +2 (green) and +3 (blue) for Co3S4. It can be clearly seen that the S atoms are distributed over the cubic closest packing and the Co with tetrahedral and octahedral stacking interstices. This structure with open channels is beneficial for electron transfer and ion diffusion.
To further study the surface chemical composition and valence electrons of the as-synthesized Co3S4, X-ray photoelectron spectroscopy (XPS) measurements were carried out.38,39 The wide XPS spectrum of Co3S4 (Fig. S2†) showed that the binding energy of S 2p, O 1s and Co 2p were determined to be 161.2 eV, 531.4 eV and 780.4 eV, respectively, with the reference binding energy at 284.8 eV for the C 1s peak. For the Co 2p region of coral-like Co3S4 (Fig. 1c), the peaks at binding energies of 780.1 and 792.6 eV were attributed to the Co 2p3/2 and Co 2p1/2 of coral-like Co3S4.40,41 There were two peaks observed at 780.3 and 796.2 due to oxidized Co species formed on the coral-like Co3S4 surface.41 Additionally, two other peaks located at 802.1 and 785.6 eV could be assigned to the satellite peaks of Co 2p, which is in accordance with the reported Co3S4 values previously.41 As shown in Fig. 1d for the S 2p region of Co3S4, it could be seen that two peaks at 161 and 162.1 eV were in good agreement with the S 2p3/2 and S 2p1/2 energy levels of Co3S4.40,41 Besides, there was a broad peak located at 167.6 eV attributed to an oxidized S species on account of air contact. Hence, the XPS analysis further demonstrated that the phase of the cobalt sulfide (CoxSy) is Co3S4, which is consistent with that of Co3S4 reported in the literature.41
To characterize the morphology and nanostructure of the product, SEM images were taken at various magnifications, and the results are presented in Fig. 2a–d. The SEM images in Fig. 2a–c reveals that the sample is comprised of numerous uniform nanosheets, which are interconnected with each other to form a 3D open coral-like microstructure. The magnified image given in Fig. 2d further displays the detailed hierarchical porous structure formation of the coral-like Co3S4. Such a porous nanoarchitecture provides fast pathways for the transport of the electrolyte ions, which is beneficial to the electrochemical performance as a supercapacitor electrode. To further investigate the unique architecture and porous features of the as-prepared Co3S4, TEM/HRTEM measurements were performed. As can been seen from Fig. 2e, the sample is assembled from interconnected nanosheets with a 3D open porous coral-like microstructure, which is in good agreement with the SEM observations. Typically, the magnified image in Fig. 2f clearly shows a large amount of mesopores on the surface of the nanosheets, mainly deriving from the release of gas during the hydrothermal process.42–44
Moreover, from the HRTEM image (Fig. 2g), the lattice fringes with an interplanar distance of around 2.83 and 4.95 Å matched well with the (311) and (511) planes of Co3S4 crystals. The corresponding fast Fourier transform (FFT) pattern further indicated the high crystallinity of the obtained Co3S4. The elemental mapping results obtained from the high-angle annular dark-field scanning TEM (HAADF-STEM) are displayed in Fig. 2i and j. It was obviously that the Co and S distributions were quite uniform with a trapezoidal shape, indicative of the coral-like Co3S4.
The N2 adsorption–desorption isotherms and Barrett–Joyner–Halenda (BJH) curves of the porous coral-like Co3S4 scratched from Ni foam are displayed in Fig. S3.† The as-prepared Co3S4 exhibited typical IUPAC type IV curve behavior with a large H3 hysteresis loop, which indicated the existence of a mesoporous structural character in the synthesized Co3S4. The corresponding pore size distribution curve (see the inset) further demonstrated the mesoporous nature. The calculated BET surface area of Co3S4 is 62 m2 g−1 and the average pore size computed by the BJH model is 3 nm. The coral-like Co3S4 with a unique hierarchical mesoporous nanostructure provides a short and fast transport pathway and further improves the faradic reaction.
