 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Arrhenius parameters for the OH-initiated degradation of methyl crotonate, methyl-3,3-dimethyl acrylate, (E)-ethyl tiglate and methyl-3-butenoate over the temperature range of 288–314 K†
Juan P.
Colomer
a,
María B.
Blanco
a,
Alicia B.
Peñéñory
a,
Ian
Barnes
b,
Peter
Wiesen
b and
Mariano A.
Teruel
*a
aInstituto de Investigaciones en Fisicoquímica de Córdoba (I.N.F.I.Q.C.), CONICET. Facultad de Ciencias Químicas, Universidad Nacional de Córdoba. Ciudad Universitaria, 5000 Córdoba, Argentina. E-mail: mteruel@fcq.un.edu.ar
bBergische Universität Wuppertal, Fakultät für Mathematik und Naturwissenschaften, Physikalische & Theoretische Chemie, 42119 Wuppertal, Germany
First published on 26th May 2016
Abstract
The relative-rate technique has been employed to obtain rate coefficients for the reactions between the OH radical and four unsaturated esters (methyl crotonate (k1), methyl-3,3-dimethyl acrylate (k2), (E)-ethyl tiglate (k3) and methyl-3-butenoate (k4)) between 288 and 314 K in 760 Torr of synthetic air. The experiments were performed in a multiple-pass environmental chamber with in situ FTIR detection of the esters and reference compounds. The rate coefficients for the reactions displayed a negative dependence with temperature and low pre-exponential factors. The following Arrhenius expressions (in units of cm3 per molecule per s) were obtained: k1 = (3.39 ± 0.78) × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(750 ± 159)/T], k2 = (2.95 ± 0.63) × 10−12
exp[(750 ± 159)/T], k2 = (2.95 ± 0.63) × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(838 ± 182)/T], k3 = (1.49 ± 0.56) × 10−12
exp[(838 ± 182)/T], k3 = (1.49 ± 0.56) × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(522 ± 114)/T] and k4 = (1.90 ± 1.25) × 10−12
exp[(522 ± 114)/T] and k4 = (1.90 ± 1.25) × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(834 ± 185)/T]. The kinetic results are in agreement with a mechanism involving mainly OH radical addition to the double with the reversible formation of an OH-adduct. Atmospheric implications are discussed with particular reference to the rate coefficients obtained as a function of the temperature.
exp[(834 ± 185)/T]. The kinetic results are in agreement with a mechanism involving mainly OH radical addition to the double with the reversible formation of an OH-adduct. Atmospheric implications are discussed with particular reference to the rate coefficients obtained as a function of the temperature.
Introduction
Unsaturated esters are emitted to the atmosphere by biogenic sources (as the emissions from agricultural crops,1 after leaf wounding2) and by anthropogenic sources including the polymer industry where they are widely used in the production of plastic and resins.3Moreover, acrylates and methacrylates are among the most prevalent industrial organic chemicals in the world as defined by the High Production Volume Chemicals list.4,5 These compounds are employed as intermediates in the manufacture of polymers and plastics and are also used as fuels and oil additives. The most important polymer types are cast acrylic sheets and molding/extrusion compounds, in addition to emulsions, dispersions, and solvent-based polymers.6
The release of these oxygenated volatile organic compounds into the atmosphere is likely to contribute to the formation of ozone, peroxy acetyl nitrate (PAN) and other components of photochemical smog formed in urban areas mainly through their reactions with OH radicals and other oxidants such as NO3 radicals and O3 molecules.7 To determine the impact of these compounds on air quality, kinetic and mechanistic data on their tropospheric degradation are needed.7
In this work, we report relative kinetic determinations of rate coefficients for the reactions of OH radicals with methyl crotonate (MC) (1), methyl-3,3-dimethyl acrylate (MDMA) (2), (E)-ethyl tiglate (ET) (3) and methyl-3-butenoate (M3B) (4) measured in an FTIR multiple-pass photoreactor between 288 and 314 K:
 | (1) |
 | (2) |
 | (3) |
 | (4) |
The rate coefficients for the reactions of these unsaturated esters with OH radicals and Cl atoms have been reported at atmospheric pressure and room temperature previously by our research group.8,9 As noted above, the atmospheric degradation of unsaturated esters is mainly initiated by reaction with OH radicals but information regarding the degradation processes is still limited. To evaluate the impact of these reactions on the formation of photo-oxidants and consequently on human health and the environment, a thorough understanding of the atmospheric oxidation processes of these compounds is needed. Therefore, in line with our previous work and to obtain a better understanding of the OH-radical mediated oxidation of these compounds in the atmosphere we report here a study on the temperature dependence of the kinetics of these reactions with OH radicals. To the best of our knowledge, this work provides the first kinetic study for the reaction of OH radicals with these unsaturated esters as a function of temperature. Furthermore, residence times of the esters studied as a function of the altitude of the troposphere are developed.
