Fluorescent paper sensor fabricated by carbazole-based probes for dual visual detection of Cu2+ and gaseous H2S†
Linlin Yanga,
Jianping Wangab,
Liang Yangab,
Cheng Zhangac,
Ruilong Zhangac,
Zhongping Zhangabc,
Bianhua Liu*ab and
Changlong Jiang*ab
aCAS Center for Excellence in Nanoscience, Institute of Intelligent Machines, Chinese Academy of Sciences, Hefei, Anhui 230031, China. E-mail: cljiang@iim.ac.cn
bState Key Laboratory of Transducer Technology, Chinese Academy of Sciences, Hefei, Anhui 230031, China
cDepartment of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, China
First published on 7th June 2016
Abstract
We report a fluorescent carbazole-based probe to achieve visual detection of Cu2+ and gaseous H2S with paper sensors in a fluorescent “on–off–on” mode. A common carbazole mother molecule is reconstructed by dual substitute and hydrazine reactions to obtain triple dentates to a single Cu2+ ion, and thus the fluorescence of the carbazole ring is strongly quenched by the captured Cu2+. Subsequently, the coordinative Cu2+ can be snatched by S2− via the formation of CuS, leading to the recovery of carbazole fluorescence. The above reactions provide the sensitive and prompt detection of Cu2+ and S2− with the limits of 65 nM and 0.29 μM, respectively. The reusability of the “on–off–on” fluorescent switch is well demonstrated by a recycling experiment with alternate additions of Cu2+ and S2−. Moreover, paper sensors are fabricated and used for the visual detection of Cu2+ and gaseous H2S with the limits of 10−6 M and 15 ppm.
Introduction
The sulfide anion (S2−) as a toxic traditional pollutant is widespread in the environment, and has a deleterious effect on human health, especially in the respiratory system.1 Sulfide anions can hydrolyze to more toxic and caustic species including hydrogen sulfide (H2S) and hydrosulfide (HS−) in buffer solution at physiological pH.2 H2S has not only been known as an environmental hazard for many decades but also it has been regarded as the third gaseous transmitter after NO and CO.3–5 Physiological levels of H2S can regulate intracellular redox status and fundamental signalling processes, including relaxation of vascular smooth muscles, mediation of neurotransmission, inhibition of insulin signaling, regulation of inflammation.6 However, abnormal levels of H2S in cells are associated with many diseases, such as Alzheimer's disease, liver cirrhosis, diabetes and Down's syndrome.7,8Fluorescence probes with the advantages of real-time measurement, high sensitivity and selectivity for the detection of H2S are remarkable over other detection methods, such as simple colorimetric assays, electrochemical sensor, monobromobimane, high pressure liquid chromatography and gas chromatography.9 The H2S-sensitive fluorescent probes are usually comprised of reaction-site modifying fluorophore and metal complex to achieve irreversible or reversible H2S-specific reactions.4,10,11 Time-consuming process and strictly reactive condition required in the former probe limit its applications. In contrast, the latter one with fast-response capability is more practical in the analysis of environment pollution.
Meanwhile, paper sensors have attracted widespread interest in environmental monitoring and explosive analysis,11,12 because they have the advantages of visual detection, portability, low cost, and feasibility of on-site assay. Some traditional methods often require tedious sample-preparation steps to indirectly detect H2S, so it is very necessary to develop a paper sensor for direct detection of H2S. Organic dyes are usually employed to fabricate test papers for visual detection of analytes owing to their high fluorescence quantum yield and easily modified properties. Carbazole and their derivatives not only have fine optical properties and high chemical stability but also possess strong emission and absorption properties.13 Although some studies on carbazole-based compounds in devices have been reported,14,15 their strong emission properties utilized as a chemosensor have attract attention just in recent years.16,17
Herein, fluorescence probe 3-N,N-(diacethydrazide)-9-ethylcarbazole (CAH) comprised of a carbazole signaling handle and diacetyl hydrazine binding moiety was reconstructed by dual substitute and hydrazine reactions to obtain triple dentates, which could easily coordinate with metal ion due to its enough nitrogen and oxygen atoms. Probe CAH exhibited highly selective response to Cu2+, and its fluorescence could be strongly quenched by Cu2+. It is well known that Cu2+ can react with sulfide anion to form a very stable species CuS (Ksp = 1.27 × 10−36).18 On basis of the fact, the coordinative Cu2+ can be snatched by H2S via the formation of CuS, leading to the recovery of carbazole fluorescence. The reusability of the “on–off–on” fluorescent switch is well demonstrated by a recycling experiment. Additionally, the application in the aspect of visually detecting Cu2+ and gaseous H2S with the paper sensors is clearly demonstrated.
Experimental
Reagents and instrumentation
3-Amino-9-ethylcarbazole was purchased from J & K Chemical Technology. Ethyl bromoacetate and 4-(2-hydroxyerhyl)piperazine-1-erhanesulfonic acid (HEPES) was provided by Sigma-Aldrich. Other reagents were received from Shanghai Chemicals Ltd. Commercially available compounds were used without further purification. Solvents were dried according to standard procedures. Ultrapure water (18 MΩ) was used for the preparation of all solutions. All reactions were magnetically stirred and monitored by thin-layer chromatography (TLC) using Spectrochem GF254 silica gel-coated plates. Column chromatography was performed using 200–300 mesh silica gel.1H NMR and 13C NMR spectra were recorded on Bruker 400 MHz Ultrashield spectrometer for using TMS as an internal standard. High-resolution mass spectra (HR-MS) were obtained using an Agilent Q-TOF 6540 mass spectrometer. UV-visible absorption spectra were measured by a Shimadu UV-2550 spectrometer. Fluorescence spectra were performed on Cary Eclipse fluorescence spectrophotometer. The Fourier transform infrared (FT-IR) spectra were obtained with a Thermo Scientific Nicolet iS10 spectrometer.
