N-Doped biochar derived from co-hydrothermal carbonization of rice husk and Chlorella pyrenoidosa for enhancing copper ion adsorption
Chao Gaia,
Yanchuan Guob,
Nana Penga,
Tingting Liua and
Zhengang Liu†
*a
aResearch Center for Eco-Environmental Sciences, Chinese Academy of Sciences, 18 Shuangqing Road, Beijing 100085, China. E-mail: zgliu@rcees.ac.cn; Tel: +86 10 62915966
bKey Laboratory of Photochemical Conversion and Optoelectronic Material, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, 29 Zhongguancun East Road, Beijing 100190, China
First published on 19th May 2016
Abstract
Biochar derived from rice husk was modified by microalgae Chlorella pyrenoidosa as a natural nitrogen-rich precursor in a hydrothermal environment for copper ion (Cu(II)) adsorption. Pristine biochar derived from hydrothermal carbonization of individual rice husks was included as a control. FTIR, SEM and BET analyses indicated that the modified biochar is more hydrophobic and basic than the pristine biochar due to the anchoring of surface nitrogenous functionalities. The adsorption of copper ions onto the pristine and modified biochar was investigated with respect to pH, adsorbent dosage, contact time, temperature, kinetics and isotherms. The results showed that modification of the biochar by nitrogen significantly increased the copper adsorption capacity from 13.12 mg g−1 for the pristine biochar to 29.11 mg g−1 for the modified biochar. Adsorption of copper ions by the modified biochar was dominated by surface complexation rather than through the electrostatic attractions that dominated adsorption for the pristine biochar.
1. Introduction
Extensive studies have been conducted to explore carbonaceous materials as adsorbents (e.g., activated carbon, graphene) capable of removing contaminants from wastewater. However, it is hard to selectively adsorb contaminants using these carbonaceous materials.1,2 Increasing attention has been focused on the surface modification of carbon materials to enhance their capacity for pollutant removal, such as the anchoring of carboxyl groups by citric acid3 or of amino groups4 by nitration onto the surface of carbon materials. Such approaches are relatively expensive and require strict pretreatments. Biochar derived from biomass is a carbon-rich material with abundant polar functional groups.5–8 Compared to other commercial carbon materials, such as activated carbon, biochar has the competitive advantages of cost-effectiveness and wide availability. It has been widely used as a promising bioadsorbent for removing contaminants from wastewater, including inorganic pollutants like heavy metals and organic contaminants, such as tetracycline/bovine serum albumin.9,10Conventional biochar is produced by oxygen-limited pyrolysis of lignocellulosic biomass, which is usually accompanied by physical/chemical activation at elevated temperatures.11,12 An alternative technology, involving sustainable biochar production from biomass, is hydrothermal carbonization, which is generally conducted in water at ambient temperatures under autogenous pressure.13–15 The surface area of the biochar derived from pyrolysis is usually higher than that of the biochar produced by hydrothermal treatment. However, the surface functional groups in the biochar produced from pyrolysis, which can provide exchange capacity, are fewer compared to those in the biochar produced by hydrothermal treatment. For example, the biochars prepared by hydrothermal carbonization are reported to have much higher oxygen functional groups (e.g. carboxylic, lactone and phenolic group) than the biochar produced from pyrolysis.9,16 According to Uchimiya et al.17 oxygen functional groups in the biochars play vital roles in binding metal ions during the adsorption process, suggesting that the biochar produced from hydrothermal processing may be a more suitable potential bioadsorbent than that produced by pyrolysis. Hydrothermal carbonization of precursory biomass can sustainably generate biochars as carbonaceous materials with attractive nanostructures and facilitate the anchoring of certain functional groups for pollutant removal.
The incorporation of nitrogen-containing functional groups can enhance the adsorption capacity of biochar for metal ions because nitrogen functionalities can efficiently complex with heavy metals due to the high stability constants of the coordination complexes.4,18 Nitrogen functionalities can be introduced either by nitrogen-containing reagents, such as trioctylamine, or by carbonization of nitrogen-rich carbon precursors like polyacrylonitrile. Microalgae of Chlorella pyrenoidosa is rich in proteins, which are natural nitrogen-containing molecules. Titirici et al.19 have demonstrated that it is possible to synthesize nitrogen-doped carbonaceous materials via the hydrothermal treatment of amino-containing carbohydrates at a temperature of 180 °C. The presence of amine groups in Chlorella pyrenoidosa has been proven in our previous work.20 This suggests that modification of biochar from lignocellulosic biomass with protein-rich microalgae as a natural nitrogen-rich precursor in a hydrothermal environment may facilitate the anchoring of nitrogen-containing groups for heavy metal removal. Additionally, considering that the peptide bond in amino acids is more stable in hydrothermal reactions than the glycosidic bond in cellulose, the natural nitrogen-containing functionalities are more easily retained on the surface of biochar after hydrothermal carbonization.