In order to gain insight into the growth process and possible formation mechanism of the porous coral-like Co3S4 nanostructures, time dependent experiments were carefully carried out, and then the products were collected at different stages and investigated by SEM and XRD. Fig. 3 shows the SEM images and XRD patterns of the typical products obtained at different reaction stages (0, 1, 2, 3 and 4 h), at the same time the other experimental conditions including the reactant concentration, pH value and temperature were kept unchanged. It can be seen that the surface of the Ni foam substrate was smooth before the reaction (Fig. 3a).
For the first hour of the hydrothermal procedure, the surface of the Ni foam showed up numerous nanoseeds at the beginning, and grew to nanoparticles at the end of this stage (Fig. 3b). When the hydrothermal time was up to 2 h, some nanosheets could be seen clearly, which were standing on the surface of the Ni foam (Fig. 3c). By prolonging the reaction time to 3 h, 2D nanosheets further grew and began to interlace with each other to form a 3D interlaced structure (Fig. 3d). And at the end of the reaction (4 h), the uniform nanostructured coral-like Co3S4 was observed as shown in Fig. 3e and f. Fig. 3g shows the XRD patterns of the above four Co3S4 samples (1, 2, 3 and 4 h). All the diffraction peaks were index to Co3S4 (JCPDS card no. 73-1703). But the lower diffraction intensity of the sample synthesized at 160 °C for 1 h indicated that the crystallinity was comparatively poor. With the increase of the reaction time, the peaks became higher and sharper, demonstrating that the crystallinity was improved.
On the basis of the XRD and SEM observations and the investigations described above, we proposed a possible “crystallization–growth–assembly–formation” growth mechanism of the nanostructured coral-like Co3S4,45–47 as illustrated in Scheme 1. In brief, nanoseed nucleation arose at the early stage of the hydrothermal procedure, which then grew into larger nanoparticles. With the increase of the reaction time, a kinetically controlled process occurred instead of the initial crystal growth stage, and some nanosheets began to grow from the nucleation site, followed by the assembly and growth of these nanosheets into interlaced nanosheets, and finally the interlaced nanosheets continued to grow and formed nanostructured coral-like Co3S4. In summary, both the phase change and morphological evolution verified that the formation of the nanocoral-like Co3S4 structure was a cooperation effect of Ostwald ripening process and heterogeneous nucleation.
 | ||
| Scheme 1 Schematic illustration of the possible “crystallization–growth–assembly–formation” growth mechanism of the nano-structured coral-like Co3S4. | ||
Influencing factors
The environmental parameters play a crucial role to determine the growth of nanocrystals.48,49 Reaction temperature is a crucial influencing factor for the growth of nanocoral-like Co3S4. It can directly affect the reaction rate and further ensure the phase and structure of the final Co3S4 product. The sample obtained at 140 °C presents an interlaced porous structure with many agglomerated nanosheets, as shown in Fig. S4a and b.† This might result from the slower sulfidation rate of Co3S4 at a lower temperature.46 When the temperature was raised to 180 °C while keeping all other reaction parameters constant, a mixture of Co3S4 sheets and bricks were produced (Fig. S4c and d†). This is because the heterogeneous nucleation growth rate would be accelerated, which makes the nucleation and growth of Co3S4 nucleus not well controlled, thus leading to the formation of bricks.46,47Further, inspired by the above discovery, we controlled the morphology of Co3S4 nanostructures by adjusting the volumetric ratio of ethanol–water (ethanol–water, R) in the mixed solvent.50 When the reaction solvent was pure water, a sample structure with a coral-like shape was uniformly grown on the substrate to form a uniform array over a large area (Fig. 2b). With the increase of the volume ratio of ethanol–water (R = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), interconnected nanoplates with much larger dimension and thickness were found in the product with a wall-like structure (Fig. S5a and b†). If the solvent was obtained by mixing equal volumes of ethanol and water (R = 20