Synthesis of intermediate 3-N,N-(diethylacetate)-9-ethylcarbazole (2)
3-Amino-9-ethylcarbazole (0.5 g, 2.38 mmol), anhydrous potassium carbonate (1.25 g, 9.52 mmol) and potassium iodide (0.790 g, 4.76 mmol) were dissolved in 20 mL of anhydrous acetonitrile. Ethyl bromoacetate (0.795 g, 4.76 mmol) in 10 mL anhydrous acetonitrile was added dropwise and the mixture was refluxed for 12 h. The solid potassium carbonate was removed by filtration and the solvent was evaporated to give a dark brown oily liquid. The liquid was purified by column chromatography (silica gel, petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 8
ethyl acetate = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtained compound 3-N,N-(diethylacetate)-9-ethylcarbazole (0.66 g, 1.86 mmol, y. 73%). 1H NMR (400 MHz, CDCl3): δ 8.01 (d, J = 7.8 Hz, 1H), 7.42 (d, J = 2.6 Hz, 1H), 7.40 (dd, J = 7.0, 1.2 Hz, 1H), 7.33 (d, J = 8.2 Hz, 1H), 7.27 (d, J = 8.8 Hz, 1H), 7.18–7.12 (m, 1H), 6.96 (dd, J = 8.8, 2.6 Hz, 1H), 4.31–4.19 (m, 10H), 1.38 (t, J = 7.2 Hz, 3H), 1.28 (t, J = 7.1 Hz, 6H). 13C NMR (100 MHz, CDCl3): 171.46, 142.01, 140.52, 134.63, 125.48, 123.58, 122.16, 118.09, 114.53, 108.96, 108.36, 105.69, 60.92, 54.78, 37.53, 14.27, 13.82. HR-MS (m/z, ESI): calculated for C22H26N2NaO4 m/z = 405.1755 [M + Na]+. Found m/z = 405.1790.
1) to obtained compound 3-N,N-(diethylacetate)-9-ethylcarbazole (0.66 g, 1.86 mmol, y. 73%). 1H NMR (400 MHz, CDCl3): δ 8.01 (d, J = 7.8 Hz, 1H), 7.42 (d, J = 2.6 Hz, 1H), 7.40 (dd, J = 7.0, 1.2 Hz, 1H), 7.33 (d, J = 8.2 Hz, 1H), 7.27 (d, J = 8.8 Hz, 1H), 7.18–7.12 (m, 1H), 6.96 (dd, J = 8.8, 2.6 Hz, 1H), 4.31–4.19 (m, 10H), 1.38 (t, J = 7.2 Hz, 3H), 1.28 (t, J = 7.1 Hz, 6H). 13C NMR (100 MHz, CDCl3): 171.46, 142.01, 140.52, 134.63, 125.48, 123.58, 122.16, 118.09, 114.53, 108.96, 108.36, 105.69, 60.92, 54.78, 37.53, 14.27, 13.82. HR-MS (m/z, ESI): calculated for C22H26N2NaO4 m/z = 405.1755 [M + Na]+. Found m/z = 405.1790.
Synthesis of compound 3-N,N-(diacethydrazide)-9-ethylcarbazole (CAH)
3-N,N-(Diethylacetate)-9-ethylcarbazole (0.5 g, 1.31 mmol) was dissolved in anhydrous ethanol (20 mL), and then excess hydrazine hydrate (2 mL) was added to the system. The mixture was refluxed for 8 h. After cooling and standing overnight, a pale-grey solid was obtained. The solid was separately washed with n-hexane and ethanol, and dried under vacuum to gain CAH in 80% yield. 1H NMR (400 MHz, d6-DMSO): δ 10.05 (s, 2H), 7.96 (d, J = 7.6 Hz, 1H), 7.50 (d, J = 8.2 Hz, 1H), 7.44 (d, J = 8.9 Hz, 1H), 7.42–7.35 (m, 1H), 7.18 (d, J = 2.4 Hz, 1H), 7.11 (dd, J = 10.9, 3.9 Hz, 1H), 6.68 (dd, J = 8.9, 2.5 Hz, 1H), 4.58–4.29 (m, 6H), 4.14 (s, 4H), 1.25 (s, 3H). 13C NMR (100 MHz, d6-DMSO): 170.23, 139.94, 133.05, 125.42, 121.87, 120.04, 117.74, 111.76, 109.59, 108.88, 101.80, 55.67, 13.66. HR-MS (m/z, ESI): calculated for C18H23N6O2 m/z = 355.1928 [M + H]+. Found m/z = 355.1882.Fluorescence response to Cu2+ and H2S
In the process of testing, the spectral determinations used the 1 cm quartz cuvettes at room temperature. The stock solution of CAH (1 and 0.5 mM) was prepared in DMSO, which was separately diluted to 30 and 5 μM with HEPES buffer (10 mM, pH = 7.4). The absorption was scanned from 250 nm to 450 nm, and the Cu2+ solutions (10 mM) were added to the 30 μM CAH solution (2 mL) in portions (total volumes of 0, 2, 4, 6, 8, 10, 12, 14, 16, 18 and 20 μL). The fluorescence spectra were scanned from 370 nm to 600 nm, and the Cu2+ solutions (1 mM) were added to the 5 μM CAH solution (2 mL) in portions (total volumes of 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22 and 24 μL). When the resultant solution was mixed thoroughly, the optical spectra were measured. The fluorescence changes of the probe in the absence/presence of Cu2+ were observed under a 365 nm UV lamp and the corresponding photographs were recorded with a digital camera.CAH–Cu2+ prepared in situ by adding 10 mM Cu2+ (2 μL) to 5 μM CAH solution (2 mL) was used as the probe for next detection of H2S. Appropriate amount of Na2S stock solution (1 mM) was added into the above-mentioned probe solution and mixed thoroughly. The final concentrations of H2S were calculated to be 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 20 and 22 μM, and fluorescence spectra were recorded. The fluorescence changes of the probe in the absence/presence of H2S were observed under a 365 nm UV lamp and the corresponding photographs were recorded with a digital camera.