Experimental
The experiments were carried out in a quartz-glass reaction chamber (1080 L) in synthetic air at a total pressure of 760 ± 10 Torr (760 Torr = 101.325 kPa) over the temperature range 288–314 K. Since a detailed description of the reaction chamber can be found in previous work10 only the basic features are outlined here.The reactor can be evacuated to 10−3 Torr using a pumping system comprised of a turbo-molecular pump backed by a double-stage rotary fore pump. To ensure a homogeneous distribution of the substrates inside the chamber three magnetically coupled Teflon mixing fans were employed. The reactor is surrounded by 32 individually switched superactinic fluorescent lamps (Philips TL05 40 W, λ = 320–480 nm, λmax = 360 nm), which are wired in parallel and spaced evenly around the reaction vessel thus allowing variation of the light intensity and consequently the photolysis frequency/radical production rate within the chamber. A multiple-reflection white-type mirror system with a base length of 5.91 ± 0.01 m is installed in the reactor for sensitive “in situ” long path absorption monitoring of reactants and products in the IR spectral range 4000–700 cm−1. The mirror system was operated with 82 infrared light traverses resulting in a total optical path length of 484.7 ± 0.8 m. The IR spectra were recorded with a spectral resolution of 1 cm−1 using a Nicolet Nexus FT-IR spectrometer, equipped with a liquid nitrogen cooled mercury–cadmium–telluride (MCT) detector. The reactor temperature was adjustable to a precision of ±1 K in the range 283 to 314 K.
In the investigations 15 spectra were recorded per experiment, whereby 64 interferograms were co-added per spectrum over 1 min, and the first five spectra were recorded in the absence of light. Hydroxyl radicals (OH) were generated in situ by photolysis of methyl nitrite (CH3ONO) in the presence of NO/O2:
 | (5) |
| CH3O + O2 → CH2O + HO2 | (6) |
| HO2 + NO → OH + NO2 | (7) |
The initial concentrations employed in the experiments for the unsaturated esters and reference compounds in ppm (1 ppm = 2.46 × 1013 molecule per cm3 at 298 K and 760 Torr of total pressure) were as follows: ∼5.3 for methyl crotonate; ∼3.9 for methyl-3,3-dimethyl acrylate; ∼3.7 for (E)-ethyl tiglate; ∼4.8 for methyl-3-butenoate and 5–22 for 1-butene. The concentration of CH3ONO was typically around 5 ppm and 3 ppm for NO.
The evolution of the reactions was monitored by following the infrared absorption frequencies of the reactants at the following infrared frequencies (in cm−1): methyl crotonate at 1190; methyl-3,3-dimethyl acrylate at 1159; (E)-ethyl tiglate at 1266; methyl-3-butenoate at 1176 and 1-butene at 912.