30), interconnected nanoplates with much larger dimension and thickness were found in the product with a wall-like structure (Fig. S5a and b†). If the solvent was obtained by mixing equal volumes of ethanol and water (R = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), the resulting product presented a uniform nanocluster morphology consisting of nanorods (Fig. S5c and d†). Fig. S5e† illustrates, with increasing the amount of ethanol to R = 30
20), the resulting product presented a uniform nanocluster morphology consisting of nanorods (Fig. S5c and d†). Fig. S5e† illustrates, with increasing the amount of ethanol to R = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, a scattered nanosheet-shaped massif-like sample was synthesized. By using only ethanol as the solvent (R = 40
10, a scattered nanosheet-shaped massif-like sample was synthesized. By using only ethanol as the solvent (R = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), accumulated nanoparticles were obtained, as shown in Fig. S5g and h.† The morphology of the prepared samples was distinctively different with the change of solvent volume ratio, suggesting the volume ratio of the solvents with different polarity and saturated vapor pressure played an important role for the formation of different morphologies of the samples due to the Kirkendall effect.51
0), accumulated nanoparticles were obtained, as shown in Fig. S5g and h.† The morphology of the prepared samples was distinctively different with the change of solvent volume ratio, suggesting the volume ratio of the solvents with different polarity and saturated vapor pressure played an important role for the formation of different morphologies of the samples due to the Kirkendall effect.51
The pH value is also of great influence on the morphology of the structure and is investigated by changing the amounts of ammonia while keeping the other conditions constant. With ammonia in the reaction solution, the surface morphology of Co3S4 changed a lot. When the pH was 7, as shown in Fig. S6a and b,† the sample exhibited a nanosheet structure with rippled silk morphology due to its ultrathin feature. With the further addition of ammonia to pH 8, sheets still existed on the surface, meanwhile some cubic-like structure was found and covered by the surface nanosheets (Fig. S6c and d†). When pH = 8.5, the resulting product exhibited a cubic-like structure with a diameter of 5–6 μm and the nanosheets disappear, as displayed in Fig. S6e and f.†
Electrochemical tests for positive electrode materials
Cyclic voltammetry (CV) and chronopotentiometry (CP) electrochemical tests were investigated first in the three-electrode measurements using 2 M KOH as the electrolyte. Fig. 4a shows the CV curves of the Co3S4 electrodes at different reaction times (1, 2, 3 and 4 h) under the voltage range of −0.2 to 0.8 V at a scan rate of 2 mV s−1. It could be clearly seen that the Co3S4 electrode at 4 h displayed the largest area CV curves compared to the other reaction times (1, 2 and 3 h), which indicated the highest capacitive capability of the Co3S4 electrode at 4 h. It is well known that specific capacitance is proportional to the average area of a CV curve. Fig. 4b shows the galvanostatic charge–discharge behaviors of the Co3S4 electrodes at different reaction times tested from −0.2 to 0.6 V at a constant current density of 0.5 A g−1. The charge–discharge duration increased with increasing of reaction time. On the other hand, through the discharge section of GCD measurements, the specific capacitance (Cs) of the Co3S4 electrode at different times was estimated according to eqn (2):52| Cs = I × Δt/(ΔV × m) | (2) |
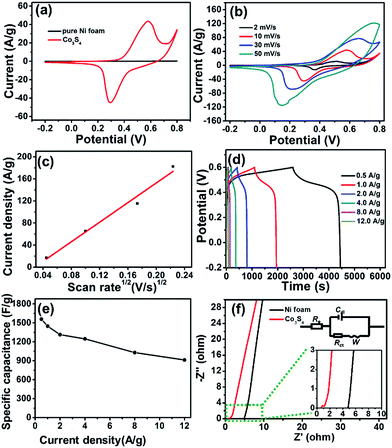
To confirm the influence of the Ni foam substrate on the electrochemical performance, the CV curves of the Ni foam and the Co3S4 electrodes (4 h) were compared under the voltage range of −0.2 to 0.8 V at a scan rate of 10 mV s−1 (Fig. 5a). Meanwhile, the CV curve of the hydrothermally treated Ni foam is shown in Fig. S8.† The shape of the CV curve based on the Co3S4 electrode had a pair of strong redox peaks, indicating the typical pseudocapacitive characteristics governed by faradaic redox reactions rather than the electric double-layer capacitance (close to an ideal rectangular shape). Up to now, it was not fully understood about the electrochemical mechanism of CoSx electrodes in alkaline electrolytes. Some relative reported literature claimed that the redox transitions of CoSx electrodes in alkaline electrolytes were very close to those of Co(OH)2 due to sulfur and oxygen being in the same family. The electrochemical redox reaction of the Co3S4 electrodes could be assumed as follows:24,29,54