Recycling experiment
The fluorescence intensity was successively recorded when 0 and 2 μL of 10 mM Cu2+ was added to 2 mL of 5 μM CAH buffer solution, and then was recorded upon the addition of 10 mM S2− (4 μL) to the same solution. The experimental process by sequentially adding Cu2+ and S2− to the same solution was repeated five times, and the volume ratios of Cu2+ and S2− were 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 2
0, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 2
0, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 6
4, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 6
4, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 10
8, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 10
8, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, 14
12, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, 14
12, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16, 18
16, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 18
20 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 μL. The corresponding photographs of fluorescence changes of the probe were observed under a 365 nm UV lamp and recorded with a digital camera.
24 μL. The corresponding photographs of fluorescence changes of the probe were observed under a 365 nm UV lamp and recorded with a digital camera.
Preparation of paper sensor for the visual detection of Cu2+ and gaseous H2S
First, several circular pieces of paper about 1 cm in diameter were separated from a common piece of filter paper by a perforator, which would be used as the substrate for immobilizing the probe. Then, 0.1 mM of the probe solution was carefully dropped onto one piece of these papers, where the solution was able to uniform distribution. After the solution was dried in air, the fluorescence indicating paper was obtained. Finally, 5 μL different concentrations of Cu2+ (0, 10−6, 10−5, 10−4 and 10−3 M) solution were separately dropped on these paper sensors. After the solution was dried, the fluorescence changes of test paper were recorded by digital camera under a 365 nm UV lamp.A wild-mouth bottle of H2S gas was collected, which was prepared by a reaction between Na2S and diluted H2SO4. Rectangular paper strips were obtained from one common piece of filter paper by means of a sharp knife and straightedge, which were used as the substrate for immobilizing the probe. When each 10 μL of CAH–Cu2+ solution (0.5 mM) was separately dropped onto these pieces of paper strip, paper sensors were obtained. The prepared paper sensors were then placed in clear glass containers (300 mL in volume) for visual detection of gaseous H2S in air. Different volumes (0, 3, 8, 13 and 24 μL) of the gas sample sucked by a micro syringe were separately injected into the same volume of containers with the paper sensors. The fluorescence changes of paper sensors were recorded by digital camera under a 365 nm UV lamp.
Results and discussion
Synthetic route and proposed mechanism
The fluorescent probe CAH was synthesized through a two-step reaction as shown in Scheme 1A. First, compound 1 was refluxed with ethyl bromoacetate in the presence of anhydrous potassium carbonate and potassium iodide for 12 h to form intermediate 2 characterized by 1H NMR, 13C NMR and HR-MS (see ESI†). Subsequently, the target compound CAH was achieved by refluxing intermediate 2 with hydrazine hydrate for 8 h. The obtained compound CAH was silver-grey solid, and its structure was characterized by 1H NMR, 13C NMR, FT-IR and HR-MS (see ESI†). CAH–Cu2+ prepared in situ by the addition of 10 μM Cu2+ to the solution of CAH (5 μM) was used as the probe for the next detection of S2−. | ||
| Scheme 1 (A) Synthetic route of probe CAH; (B) the proposed mechanism of CAH to successively detect Cu2+ and S2−. | ||
Fluorescent probe CAH by reconstruction of fluorophore carbazole to obtain triple dentates was able to efficiently trap Cu2+, and its fluorescence was strongly quenched by Cu2+. Subsequently, Cu2+ could be easily snatched by S2− via the formation of an extremely stable species CuS to achieve the release and fluorescence recovery of probe. Interestingly, the fluorescent “on–off–on” process could repeatedly occur by the alternate addition of Cu2+ and S2− to the solution of probe. The most possible sensing mechanism for the detection of Cu2+ and S2− was proposed and described in Scheme 1B.