Materials
The following chemicals, with purities as given by the manufacturer, were used in the experiments without further purification: synthetic air (Air Liquide, 99.999%), methyl crotonate (Aldrich, 98%), methyl-3,3-dimethyl acrylate (Aldrich, 97%), (E)-ethyl tiglate (Aldrich, >98%), methyl-3-butenoate (Aldrich, 95%), 1-butene (Messer Griesheim, 99%), NO (Messer Griesheim, 99%).Methyl nitrite was synthesized according to the method of Taylor et al.11 by the dropwise addition of 50% H2SO4 to a saturated solution of NaNO2 in methanol and was purified by vacuum distillation.
Results and discussion
Rate coefficients for the reactions of OH radicals with MC, MDMA, ET and M3B were determined over the range 288–314 K by comparing their rates of decay with that of the corresponding decay of a reference compound:| OH + unsaturated ester → products | (8) |
| OH + ref → products | (9) |
Provided that the reference compound and the reactant are lost only by reactions (8) and (9), then it can be shown that:
 | (10) |
Eqn (10) is only valid when the ester and the reference are removed solely by reaction with OH radicals. To verify this assumption, various tests were performed to ascertain whether or not photolysis, wall loss and reaction of the unsaturated esters and reference compounds with methyl nitrite/NO were negligible. The tests showed that all of these loss processes were negligible over the time period of the experiments, i.e. photolysis of the ester and reference compounds in the absence of the OH source was negligible and no significant wall loss of either the ester or reference compound was observed on leaving the compounds to stand in the dark in the reactor in the presence of the OH radical precursor. The IR spectra for the unsaturated esters alone and for the mixture before photolysis and during the kinetic experiments are provided now in the ESI file (Fig. S1 to S12†).
Temperature dependencies of the ester OH radical rate coefficients
Examples of the kinetic data obtained in the temperature range 288–314 K for the reactions of OH radicals with methyl crotonate, methyl-3,3-dimethyl acrylate, (E)-ethyl tiglate and methyl-3-butenoate relative to that of OH 1-butene kinetic are shown plotted according to eqn (10) in Fig. 1–4. The plots show reasonable linear correlations with near zero intercepts for all the esters at the three temperatures shown. Similar good linear correlations were also obtained for the other two temperatures investigated; these have been omitted from the plots for clarity. | ||
| Fig. 1 Plot of the kinetic data for the reaction of OH radicals with methyl crotonate obtained at 288, 291 and 311 K, using 1-butene as reference hydrocarbon. | ||
 | ||
| Fig. 2 Plot of the kinetic data for the reaction of OH radicals with methyl-3,3-dimethyl acrylate obtained at 289, 302 and 313 K, using 1-butene as reference hydrocarbon. | ||
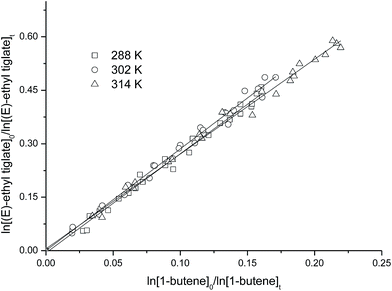 | ||
| Fig. 3 Plot of the kinetic data for the reaction of OH radicals with (E)-ethyl tiglate obtained at 288, 302 and 314 K, using 1-butene as reference hydrocarbon. | ||
 | ||
| Fig. 4 Plot of the kinetic data for the reaction of OH radicals with methyl-3-butenoate obtained at 289, 301 and 313 K, using 1-butene as reference hydrocarbon. | ||