| Co3S4 + OH− ↔ Co3S4OH + e− |
| Co3S4OH + OH− ↔ Co3S4O + H2O + e− |
In contrast, the CV curve of the Ni foam substrate measured under the same conditions was similar to a straight line without redox reaction. As is known, the specific capacitance was proportional to the area under the CV curve. Hence, the Ni foam is negligible for the Co3S4 electrode, owing to the negligible contribution to the total electrochemical capacitance.
Fig. 5b illustrates the typical CV curves of the Co3S4 electrode (4 h) with various sweep rates ranging from 2 to 50 mV s−1 in the potential range of −0.2 to 0.8 V. Obviously, it could be observed that the redox peak positions shifted with increasing scan rate, due to the limiting charge transfer step of the reaction. Furthermore, Fig. 5c shows the curve of the relationship between anodic current density and scan rate. Obviously, there was a good linear relationship, revealing an excellent rate capability for the Co3S4 electrode, which could be attributed to the abundant surface area of the nanocoral-like hierarchical porous structure for providing fast ionic/diffusion pathways within the electrode.
Rate capability is a crucial factor to evaluate the applications of supercapacitors. Fig. 5d presents the GCD behaviors of the as-prepared Co3S4 electrode (4 h) tested from −0.2 to 0.6 V at different current densities. The GCD curves were well manifested as a plateau, suggesting a faradaic redox reaction at the interface between electrode and electrolyte, which was consistent with the CV curves. Meanwhile, with the increase of the current density, this plateau shifted to a higher potential due to the stronger polarization. Moreover, the voltage–time profiles displayed symmetric charge–discharge features at different current densities, suggesting outstanding pseudocapacitive characteristics and excellent reversible redox reaction behavior. According to eqn (2), the C values of the porous coral-like Co3S4 electrode were calculated to be about 1559, 1447, 1312, 1245, 1028 and 921 F g−1 at current densities of 0.5, 1, 2, 4, 8 and 12 A g−1, respectively (Fig. 5e). It was found that the electrode still revealed a high capacitance of 921 F g−1 with current density as high as 12 A g−1, demonstrating a superior rate capability.
To further study the reaction mechanism of the Co3S4 electrode (4 h) during the charge–discharge process, electrochemical impedance spectroscopy (EIS) measurements were performed in the frequency range of 100 kHz to 10 mHz at open circuit potential with an ac perturbation of 5 mV. Additionally, to confirm the influence of the Ni foam substrate on the impedance, the Nyquist plots of the Co3S4 electrode and pure Ni foam were compared in Fig. 5f, where Z′ (x-axis) and Z′′ (y-axis) represent the real and imaginary parts of the impedance, respectively. The inset of Fig. 5f shows the equivalent circuit in accordance with the Nyquist plot where Rs is the solution resistance, Cdl represents the constant phase element accounting for a double-layer capacitance, CF representsd the faradaic capacitance, W is the Warburg impedance and Rct is the faradaic interfacial charge-transfer resistance.55 As observed from Fig. 5f, the Nyquist plots of the Co3S4 electrode exhibited a small depressed semicircle at a higher frequency followed by the presence of straight lines at the low frequency region. In contrast, the Ni foam only displayed straight lines and no semi-arc in the impedance spectrum, suggesting that the influence of Ni foam substrate on the impedance could be negligible. The electrode inter resistance (Rs) was calculated from the point of intersection with the x-axis in the range of high frequency and the charge transfer resistance (Rct) was counted from the span of the single semi-circle along the x-axis from high to low frequency region.56 The calculated Rs and Rct values were 0.6 and 0.3 Ω, which indicated that the good conductivity of the Co3S4 electrode, due to its porous coral-like structure, played an important role for its high-performance in the CV and GCD curves (Fig. 5).