Characterization of the probe structure
In the FT-IR spectrum of CAH (Fig. S1A†), the peak at 3432 cm−1 indicated the existence of N–H bond from the acethydrazide. The overlapped broad peaks at 1650 and 1640–1618 cm−1 could be attributed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and bending vibrations of N–H bond in the acethydrazide, respectively. The peak at 1401 cm−1 originated from the stretching vibration of C–N bond. Upon the formation of complex CAH–Cu2+, N–H bond vibration of CAH at 3432 cm−1 broadened and shifted to 3347 cm−1, which might due to the stretching vibration of O–H bond (Fig. S1B†). At the same time, a new peak was found at 660 cm−1 which ascribed to the out-of-plane ring bend of O–H bond. Compared with the vibration of C
O bond and bending vibrations of N–H bond in the acethydrazide, respectively. The peak at 1401 cm−1 originated from the stretching vibration of C–N bond. Upon the formation of complex CAH–Cu2+, N–H bond vibration of CAH at 3432 cm−1 broadened and shifted to 3347 cm−1, which might due to the stretching vibration of O–H bond (Fig. S1B†). At the same time, a new peak was found at 660 cm−1 which ascribed to the out-of-plane ring bend of O–H bond. Compared with the vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1650 cm−1, the corresponding peak of complex CAH–Cu2+ became weaker and shifted to 1625 cm−1. The emerged strong peak at 1148 cm−1 was originated from the stretching vibration of C–O bond.19 The results implied the structure changes from keto to enol form, and the oxygen atom of enol form involved in the formation of coordination bond. In addition, the new peaks at 602 and 423 cm−1 confirmed the formation of Cu–O and Cu–N bond,20 which further manifested compound CAH coordinating with Cu2+ by the nitrogen and oxygen atom. The HR-MS spectrum of CAH showed a molecular-ion peak [M + H]+ at m/z 355.19 (Fig. S2†). Adding Cu2+ into the solution, the fluorescence of CAH was rapidly quenched due to the formation of non-fluorescent complex, CAH–Cu2+. The mass spectrum of complex CAH–Cu2+ showed a prominent peak at m/z = 417.21, corresponding to [CAH–Cu2+] (Fig. S3A†). Successively, the addition of Na2S to the CAH–Cu2+ solution was able to promptly recover its fluorescence through removal of Cu2+ and regeneration of probe CAH. The parent peak [(CAH–Cu2+) − Cu2+ + H]+ of CAH–Cu2+/Na2S solution was located at m/z 355.19 (Fig. S3B†), which was equivalent to that of probe CAH. Furthermore, CAH–Cu2+ formed between CAH and Cu2+ was found to be 1
O at 1650 cm−1, the corresponding peak of complex CAH–Cu2+ became weaker and shifted to 1625 cm−1. The emerged strong peak at 1148 cm−1 was originated from the stretching vibration of C–O bond.19 The results implied the structure changes from keto to enol form, and the oxygen atom of enol form involved in the formation of coordination bond. In addition, the new peaks at 602 and 423 cm−1 confirmed the formation of Cu–O and Cu–N bond,20 which further manifested compound CAH coordinating with Cu2+ by the nitrogen and oxygen atom. The HR-MS spectrum of CAH showed a molecular-ion peak [M + H]+ at m/z 355.19 (Fig. S2†). Adding Cu2+ into the solution, the fluorescence of CAH was rapidly quenched due to the formation of non-fluorescent complex, CAH–Cu2+. The mass spectrum of complex CAH–Cu2+ showed a prominent peak at m/z = 417.21, corresponding to [CAH–Cu2+] (Fig. S3A†). Successively, the addition of Na2S to the CAH–Cu2+ solution was able to promptly recover its fluorescence through removal of Cu2+ and regeneration of probe CAH. The parent peak [(CAH–Cu2+) − Cu2+ + H]+ of CAH–Cu2+/Na2S solution was located at m/z 355.19 (Fig. S3B†), which was equivalent to that of probe CAH. Furthermore, CAH–Cu2+ formed between CAH and Cu2+ was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in stoichiometry, which was proved by Job's plot (Fig. S4†). All the results were able to well elucidate the mechanism of probe for the successive detection of Cu2+ and S2−.
1 in stoichiometry, which was proved by Job's plot (Fig. S4†). All the results were able to well elucidate the mechanism of probe for the successive detection of Cu2+ and S2−.
Fluorescence turn-off detection of Cu2+
The fluorescent spectra of probe CAH centered at 425 nm were recorded upon the excitation wavelength of 350 nm in HEPES buffer (10 mM HEPES, 1% DMF as cosolvent, pH = 7.2) at ambient temperature. Fluorescence intensity (I425 nm) changes of CAH (5 μM) treated with different concentrations of Cu2+ were monitored over time. As shown in Fig. S5,† the intensity of CAH rapidly decreased and nearly reached the minimum value within 10 min upon the addition of 5.0, 7.5 and 10 μM Cu2+. The fluorescence intensity of CAH did not change for 30 min, demonstrating Cu2+ could efficiently quench the fluorescence of probe. Therefore, the subsequent fluorescence studies were carried out after an equilibration of 10 min.The interaction between probe CAH and Cu2+ was examined by UV-vis absorption spectra. As shown in Fig. 1, free CAH (30 μM) in HEPES buffer showed three absorption bands centered at 275, 308 and 375 nm, which could be assigned as n–π* and π–π* transitions.21 Upon the addition of Cu2+ (0–60 μM), the absorption peak at 308 nm was gradually decreased, and the peaks at 275 and 375 nm separately shifted to shorter wavelengths 265 and 355 nm. Meanwhile, a new absorption peak around 400 nm was observed, indicating the formation of complex CAH–Cu2+.