Contamination of water streams by toxic heavy metal ions, such as Hg2+, Ni2+, Pb2+, Cr3+, Cu2+, and Zn2+, has become a worldwide environmental problem. In this study, copper was chosen as a representative heavy metal contaminant in wastewater. We modified the biochar by the anchoring of nitrogenous functional groups and characterized the pristine and modified biochars by SEM, FTIR, and BET. The adsorption behavior of Cu(II) as a function of different adsorption conditions was studied. This work aims to provide a new and cost-effective approach to the preparation of adsorbents with an improved performance in heavy metal removal from wastewater.
2. Materials and methods
2.1. Adsorbent preparation
The pristine biochar was produced by hydrothermal carbonization of individual rice husk, RH, as lignocellulosic biomass in subcritical water. The rich husk was locally obtained. Modified biochar was prepared in a hydrothermal environment in the presence of the nitrogen-rich microalgae, Chlorella pyrenoidosa, CP, which was purchased from a health-food store (NOW FOODS, Bloomingdale, IL).Hydrothermal experiments were conducted in a high-pressure batch reactor with a 100 mL stainless steel autoclave. Typically, feedstock slurries (30 g) with precursory biomass and deionized water were charged into the reactor. Then, the sealed reactor was heated up to 200 °C by an electric heater and the corresponding pressure at final temperature was 1.7 MPa. After a residence time of 60 min, the reactor was cooled down to ambient temperature and opened. The solid fraction was separated from the resultant mixture by vacuum filtration using a 55 mm glass-fiber filter (Whatman®) and then oven dried at 105 °C for 12 h; this is regarded as the biochar. All hydrothermal experiments were conducted in triplicate. The biochar samples were mixed together and then the biochar was ground and sieved. The fraction of 100–120 mesh was used for characterization and subsequent adsorption experiments.
In this study, the yield ratio YRi (i = RH or CP) is applied to quantify the effect of co-carbonization on the yield of biochar, which is defined as:
 | (1) |
 | (2) |
2.2. Adsorbent characterization
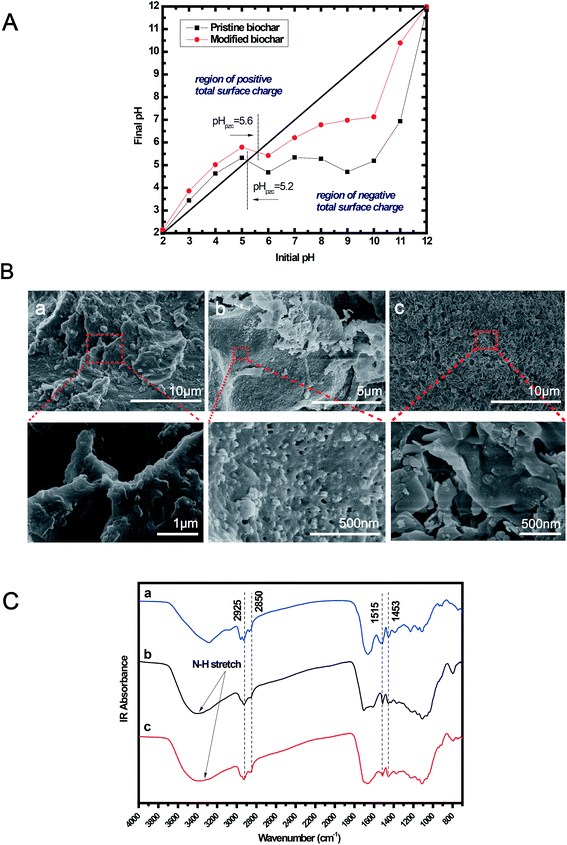
The percentage contents of carbon, hydrogen, oxygen and nitrogen in the pristine and modified biochar were analyzed using a CE-440 elemental analyzer (Exeter Analytical Inc., USA). The textural, morphological, and surface functionality of the biochar before and after modification were characterized by BET, SEM, and FTIR. A Scanning Electron Microscope (SEM, HR-FE-SEM SU8020, HITACHI, Japan) was used to observe the surface morphologies of the biochars. Fourier Transform Infrared Spectroscopy (FTIR, Thermo Nicolet Nexus 670, USA) was applied to characterize the surface functional groups of the biochars. The BET surface areas of the biochar samples were determined from nitrogen adsorption isotherms at 77 K using an ASAP-2010 analyzer (Micromeritics, USA). A Boehm titration approach10 using different alkali solutions (e.g. NaOH, Na2CO3, and NaHCO3) was applied to determine the content of major acidic oxygen-containing functional groups in the biochars, including phenolic, lactone and carboxylic. The point of zero charge (pHpzc) of the biochars was measured by the pH drift method,21 using a 0.01 M NaCl solution with an initial pH ranging from 2 to 12, which was prepared by adding either HCl or NaOH. Dissolved carbon dioxide in the solution was removed by bubbled nitrogen until the initial pH of the solution stabilized. Then, 0.15 g of biochar was added to 25 mL of NaCl solution and shaken for 24 h to measure the final pH. The pHpzc was determined when the final pH was equal to the initial pH. The total organic carbon content (TOC) of the solution after adsorption was measured using a Vario TOC analyzer (Elementar, Germany).2.3. Adsorption experiments