Asymmetric supercapacitor
To better evaluate the possibility of using the porous coral-like Co3S4 electrode to fabricate practical supercapacitor devices, an asymmetric supercapacitor was constructed. The porous coral-like Co3S4 electrode in this work was attractive as the positive electrode for the asymmetric device. Here we investigated the electrochemical performance of the device by galvanostatic charge–discharge, cyclic voltammetry, and stability using activated carbon (AC) as the negative electrode in 2 M KOH aqueous electrolyte. Meanwhile, a schematic illustration of the asymmetric supercapacitor is presented in Fig. 6a. Prior to fabricating the Co3S4//AC asymmetric supercapacitor, a series of CV measurements with different voltage windows at 10 mV s−1 were performed to estimate the best operating potential of the asymmetric supercapacitor (Fig. 6b). It was expected that the stable potential window of the asymmetric supercapacitor could be extended to 1.6 V without obvious polarization phenomenon. The electrochemical properties of the asymmetric supercapacitor cell were measured and are shown in Fig. 6c–f. Fig. 6c and d show the CV and GCD curves of Co3S4//AC asymmetric supercapacitors tested at room temperature at various scan rates and charge–discharge current densities, which demonstrated that the as-fabricated device displayed high capacitive behavior with both contribution of electric double-layer capacitance (ions adsorption) and pseudocapacitance (fast surface redox process). Its capacity (C) values were calculated to be 167.9, 147.0, 132.7, 119.2 and 112.3 F g−1 at current densities of 0.3, 0.5, 1.0, 3.0 and 6.0 A g−1, respectively, as shown in Fig. 6e. Such an asymmetric supercapacitor revealed a high rate capability, maintaining 67.0% retention at a current density of 6.0 A g−1 (vs. current density of 0.3 A g−1), which was attributed to high rate performance of both porous coral-like Co3S4 electrode and AC. The GCD measurement was used to evaluate the durability of the as-fabricated asymmetric device. Fig. 6f illustrates the capacity retention as a function of the cycle number for the as-obtained asymmetric device at a current density of 3.0 A g−1. Notably, this reveals a long cycle life with a capacitance loss of only ∼10% even after repeating the GCD test up to 5000 times. It is reasonable to conclude that the asymmetric supercapacitor cell presented superior long-term electrochemical stability on account of its good conductivity and strong adherence with the 3D Ni foam substrate.On the basis of the above data, the relationship between energy (E) and power (P) densities of Co3S4//AC asymmetric supercapacitors was presented in Ragone plots, as shown in Fig. 7. E and P were calculated based on the following formulas:12,34
| E = 0.5 × Ccell × (ΔV)2/3.6 | (3) |
| P = E × 3600/(Δt) | (4) |
 | ||
| Fig. 7 Ragone plots of symmetric and asymmetric supercapacitors based on our work Co3S4//AC, AC//AC and other electrodes preciously reported. | ||
According to the above mentioned tests and discussions, the superior electrochemical performance of the nanocoral-like Co3S4 electrode and asymmetric supercapacitor based on a Co3S4 cathode and AC anode could be attributed to the following points: (1) the hierarchical porous structure of the nanocoral-like Co3S4 electrode provided fast pathways for electrolyte ion diffusion; (2) the close contact between the Co3S4 and conductive Ni foam current collector improved the electronic conductivity of the electrode; (3) the in situ growth of Co3S4 on Ni foam with good adhesion enhanced the stability of the integrated electrode; and (4) for the asymmetric supercapacitor, the AC negative material with high specific surface area and narrow pore size distribution offers high energy storage capacity during the rapid charge–discharge process at large current density.