 | ||
| Fig. 1 UV-vis spectra of CAH (30 μM) upon the addition of Cu2+ (0–100 μM) in HEPES buffer solution (10 mM HEPES, 1% DMF, pH = 7.4). | ||
Fluorescent spectra of probe CAH centered at 425 nm were recorded upon excitation wavelength of 350 nm in HEPES buffer (10 mM HEPES, 1% DMF as cosolvent, pH = 7.4) at ambient temperature. In terms of fluorescence emission, CAH emitted a bright blue fluorescence with a quantum yield of 0.40 (Φ1, Fig. S6 and Table S1†).22 In order to evaluate Cu2+-responsive nature of CAH, fluorescence titration with Cu2+ in varying concentrations was conducted. As shown in Fig. 2A, upon the addition of Cu2+ with increasing amounts (0–10 μM), the fluorescence intensity of CAH was gradually diminished by the formation of complex CAH–Cu2+ (Φ2 = 0.003). When 10 μM of Cu2+ was added to the solution of CAH, dramatic fluorescent quenching (quenching efficiency (I0 − I)/I0 × 100 = 98%, I425 nm) was observed, meaning that Cu2+ could effectively quenched the fluorescence of CAH. Fluorescence quenching of the probe may occur by the excitation energy transfer from the ligand to the metal d-orbital and/or ligand to metal charge transfer (LMCT).23 The decrease in emission band at 425 nm was in a good linear relationship (R2 = 0.9967) between fluorescence intensity and Cu2+ concentration in the range from 1.0 × 10−6 to 8.0 × 10−6 M with 5 μM probe (Fig. 2B). The binding constant (K) derived from the fluorescence titration data was found to be 2.8 × 102 (R2 = 0.9971, Fig. S7†) using Benesi–Hildebrand plot.24 The limit of detection (LOD) for Cu2+ was estimated to be 65 nM in HEPES buffer based on LOD = 3δ/k (Fig. S8†),25 which is below the maximum permissive level of Cu2+ in drinking water (20 μM) set by the U.S. Environmental Protection Agency.26
Selectivity of probe CAH for Cu2+
For a fluorescent sensor, it is very important to possess high sensitivity for Cu2+ over other metal ions, and still retain good selective response to Cu2+ even in the presence of disruptors. The fluorescence spectra of CAH (5 μM) response to various cations were examined as shown in Fig. 2 (black bars). Results demonstrated that the addition of other ions had negligible influences on the fluorescence intensity of CAH, such as Li+, Na+, K+, Ag+, Zn2+, Mn2+, Cd2+, Hg2+, Mg2+, Ca2+, Ba2+, Co2+, Al3+, Pb2+, Ni2+, Fe2+ and Fe3+, even if their concentrations reached up to 50 μM. In contrast, dramatic fluorescence quenching of CAH was observed upon the addition of 10 μM Cu2+, demonstrating that the probe had a great sensitivity and selectivity for Cu2+. Further interference experiments were performed to investigate whether other coexistent metal ions had significant effects on the selectivity of CAH for Cu2+. The results of Fig. 2 (black bars) showed no significant influence on the fluorescence intensity of probe in the presence of other cations. However, the addition of 10 μM Cu2+ to the above solutions of CAH with 50 μM various ions, the fluorescence intensity was almost completely quenched, as shown in Fig. 2 (red bars). It was consistent with the phenomenon generated by the separate addition of 10 μM Cu2+, implying that CAH had a better sensitivity for Cu2+ than other ions. Therefore, the results mentioned-above demonstrated that CAH was able to serve as a potential chemsensor for Cu2+ with high selectivity and anti-interference performance (Fig. 3).Fluorescent turn-on detection of S2−
Time-dependent changes in the fluorescence intensity (I425 nm) of CAH–Cu2+ (5 μM) were separately monitored in the presence of 10, 15 and 20 μM S2− as shown in Fig. 4. After the addition of S2−, the fluorescence of probe immediately recovered and reached a maximum within 1 min. The recovered fluorescence intensity at 425 nm still kept stable and constant for 30 min. Together with the stability of CAH–Cu2+ and CAH in HEPES buffer solution, it is feasible and applicable for probe to detect S2− in the environment.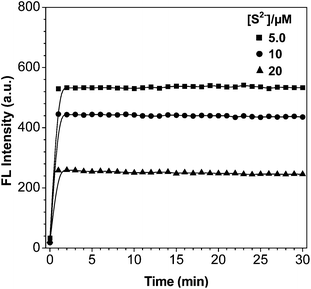 | ||
| Fig. 4 Time-dependent fluorescence intensity (λex = 350 nm, λem = 425 nm) change of CAH–Cu2+ (5 μM) with S2− (5, 10 and 20 μM) in HEPES buffer solution (10 mM HEPES, 1% DMF, pH = 7.4). | ||
The fluorescence spectra of CAH–Cu2+ upon the addition of different concentrations of S2− were obtained. As the increasing concentrations of S2− (0–20 μM) were added, the fluorescence intensity (I425 nm) of probe was gradually recovered from dark to blue (Fig. 5A). The fluorescence intensity increased in a S2− concentration-dependent way as displayed in Fig. 5B. The enhancement of intensity (I425 nm) was in a good linear relationship (R2 = 0.9984) between fluorescence intensity and the concentrations of S2− in the range from 0 to 1.5 × 10−5 M with the detection limit of 0.29 μM in HEPES buffer (Fig. S9†). The results proved that the probe had a high sensitivity for the detection of S2−.