Table 1 lists the rate coefficient ratios obtained at each of the five temperatures investigated from linear least-squares analyses of the kinetic data plots and the absolute rate coefficients obtained with OH for each unsaturated ester derived from the rate ratios. Table 1 also lists the Arrhenius parameters obtained from linear fits to plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k versus 1/T for each ester as shown in Fig. 5. The rate coefficient ratios listed in Table 1 are the average values obtained from at least 2–3 experiments per compound and per temperature. The rate coefficients for the reactions of OH radicals with the unsaturated esters were placed on an absolute basis using rate coefficients for the reaction of OH with 1-butene calculated for the respective temperatures from the Arrhenius expression (in cm3 per molecule per s units): k(OH+1-butene) = 6.55 × 10−12
k versus 1/T for each ester as shown in Fig. 5. The rate coefficient ratios listed in Table 1 are the average values obtained from at least 2–3 experiments per compound and per temperature. The rate coefficients for the reactions of OH radicals with the unsaturated esters were placed on an absolute basis using rate coefficients for the reaction of OH with 1-butene calculated for the respective temperatures from the Arrhenius expression (in cm3 per molecule per s units): k(OH+1-butene) = 6.55 × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(4.67/T) cm3 per molecule per s.12
exp(4.67/T) cm3 per molecule per s.12
| T (K) | k unsaturated ester/kref | k unsaturated ester × 10−11 (cm3 per molecule per s) | Reactant | −Ea/R (K) | A/10−12 (cm3 per molecule per s) |
|---|---|---|---|---|---|
| 288 | 1.37 ± 0.01 | 4.55 ± 0.96 | Methyl crotonate | 750 ± 159 | 3.39 ± 0.78 |
| 291 | 1.41 ± 0.02 | 4.61 ± 0.98 | |||
| 305 | 1.21 ± 0.03 | 3.68 ± 0.82 | |||
| 308 | 1.35 ± 0.03 | 4.03 ± 0.90 | |||
| 311 | 1.32 ± 0.02 | 3.87 ± 0.84 | |||
| 289 | 1.69 ± 0.03 | 5.58 ± 1.21 | Methyl-3,3-dimethyl acrylate | 838 ± 182 | 2.95 ± 0.63 |
| 291 | 1.53 ± 0.02 | 5.00 ± 1.06 | |||
| 302 | 1.54 ± 0.02 | 4.74 ± 1.01 | |||
| 308 | 1.55 ± 0.02 | 4.63 ± 0.99 | |||
| 313 | 1.44 ± 0.03 | 4.20 ± 0.93 | |||
| 288 | 2.78 ± 0.05 | 9.23 ± 1.31 | (E)-Ethyl tiglate | 522 ± 114 | 0.149 ± 0.06 |
| 291 | 2.62 ± 0.04 | 8.55 ± 1.24 | |||
| 302 | 2.85 ± 0.05 | 8.78 ± 1.21 | |||
| 308 | 2.66 ± 0.07 | 7.93 ± 1.12 | |||
| 314 | 2.66 ± 0.04 | 7.73 ± 1.10 | |||
| 289 | 1.05 ± 0.02 | 3.47 ± 0.77 | Methyl-3-butenoate | 834 ± 185 | 1.90 ± 1.25 |
| 291 | 1.05 ± 0.02 | 3.41 ± 0.74 | |||
| 301 | 0.86 ± 0.01 | 2.67 ± 0.55 | |||
| 308 | 1.03 ± 0.02 | 3.06 ± 0.68 | |||
| 313 | 0.93 ± 0.01 | 2.73 ± 0.59 |
 | ||
| Fig. 5 Arrhenius plots of the kinetic data obtained in this study between 288 and 314 K for the reactions of OH radicals with methyl crotonate (△); methyl-3,3-dimethyl acrylate (□); (E)-ethyl tiglate (○) and methyl-3-butenoate (◊). The filled symbols correspond to previous measurements from our group for the title reactions at 298 K.8,9 | ||
The errors quoted for the rate coefficients given in Table 1 are a combination of 2σ statistical errors from the linear fit analyses of the plots and an additional 20% error allocated to potential errors in the recommended value of the rate coefficient for the reference reaction. The errors for the ratios kunsaturated ester/kref are only 2σ statistical errors.