Conclusions
In summary, a hierarchical porous coral-like Co3S4 supported on 3D Ni foam was fabricated by a facile one-step hydrothermal treatment. Such a Co3S4 electrode provided ultrahigh capacity values of 1559 F g−1 at a current density of 0.5 A g−1, and presented an outstanding rate capability. In addition, an asymmetric supercapacitor based on a Co3S4//AC electrode further demonstrated a high energy density of 60.1 W h kg−1 at a power density of 418.2 W kg−1. Meanwhile, the device also displayed a superior cyclic durability (after more than 5000 charge–discharge cycles, the device maintains ∼90% of its initial specific capacitance). The desirable performance of the Co3S4 electrode could be mainly ascribed to the unique porous Co3S4 coral-like structure, which could obtain high aspect ratio, high interfacial area and shorten electron and ion pathways and therefore improve the electrochemical reaction. Therefore, these above results ensure the potential application of this unique structure for high performance supercapacitor electrodes.Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (No. 50974045) and the Fundamental Research Funds for the Central Universities (Grant No. HIT.NSRIF.2017024).Notes and references
- B. Wang, W. Al Abdulla, D. Wang and X. S. Zhao, Energy Environ. Sci., 2015, 8, 869 CAS.
- E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774 RSC.
- A. Burke, J. Power Sources, 2000, 91, 37 CrossRef CAS.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28 CrossRef CAS PubMed.
- H. Huo, Y. Zhao and C. Xu, J. Mater. Chem. A, 2014, 2, 15111 CAS.
- T. Stimpfling and F. Leroux, Chem. Mater., 2010, 22, 974 CrossRef CAS.
- X. Y. Lang, A. Hirata, T. Fujita and M. W. Chen, Nat. Nanotechnol., 2011, 6, 232 CrossRef CAS PubMed.
- X. Xia, D. Chao, Z. Fan, C. Guan, X. Cao, H. Zhang and H. J. Fan, Nano Lett., 2014, 14, 1651 CrossRef CAS PubMed.
- X. Wang, X. Lu, B. Liu, D. Chen, Y. Tong and G. Shen, Adv. Mater., 2014, 26, 4763 CrossRef CAS PubMed.
- X. Xiao, T. Ding, L. Yuan, Y. Shen, Q. Zhong, X. Zhang, Y. Cao, B. Hu, T. Zha, L. Gong, J. Chen, Y. Tong, J. Zhou and Z. L. Wang, Adv. Energy Mater., 2012, 2, 1328 CrossRef CAS.
- V. Khomenko, E. Raymundo-Piñero and F. Béguin, J. Power Sources, 2008, 177, 643 CrossRef CAS.
- H. Q. Wang, Z. S. Li, Y. G. Huang, Q. Y. Li and X. Y. Wang, J. Mater. Chem., 2010, 20, 3883 RSC.
- J. H. Park and O. O. Park, J. Power Sources, 2002, 111, 185 CrossRef CAS.
- X.-Z. Wang, N. Liu, H. Hu, X.-P. Wang and J.-S. Qiu, Carbon, 2014, 76, 471 CrossRef.
- Y. Gu, Y. Xu and Y. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 801 CAS.
- B. Wang, D. Wang, Q. Wang, T. Liu, C. Guo and X. Zhao, J. Mater. Chem. A, 2013, 1, 135–144 CAS.
- B. N. Reddy, M. Deepa and A. G. Joshi, Phys. Chem. Chem. Phys., 2014, 16, 2062 RSC.