Selectivity of probe CAH–Cu2+ for S2−
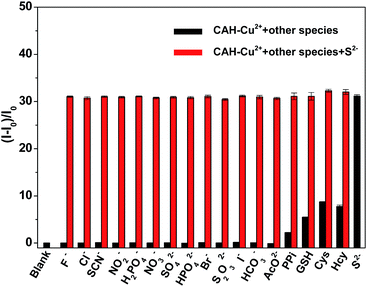
The fluorescence responses of CAH–Cu2+ to other anions and biothiol, such as F−, Cl−, SCN−, NO2−, H2PO4−, NO3−, SO42−, HPO42−, Br−, S2O32−, I−, HCO3−, AcO−, PPi, GSH, Cys and Hcy were also examined (Fig. 6, black bars). Obviously, other anions had slight influences on the fluorescence intensity compared with S2− even if the concentrations of these anions reached up to 50 μM, indicating that probe CAH–Cu2+ possessed a predominant selectivity for S2− over other anions. Although probe CAH–Cu2+ separately gave about 2-fold, 5-fold, 8-fold and 7-fold fluorescence enhancement for PPi, GSH, Cys and Hcy, there were more than 31-fold fluorescence enhancements when the system was treated with S2−. The result manifested probe CAH–Cu2+ was capable of distinguishing S2− from PPi, GSH, Cys and Hcy. Additionally, it was very important to explore whether CAH–Cu2+ could still retain highly sensitive response to S2− in the presence of other interfering species. The interference experiments were carried out in the presence of other potential coexisting ions at relatively high concentration. Fig. 6 (black bars) showed that the probe was non-responsive to other anions, and just a minor fluorescence enhancement was induced in the presence of PPi, GSH, Cys and Hcy. Whereafter, the fluorescence of probe recovered immediately upon the addition of S2− to the above solution, which is consistent with the result generated by the separate addition of S2− (Fig. 6, red bars). The results implied that the probe CAH–Cu2+ could identify S2− over other anions and biothiols with high sensitivity and selectivity in assay conditions.Effect of pH
The fluorescence intensity of probes CAH and CAH–Cu2+ in various pH values was measured, because a good stability of probe in suitable pH values is very important for its practical applications. As shown in Fig. 7, the fluorescence of CAH was weak in the acidic environment due to the protonation of amino group, leading to a weak coordination ability of Cu2+. When the pH was increased from 6 to 9, satisfactory fluorescence intensity of CAH and Cu2+-quenching abilities were exhibited. At pH about 7, the fluorescence intensity of CAH–Cu2+ reached its minimum value, indicating probe possessed the highest sensing ability in physiological pH. Probe CAH–Cu2+ in weak acidic and near neutral media is favorable for sensing assay of S2− in environment. | ||
Fig. 7 Fluorescence intensity of 5 μM CAH ( ), CAH + 10 μM Cu2+ ( ), CAH + 10 μM Cu2+ ( ) and CAH–Cu2+ + 20 μM S2− ( ) and CAH–Cu2+ + 20 μM S2− ( ) at various pH in HEPES buffer solution (10 mM HEPES, 1% DMF as cosolvent). ) at various pH in HEPES buffer solution (10 mM HEPES, 1% DMF as cosolvent). | ||
Reusability and application of probe
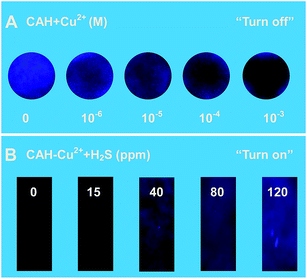
On the basis of the fact that the fluorescence of probe CAH was quenched and recovered by adding Cu2+ and S2−, the reusability of fluorescence system was investigated by repeating the processes of binding and release. As shown in Fig. 8, the fluorescence could be turn off and on with alternate addition of Cu2+ and S2−, the recovered fluorescence intensity could roughly keep consistent even recycled for 5 times. The fluorescence changes of probe in the absence and presence of Cu2+/S2− were separately observed, and the corresponding photographs (Fig. 8) were recorded. The mentioned-above results implied that the fluorescent switch could be developed as a regeneratable fluorescence sensor: “on–off” type for Cu2+and “off–on” type for S2−.To achieve visual detection of Cu2+ in aqueous solution, a paper-based sensor made of filter paper has been developed. First, 2.0 μL of probe solution (0.1 mM) was carefully dropped onto a piece of filter paper. After the solution was dried in air, the fluorescence indicating paper was obtained, forming a spot about 1 cm in diameter. Then, different concentrations of Cu2+ were dropped on the test paper. As shown in Fig. 9A, we could see an obvious brightness change from blue to dark under a UV lamp. The detection limit for Cu2+ was about 10−6 M. Probe CAH–Cu2+ was capable of fast response to S2−, which prompted us to expect feasibility of directly detecting gaseous H2S. To conveniently and efficiently detect gaseous H2S, a paper sensor was fabricated by dropping 10 μL of 0.5 mM probe solution on a piece of paper. Such prepared paper sensors were exposed to gas samples containing different amounts of H2S for 1 min, and then illuminated under a UV lamp. It was obvious that the fluorescence brightness changes gradually became clear with increasing concentrations of H2S, and the visual detection limit was about 15 ppm as shown in Fig. 9B. The result demonstrated that the paper sensor immobilized with probe CAH–Cu2+ was able to serve as a simple way to direct and visual detection of gaseous H2S. Furthermore, the reusability of paper sensor was verified by successively adding Cu2+ (10−3 M) and being exposed in gaseous H2S (120 ppm) for 2 times (Fig. S10†). The result implied that the fluorescent switch could also be developed as a regeneratable paper sensor: “on–off” type for Cu2+ and “off–on” type for gaseous H2S.