For the four unsaturated esters studied MC, MDMA, ET and M3B the reaction rate coefficients were found to decrease slightly with increasing temperature over the range of temperature investigated. The Arrhenius expressions presented below describe reasonably well the OH-radical kinetic temperature dependence of the esters in the temperature range 288–314 K with k in units of cm3 per molecule per s:
k1(OH+MC) = (3.39 ± 0.78) × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(750 ± 159)/T] exp[(750 ± 159)/T] |
k2(OH+MDMA) = (2.95 ± 0.63) × 10−11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(838 ± 182)/T] exp[(838 ± 182)/T] |
k3(OH+ET) = (1.49 ± 0.56) × 10−11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(522 ± 114)/T] exp[(522 ± 114)/T] |
k4(OH+M3B) = (1.90 ± 1.25) × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(834 ± 185)/T] exp[(834 ± 185)/T] |
The errors in the activation term and the pre-exponential factor are the 2σ random statistical errors from fits to the data presented in Table 1 and plotted in Fig. 5.
The weak negative temperature behavior illustrated by the Arrhenius analysis can be attributed to the existence of a stable van der Waals pre-reactive complex in the entrance of the reaction channel which explains satisfactorily the observed temperature dependence of the reactions of OH with the unsaturated esters.13 This is the generally accepted mechanism for OH-radical addition to alkenes12,14,15 and for unsaturated VOCs in general.16 The weak negative activation energies found for reactions (1) to (4) are also in agreement with findings from our studies on the reactions of OH radicals with other unsaturated esters, i.e. methyl methacrylate (Ea/R = 921 K), butyl methacrylate (Ea/R = 413 K), butyl acrylate (Ea/R = 1117 K) and vinyl acetate (Ea/R = 540 K).17
Comparison with previous kinetics determinations at room temperature
Although, there are no prior experimental determinations of the Arrhenius parameters for the reactions studied, the room temperature rate coefficients for the reactions of OH radicals with methyl crotonate, methyl-3,3-dimethyl acrylate, (E)-ethyl tiglate and methyl-3-butenoate at 298 K were previously reported by our group.8,9Teruel et al.8 reported a rate coefficient of (4.65 ± 0.65) × 10−11 cm3 per molecule per s for the reaction of OH with methyl crotonate at 298 K and 750 Torr total pressure measured using the relative kinetic method and gas chromatography/flame ionization detection (RR-GC/FID) for the analyses. This value is in very good agreement with the values of (4.61 ± 0.98) × 10−11 cm3 per molecule per s and (3.68 ± 0.82) × 10−11 cm3 per molecule per s reported in this work for the reaction at 291 and 305 K, respectively.
On the other hand, Colomer et al.9 have performed relative kinetic determinations of the reactions of OH with methyl-3,3-dimethyl acrylate, (E)-ethyl tiglate and methyl-3-butenoate using FTIR at 298 K and 760 Torr of synthetic air. They reported rate coefficients (in cm3 per molecule per s) of (4.46 ± 1.05) × 10−11, (8.32 ± 1.93) × 10−11 and (3.16 ± 0.57) × 10−11 for the reactions of MDMA, ET and M3B, respectively. The values of k at 298 K are also in agreement with the rate coefficient values (in units of 10−11 cm3 per molecule per s) reported in this work at temperatures just below and above 298 K (see Table 1); (5.00 ± 1.06 and 4.74 ± 1.01) for MDMA; (8.55 ± 1.24 and 8.78 ± 1.21) for ET and (3.41 ± 0.74 and 2.67 ± 0.55) for M3B.
Our previously reported rate coefficient values obtained at 298 K for the title reactions are represented in Fig. 5 as filled symbols for comparison with the present results. These values were not included in the determination of the Arrhenius parameters for the reactions.