- C. W. Kung, H. W. Chen, C. Y. Lin, K. C. Huang, R. Vittal and K. C. Ho, ACS Nano, 2012, 6, 7016 CrossRef CAS PubMed.
- T. Zhu, B. Y. Xia, L. Zhou and X. W. Lou, J. Mater. Chem., 2012, 22, 7851 RSC.
- B. Hu, X. Qin, A. M. Asiri, K. A. Alamry, A. O. Al-Youbi and X. Sun, Electrochem. Commun., 2013, 28, 75 CrossRef CAS.
- R. Ramachandran, S. Felix, M. Saranya, C. Santhosh, V. Velmurugan, B. P. C. Ragupathy, S. K. Jeong and A. N. Grace, IEEE Trans. Nanotechnol., 2013, 12, 985 CrossRef CAS.
- H. Tang, J. Wang, H. Yin, H. Zhao, D. Wang and Z. Tang, Adv. Mater., 2015, 27, 1117 CrossRef CAS PubMed.
- D. Ghosh and C. K. Das, ACS Appl.Mater. Interfaces, 2015, 7, 1122 CAS.
- F. Tao, Y.-Q. Zhao, G.-Q. Zhang and H.-L. Li, Electrochem. Commun., 2007, 9, 1282 CrossRef CAS.
- Q. Chen, H. Li, C. Cai, S. Yang, K. Huang, X. Wei and J. Zhong, RSC Adv., 2013, 3, 22922 RSC.
- Q. Wang, L. Jiao, H. Du, Y. Si, Y. Wang and H. Yuan, J. Mater. Chem., 2012, 22, 21387 RSC.
- P. Justin and G. Ranga Rao, Int. J. Hydrogen Energy, 2010, 35, 9709 CrossRef CAS.
- K.-J. Huang, J.-Z. Zhang, G.-W. Shi and Y.-M. Liu, Mater. Lett., 2014, 131, 45 CrossRef CAS.
- J.-Y. Lin and S.-W. Chou, RSC Adv., 2013, 3, 2043 RSC.
- H. Chen, L. Hu, Y. Yan, R. Che, M. Chen and L. Wu, Adv. Funct. Mater., 2013, 3, 1636 CAS.
- Q. T. Qu, Y. Shi, S. Tian, Y. H. Chen, Y. P. Wu and R. Holze, J. Power Sources, 2009, 194, 1222 CrossRef CAS.
- Y. Cao, M. Zhu, P. Li, R. Zhang, X. Li, Q. Gong, K. Wang, M. Zhong, D. Wu, F. Lin and H. Zhu, Phys. Chem. Chem. Phys., 2013, 15, 19550 RSC.
- V. Khomenko, E. Raymundo-Piñero and F. Béguin, J. Power Sources, 2006, 153, 183 CrossRef CAS.
- Z. Fan, J. Yan, T. Wei, L. Zhi, G. Ning, T. Li and F. Wei, Adv. Funct. Mater., 2011, 21, 2366 CrossRef CAS.
- G. Liu, B. Wang, L. Wang, Y. Yuan and D. Wang, RSC Adv., 2016, 6, 7129 RSC.
- Z. Chen, W. Ren, L. Gao, B. Liu, S. Pei and H.-M. Cheng, Nat. Mater., 2011, 10, 424 CrossRef CAS PubMed.
- B. Guan, D. Guo, L. Hu, G. Zhang, T. Fu, W. Ren, J. Li and Q. Li, J. Mater. Chem. A, 2014, 2, 16116 CAS.
- N. Mahmood, C. Zhang, J. Jiang, F. Liu and Y. Hou, Chemistry, 2013, 19, 5183 CrossRef CAS PubMed.
- Y. Pan, Y. Liu and C. Liu, Appl. Surf. Sci., 2015, 357, 1133 CrossRef CAS.
- S.-J. Bao, Y. Li, C. M. Li, Q. Bao, Q. Lu and J. Guo, Cryst. Growth Des., 2008, 8, 3745 CAS.