Conclusions
In summary, a carbazole-based fluorescence probe to present an “on–off–on” type fluorescence response mode for sequential detection of Cu2+ and S2− has been developed. High selectivity and sensitivity of probe response to Cu2+ are barely affected by coexistence of other interfering analytes. The subsequent addition of S2− can effectively snatch Cu2+ from the complex CAH–Cu2+ to immediately recover the quenched fluorescence by releasing free probe. The detection limits of Cu2+ and S2− can reach 65 and 290 nM, which are lower than that of most probes. The good reusability of the fluorescent sensor for cyclical detection of Cu2+ and S2− is fully demonstrated by its recycling experiment. Additionally, paper sensors were applied to visually detect Cu2+ and gaseous H2S with the limit of 10−6 M and 15 ppm.Acknowledgements
This work was supported by National Basic Research Program of China (2015CB932002), China-Singapore Joint Project (2015DFG92510), National Natural Science Foundation of China (No. 21335006, 21475135, 21375131 and 21277145), Science and Technology Service Network Initiative (KFJ-SW-STS-172) and Natural Science Foundation of Anhui Province (1408085MKL52).Notes and references
- R. F. Huang, X. W. Zheng and Y. J. Qu, Anal. Chim. Acta, 2007, 582, 267 CrossRef CAS PubMed.
- K. Park, H. Lee, S. Phelan, S. Liyanaarachchi, N. Marleni, D. Navaratna, V. Jegatheesan and L. Shu, Int. Biodeterior. Biodegrad., 2014, 95, 251 CrossRef CAS.
- A. R. Lippert, E. J. New and C. J. Chang, J. Am. Chem. Soc., 2011, 133, 10078 CrossRef CAS PubMed; K. Sasakura, K. Hanaoka, N. Shibuya, Y. Mikami, Y. Kimura, T. Komatsu, T. Ueno, T. Terai, H. Kimura and T. Nagano, J. Am. Chem. Soc., 2011, 133, 18003 CrossRef PubMed.
- H. Peng, Y. Cheng, C. Dai, A. L. King, B. L. Predmore, D. J. Lefer and B. Wang, Angew. Chem., Int. Ed., 2011, 50, 9672 CrossRef CAS PubMed; C. Liu, J. Pan, S. Li, Y. Zhao, L. Y. Wu, C. E. Berkman, A. R. Whorton and M. Xian, Angew. Chem., Int. Ed., 2011, 50, 10327 CrossRef PubMed.
- J. Liu, Y. Q. Sun, J. Zhang, T. Yang, J. Cao, L. Zhang and W. Guo, Chem.–Eur. J., 2013, 19, 4717 CrossRef CAS PubMed; G. D. Yang, L. Y. Wu, B. Jiang, W. Yang, J. S. Qi, K. Cao, Q. H. Meng, A. K. Mustafa, W. T. Mu, S. M. Zhang, S. H. Snyder and R. Wang, Science, 2008, 322, 587 CrossRef PubMed.
- G. D. Yang, L. Y. Wu, B. Jiang, W. Yang, J. S. Qi, K. Cao, Q. H. Meng, A. K. Mustafa, W. T. Mu, S. M. Zhang, S. H. Snyder and R. Wang, Science, 2008, 322, 587 CrossRef CAS PubMed; K. Abe and H. Kimura, J. Neurosci., 1996, 16, 1066 Search PubMed; Y. Kaneko, Y. Kimura, H. Kimura and I. Niki, Diabetes, 2006, 55, 1391 CrossRef PubMed.
- K. Eto, T. Asada, K. Arima, T. Makifuchi and H. Kimura, Biochem. Biophys. Res. Commun., 2002, 293, 1485 CrossRef CAS PubMed; S. Fiorucci, E. Antonelli, A. Mencarelli, S. Orlandi, B. Renga, G. Rizzo, E. Distrutti, V. Shah and A. Morelli, Hepatology, 2005, 42, 539 CrossRef PubMed.
- W. Yang, G. Yang, X. Jia, L. Wu and R. Wang, J. Physiol., 2005, 569, 519 CrossRef CAS PubMed; P. Kamoun, M. C. Belardinelli, A. Chabli, K. Lallouchi and B. Chadefaux-Vekemans, Am. J. Med. Genet., Part A, 2003, 116, 310 CrossRef PubMed.
- X. Hu and B. Mutus, Rev. Anal. Chem., 2013, 32, 247 Search PubMed; X. Shen, C. B. Pattillo, S. Pardue, S. C. Bir, R. Wang and C. G. Kevil, Free Radical Biol. Med., 2011, 50, 1021 CrossRef CAS PubMed; T. Ubuka, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2002, 781, 227 CrossRef.
- F. B. Yu, P. Li, P. Song, B. S. Wang, J. Z. Zhao and K. L. Han, Chem. Commun., 2012, 48, 2852 RSC; F. Hou, L. Huang, P. Xi, J. Cheng, X. Zhao, G. Xie, Y. Shi, F. Cheng, X. Yao, D. Bai and Z. Zeng, Inorg. Chem., 2012, 51, 2454 CrossRef CAS PubMed.