Tropospheric OH radical reaction loss rates for the unsaturated esters
The temperature dependencies of the OH-rate coefficients reported in this work were used to calculate the troposphere rate of loss of the unsaturated esters with respect to reaction with OH radicals as a function of altitude. At a given temperature (T) corresponding to a given altitude in the troposphere, the rate of loss of the unsaturated esters is defined as the product between the OH rate coefficient (T) and [OH] at this altitude.Using a 12 h daytime average global tropospheric OH radical concentration18 of [OH] = 2 × 106 molecule per cm3, the Arrhenius parameters reported in this work and considering a lapse rate in the troposphere of −6.5 K km−1,19 we have calculated the temperature profiles between 0 and 10 km. Table 2 shows the OH removal rates for the esters studied as a function of altitude in the troposphere assuming a temperature of 298.15 K at 0 km. The results shown in Table 2 are plotted in Fig. 6.
| Altitude (km) | T (K) | k OH+MC(T)[OH] (s−1) | k OH+MDMA(T)[OH] (s−1) | k OH+ET(T)[OH] (s−1) | k OH+M3B(T)[OH] (s−1) |
|---|---|---|---|---|---|
| 0 | 298.15 | 8.38 × 10−5 | 9.80 × 10−5 | 1.72 × 10−4 | 6.24 × 10−5 |
| 1 | 291.65 | 8.88 × 10−5 | 1.04 × 10−4 | 1.78 × 10−4 | 6.64 × 10−5 |
| 2 | 285.15 | 9.40 × 10−5 | 1.11 × 10−4 | 1.86 × 10−4 | 7.08 × 10−5 |
| 3 | 278.65 | 1.00 × 10−4 | 1.19 × 10−4 | 1.94 × 10−4 | 7.58 × 10−5 |
| 4 | 272.15 | 1.07 × 10−4 | 1.28 × 10−4 | 2.02 × 10−4 | 8.14 × 10−5 |
| 5 | 265.65 | 1.14 × 10−4 | 1.38 × 10−4 | 2.12 × 10−4 | 8.78 × 10−5 |
| 6 | 259.15 | 1.22 × 10−4 | 1.50 × 10−4 | 2.24 × 10−4 | 9.50 × 10−5 |
| 7 | 252.65 | 1.32 × 10−4 | 1.63 × 10−4 | 2.36 × 10−4 | 1.03 × 10−4 |
| 8 | 246.15 | 1.43 × 10−4 | 1.78 × 10−4 | 2.48 × 10−4 | 1.13 × 10−4 |
| 9 | 239.65 | 1.55 × 10−4 | 1.95 × 10−4 | 2.64 × 10−4 | 1.23 × 10−4 |
| 10 | 233.15 | 1.69 × 10−4 | 2.14 × 10−4 | 2.80 × 10−4 | 1.36 × 10−4 |
 | ||
| Fig. 6 Hydroxyl radical (OH) reaction loss rates for methyl crotonate (●), methyl-3,3-dimethyl acrylate (▼), (E)-ethyl tiglate (■) and methyl-3-butenoate (▲) as a function of altitude (km). | ||
The loss rates (in s−1) of the esters at sea level (0 km) are 6.24 × 10−5, 8.38 × 10−5, 9.80 × 10−5 and 1.72 × 10−4 and near to the tropopause (∼10 km) around 1.36 × 10−4, 1.69 × 10−4, 2.14 × 10−4 and 2.80 × 10−4 for M3B, MC, MDMA and ET, respectively.
The loss rate of ethyl tiglate is the largest and that of methyl-3-butenoate is the smallest at all altitudes while the other two unsaturated esters have intermediate values, as expected taking into account the larger and lower rate coefficients kOH(T) for ET and M3B, respectively.