- A. N. Grace, R. Ramachandran, M. Vinoba, S. Y. Choi, D. H. Chu, Y. Yoon, S. C. Nam and S. K. Jeong, Electroanalysis, 2014, 26, 199 CrossRef CAS.
- M. Kim and J. Kim, Phys. Chem. Chem. Phys., 2014, 16, 11323 RSC.
- B. Wang, A. Liu, W. A. Abdulla, D. Wang and X. S. Zhao, Nanoscale, 2015, 7, 8819–8828 RSC.
- S. Peng, L. Li, H. B. Wu, S. Madhavi and X. W. D. Lou, Adv. Eng. Mater., 2015, 5, 2 Search PubMed.
- G. C. Xi, K. Xiong, Q. B. Zhao, R. Zhang, H. B. Zhang and Y. T. Qian, Cryst. Growth Des., 2006, 6, 577 CAS.
- R. Jin, G. Chen, J. Pei, C. Yan, X. Zou, M. Deng and S. Sun, CrystEngComm, 2012, 14, 2327 RSC.
- J. Ma, D. Lei, L. Mei, X. Duan, Q. Li, T. Wang and W. Zheng, CrystEngComm, 2012, 14, 832 RSC.
- J. Ma, Y. Wang, Y. Wang, Q. Chen, J. Lian and W. Zheng, J. Phys. Chem. C, 2009, 113, 13588 CAS.
- J. Ma, J. Yang, L. Jiao, T. Wang, J. Lian, X. Duan and W. Zheng, Dalton Trans., 2011, 40, 10100 RSC.
- L. Yu, L. Zhang, H. B. Wu, G. Zhang and X. W. Lou, Energy Environ. Sci., 2013, 6, 2664 CAS.
- Y. Zhang, M. Ma, J. Yang, C. Sun, H. Su, W. Huang and X. Dong, Nanoscale, 2014, 6, 9824 RSC.
- Z. Tang, C.-h. Tang and H. Gong, Adv. Funct. Mater., 2012, 22, 1272 CrossRef CAS.
- R. R. Salunkhe, J. Lin, V. Malgras, S. X. Dou, J. H. Kim and Y. Yamauchi, Nano Energy, 2015, 11, 211 CrossRef CAS.
- C. Yuan, X. Zhang, B. Gao and J. Li, Mater. Chem. Phys., 2007, 101, 148 CrossRef CAS.
- W. Chen, C. Xia and H. N. Alshareef, ACS Nano, 2014, 8, 9531 CrossRef CAS PubMed.
- Y. Li, L. Cao, L. Qiao, M. Zhou, Y. Yang, P. Xiao and Y. Zhang, J. Mater. Chem. A, 2014, 2, 6540 CAS.
- X. Ren, C. Guo, L. Xu, T. Li, L. Hou and Y. Wei, ACS Appl. Mater. Interfaces, 2015, 7, 19930 CAS.
- Y. Xu, Z. Lin, X. Huang, Y. Liu, Y. Huang and X. Duan, ACS Nano, 2013, 7, 4042 CrossRef CAS PubMed.
- M. Kaempgen, C. K. Chan, J. Ma, Y. Cui and G. Gruner, Nano Lett., 2009, 9, 1872 CrossRef CAS PubMed.
- Y. He, W. Chen, X. Li, Z. Zhang, J. Fu, C. Zhao and E. Xie, ACS Nano, 2013, 7, 174 CrossRef CAS PubMed.
- Y. Xiao, Y. Cao, Y. Gong, A. Zhang, J. Zhao, S. Fang, D. Jia and F. Li, J. Power Sources, 2014, 246, 926 CrossRef CAS.
- M. Huang, Y. Zhang, F. Li, L. Zhang, Z. Wen and Q. Liu, J. Power Sources, 2014, 252, 98 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10427d |
| This journal is © The Royal Society of Chemistry 2016 |