Dopamine carbon nanodots as effective photothermal agents for cancer therapy
Abstract
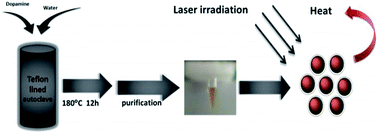
Dopamine carbon nanodots (DA CNDs) with an average diameter of approximately 23 nm were prepared through a facile hydrothermal method without adding any passivating agents. The as-prepared DA CNDs have high photothermal conversion efficiency (35%), excellent photostability and thermal stability. More importantly, DA CNDs exhibit significant photothermal therapeutic effects toward human cervical cancer (HeLa) cells under laser irradiation, while no appreciable dark cytotoxicity was observed even in high concentrations of DA CNDs aqueous solution. These results indicate that DA CNDs possess great potential to be effective photothermal agents for cancer therapy.

 Please wait while we load your content...
Please wait while we load your content...