Atmospheric implications
As stated in our previous studies the main tropospheric fate for methyl crotonate, methyl-3,3-dimethyl acrylate, (E)-ethyl tiglate and methyl-3-butenoate will be reaction with OH radicals resulting in lifetimes of 3 hours for methyl crotonate and methyl-3,3-dimethyl acrylate8,9 and 2 and 4 hours for (E)-ethyl tiglate and methyl-3-butenoate, respectively.9 Using the Arrhenius expressions reported in this work, we have calculated the atmospheric lifetimes of the unsaturated esters for different temperatures and their variations with altitude.With the rate coefficients obtained at different temperatures for the reactions studied, we have estimated the lifetimes for the four unsaturated VOCs studied as a function of altitude.
The calculations show that the estimated lifetimes of these unsaturated esters with respect to reaction with OH decrease with altitude (from sea level to near the tropopause) by around 50% for methyl crotonate (with lifetimes from 3.30 to 1.64 hours, respectively), 46% for methyl-3,3-dimethyl acrylate (with lifetimes from 2.83 to 1.30 hours, respectively), and methyl-3-butenoate (with lifetimes from 4.45 to 2.04 hours, respectively) and 60% for (E)-ethyl tiglate (with lifetimes from 1.61 to 0.99 hours, respectively) reflecting the decrease of temperature with altitude and associated decrease in reaction rates of the reactions. The temperature dependent rate coefficients determined in this study will allow a better representation in three-dimensional climate models where temperature variations in reactions are considered.20
The OH-radical initiated tropospheric degradation of the unsaturated esters studied is expected to result mainly in the formation of oxygenated VOCs such as aldehydes and dicarbonyls compounds, which will be subject to further reaction with OH and also photolysis. The exact identification and yields of the products formed from the reaction of OH radicals with the compounds studied here still remain to be investigated. The high reactivity and thus short atmospheric lifetimes of the unsaturated esters and also their primary products implies they will contribute significantly to formation of ozone and other photooxidants in the atmosphere close to their emission sources if emitted in large quantities.
The photochemical ozone creation potential (POCP) is a modelling method used to estimate the ozone creation potential of VOCs relative to that of ethene which is given the value 100.21–24 To make POCP values more readily calculable without resource to computer modelling Derwent et al.23 and Jenkin et al.24 have developed a simplified estimation procedure which uses fundamental molecular properties molecular weight, number of reactive bonds, and rate coefficient for reaction with OH radicals to provide estimated POCPs (εPOCP) using an analytical expression. This analytical expression gives εPOCP values which are in reasonable agreement for a wide range of different compound classes.
This estimate method gives values of εPOCP for MC, MDMA, ET and M3B of around 57, 62, 66 and 57, respectively, indicating that these unsaturated esters will contribute significantly to the formation of tropospheric ozone.
Conclusions
Arrhenius expressions for the reactions of OH radicals with the unsaturated esters MC, MDMA, ET and M3B have been reported over the temperature range of 288–314 K. For the four unsaturated esters studied the reaction rate coefficients were found to decrease slightly with increasing temperature over the range of temperature investigated.The faint negative temperature dependence observed in the Arrhenius analysis can be attributed to the existence of a stable van der Waals pre-reactive complex in the entrance of the reaction channel which explains satisfactorily the observed temperature behavior of the reactions of OH with these unsaturated esters.
For the four unsaturated ester studied the main degradation pathway will be reaction with OH radicals. Employing the Arrhenius expressions obtained in this work, the atmospheric lifetimes of these unsaturated esters with respect to reaction with OH were calculated as a function of temperature and also their variation with altitude between 0 and 10 km. The values obtained decrease with altitude, reflecting the decline of temperature with altitude and associated decrease in reaction rates of the reactions.
Acknowledgements
The authors wish to acknowledge the EU project EUROCHAMP2, SECYT (Argentina), CONICET (Argentina), ANPCyT-FONCYT (Argentina), SECyT-UNC (Córdoba, Argentina) for financial support of this research.References
- J. Arey, A. M. Winer, R. Atkinson, S. M. Aschmann, W. D. Long and C. L. Morrison, Atmos. Environ., 1991, 25, 1063 CrossRef.