- M. T. Sun, H. Yu, H. H. Li, H. D. Xu, D. J. Huang and S. H. Wang, Inorg. Chem., 2015, 54, 3766 CrossRef CAS PubMed.
- Y. h. Wang, C. Zhang, X. C. Chen, B. Yang, L. Yang, C. L. Jiang and Z. P. Zhang, Nanoscale, 2016, 8, 5977 RSC; K. Zhang, H. B. Zhou, Q. S. Mei, S. H. Wang, G. J. Guan, R. Y. Liu, J. Zhang and Z. P. Zhang, J. Am. Chem. Soc., 2011, 133, 8424 CrossRef CAS PubMed; C. Yuan, K. Zhang, Z. P. Zhang and S. H. Wang, Anal. Chem., 2012, 84, 9792 CrossRef PubMed.
- J. V. Grazulevicius, P. Strohriegl, J. Pielichowski and K. Pielichowski, Prog. Polym. Sci., 2003, 28, 1297 CrossRef CAS; J.-F. Morin, M. Leclerc, D. Ades and A. Siove, Macromol. Rapid Commun., 2005, 26, 761 CrossRef; N. Blouin and M. Leclerc, Acc. Chem. Res., 2008, 41, 1110 CrossRef PubMed.
- U. H. F. Bunz, Chem. Rev., 2000, 100, 1605 CrossRef CAS PubMed; P. Kundu, K. R. J. Thomas, J. T. Lin, Y.-T. Tao and C.-H. Chien, Adv. Funct. Mater., 2003, 13, 445 CrossRef; Y. Ohmori, H. Kajii, T. Sawatani, H. Ueta and K. Yoshino, Thin Solid Films, 2001, 393, 407 CrossRef.
- N. Koumura, Z. S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, J. Am. Chem. Soc., 2006, 128, 14256 CrossRef CAS PubMed; D. Kim, J. K. Lee, S. O. Kang and J. Ko, Tetrahedron, 2007, 63, 1913 CrossRef; C. Teng, X. Yang, C. Yuan, C. Li, R. Chen, H. Tian, S. Li and L. Sun, Org. Lett., 2009, 11, 5542 CrossRef PubMed.
- W. J. Zhu, L. L. Yang, M. Fang, Z. Y. Wu, Q. Zhang, F. F. Yin, Q. Huang and C. Li, J. Lumin., 2015, 158, 38 CrossRef CAS; G. Wang, H. Chen, X. L. Chen and Y. M. Xie, RSC Adv., 2016, 6, 18662 RSC.
- D. X. Li, X. Sun, J. M. Huang, Q. Wang, Y. Feng, M. Chen, X. M. Meng, M. Z. Zhu and X. Wang, Dyes Pigm., 2016, 125, 185 CrossRef CAS; S. Goswami, S. Paul and A. Manna, Dalton Trans., 2013, 42, 10097 RSC; A. K. Mahapatra, J. Roy and P. Sahoo, Tetrahedron Lett., 2011, 52, 2965 CrossRef.
- R. C. Weast, CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, FL, 69th edn, 1988 CAS; Y. F. Zhu, D. H. Fan and W. Z. Shen, J. Phys. Chem. C, 2008, 112, 10402 CAS.
- K. X. Yao, X. M. Yin, T. H. Wang and H. C. Zeng, J. Am. Chem. Soc., 2010, 132, 6131 CrossRef CAS PubMed; L. Hu, L. P. Zhu, H. P. He, L. Zhang and Z. Z. Ye, J. Mater. Chem. C, 2015, 3, 1330 RSC.
- J. Ouyang, H. Hong, Y. Zhao, H. Q. Shen, C. Shen, C. Y. Zhang and J. F. Zhang, Nitric Oxide, 2008, 19, 42 CrossRef CAS PubMed.
- Z. P. Liu, Z. L. Zhang, X. Q. Wang, W. J. He and Z. J. Guo, Org. Lett., 2012, 14, 4378 CrossRef CAS PubMed.
- Q. S. Mei, K. Zhang, G. J. Guan, B. H. Liu, S. H. Wang and Z. P. Zhang, Chem. Commun., 2010, 46, 7319 RSC.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS.
- R. Koteeswari, P. Ashokkumar, E. J. P. Malar, V. T. Ramakrishnan and P. Ramamurthy, Chem. Commun., 2011, 47, 7695 RSC; K. Chirantan, D. A. Manab, R. Aiyagari and D. Gopal, Inorg. Chem., 2013, 52, 743 CrossRef CAS PubMed.
- J. A. Dean, Langès Handbook of Chemistry, McGraw-Hill, New York, 15th edn, 1999 Search PubMed.
- R. Wang, F. B. Yu, L. X. Chen, L. J. Wang and W. W. Zhang, Chem. Commun., 2012, 48, 11757 RSC; W. M. Xuan, R. Pan, Y. T. Cao, K. J. Liu and W. Wang, Chem. Commun., 2012, 48, 10669 RSC; S. K. Das, C. S. Lim, S. Y. Yang, J. H. Han and B. R. Cho, Chem. Commun., 2012, 48, 8395 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: UV/Vis absorption, detection limit, fluorescence quantum yield and other additional data, 1H NMR, 13C NMR, and HR-MS. See DOI: 10.1039/c6ra10293j |
| This journal is © The Royal Society of Chemistry 2016 |