- R. Fall, T. Karl, A. Hansel, A. Jordan and W. Lindinger, J. Geophys. Res., 1999, 104, 963 CrossRef.
- T. E. Graedel, Chemical compounds in the atmosphere, Academic Press, New York, 1978 Search PubMed.
- S. Green, A. Goldberg and J. Zurlo, Toxicol. Sci., 2001, 63, 6 CrossRef CAS PubMed.
- http://www.cs3-hq.oecd.org/scripts/hpv/index.asp .
- S. Srivastava, Des. Monomers Polym., 2009, 12, 1 CrossRef CAS.
- A. Mellouki, G. L. Bras and H. Sidebottom, Chem. Rev., 2003, 103, 5077 CrossRef CAS PubMed.
- M. A. Teruel, J. Benitez-Villalba, N. Caballero and M. B. Blanco, J. Phys. Chem. A, 2012, 116, 6127 CrossRef CAS PubMed.
- J. P. Colomer, M. B. Blanco, A. B. Peñéñory, I. Barnes, P. Wiesen and M. A. Teruel, Atmos. Environ., 2013, 79, 546 CrossRef CAS.
- I. Barnes, K. H. Becker and N. Mihalopoulos, J. Atmos. Chem., 1994, 18, 267 CrossRef CAS.
- W. D. Taylor, T. D. Allston, M. J. Moscato, G. B. Fazekas, R. Kozlowski and G. A. Takacs, Int. J. Chem. Kinet., 1980, 12, 231 CrossRef CAS.
- J. G. Calvert, R. Atkinson, J. A. Kerr, S. Madronich, G. K. Moortgat, T. J. Wallington and G. Yarwood, The mechanisms of atmospheric oxidation of the alkenes, Oxford University Press, New York, 2000 Search PubMed.
- D. L. Singleton and R. J. Cvetanovic, J. Am. Chem. Soc., 1976, 98, 6812 CrossRef CAS.
- R. Atkinson, J. Phys. Chem. Ref. Data, 1997, 26, 215 CrossRef CAS.
- R. Atkinson, Atmos. Environ., 2000, 34, 2063 CrossRef CAS.
- R. Atkinson and J. Arey, Chem. Rev., 2003, 103, 4605 CrossRef CAS PubMed.
- M. B. Blanco, I. Bejan, I. Barnes, P. Wiesen and M. A. Teruel, J. Phys. Chem. A, 2009, 113, 5958 CrossRef CAS PubMed.
- R. Hein, P. J. Crutzen and M. Heimann, Global Biogeochem. Cycles, 1997, 11, 43 CrossRef CAS.
- M. Beychok, http://www.eoearth.org/view/article/170859, 2013.
- A. Guenther, C. N. Hewitt, D. Erickson, R. Fall, C. Geron, T. Graedel, P. Harley, L. Klinger, M. Lerdau, W. A. McKay, T. Pierce, B. Scholes, R. Steinbrecher, R. Tallamraju, J. Taylor and P. Zimmerman, J. Geophys. Res.: Atmos., 1995, 100, 8873 CrossRef CAS.
- R. G. Derwent, M. E. Jenkin and S. M. Saunders, Atmos. Environ., 1996, 30, 181 CrossRef CAS.
- R. G. Derwent, M. E. Jenkin, S. M. Saunders and M. J. Pilling, Atmos. Environ., 1998, 32, 2429 CrossRef CAS.
- R. G. Derwent, M. E. Jenkin, N. R. Passant and M. J. Pilling, Atmos. Environ., 2007, 41, 2570 CrossRef CAS.
- M. E. Jenkin, Photochemical Ozone and PAN Creation Potentials: Rationalisation and methods of estimation. AEA Technology plc, Report AEAT-4182/20150/003, AEA Technology plc., National Environmental Technology Centre, Culham, Oxfordshire OX14 3DB, UK, 1998 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10279d |
| This journal is © The Royal Society of Chemistry 2016 |