The prepared pristine and modified biochar were applied as sorbents to remove Cu(II) from aqueous solution. Cu(II) stock solution (1000 mg L−1) was prepared by dissolving analytical grade copper nitrate (Cu(NO3)2·3H2O) in deionized water. The solutions were further diluted to different concentrations based on the experimental design. The initial solution pH was adjusted using 0.1 mol L−1 HCl or NaOH. A certain amount of biochar was mixed with 10 mL d Cu(II) solution in flasks. Then the flasks were put in the shaker bath and shaken at 150 rpm at the required temperature. Afterwards, the contents of the flasks were filtered immediately using a 0.45 μm pore size glass filter (Whatman®). The concentration of copper in the filtrates was measured by inductively coupled plasma emission spectroscopy, OPTIMA 2000 (USA). The copper concentrations adsorbed by the biochar were determined from the difference between the initial and final copper concentrations in the solution.The amount of copper sorption onto the biochar, qe, was calculated based on the following equation:
 | (3) |
The percentage of copper removal, S%, was determined as:
 | (4) |
In addition to the physical and chemical properties of the biochars, adsorption characteristics are also affected by various factors. This study concerned the effects of the initial solution pH, the adsorbent dosage, temperature, contact time and the initial copper concentration on copper sorption on pristine and modified biochar. All sorption experiments were conducted in duplicate and average values were reported.
3. Results and discussion
3.1. Yield of the modified biochar from hydrothermal processing
A YRi of 1 means that the biochar production is not affected by co-carbonization, while values of YRi greater or less than 1 imply biochar promotion or inhibition, respectively. Fig. 1 shows that the biochar yield was inhibited when the value of RRH was 0.2 and 0.4, while a reverse trend could be observed when the value of YRRH was 0.6 and 0.8. This implies the existence of synergistic interactions between lignocellulosic biomass and microalgae in a hydrothermal environment, which increase the mass ratio of lignocellulosic biomass to a certain extent, promoting the biochar production. In a previous study,22 we investigated the effect of the microalgae/rice husk mass ratio on the yields of solid products during a co-liquefaction process in hydrothermal media at 300 °C. It was observed that the solid product yields increased with the fraction of rice husk. Taking into account the differences in the temperatures of hydrothermal processing, the results of this study are consistent with the results of previous work.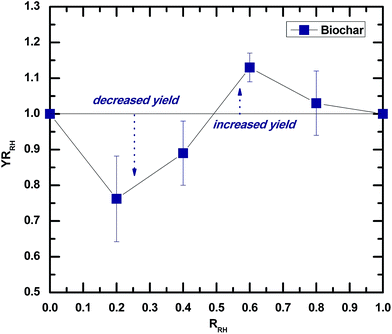 | ||
| Fig. 1 Yield ratio of biochar from co-carbonization versus mixture composition of rice husk and Chlorella pyrenoidosa. | ||
A possible reason for this may be that interactions between the major compositions of lignocellulosic biomass (i.e., cellulose, hemicelluloses, and lignin) and microalgae (i.e., protein and lipid) in a hydrothermal environment are significantly affected by the mass ratio of lignocellulosic biomass and microalgae. For instance, under hydrothermal conditions, reduced sugar and non-reduced sugar from hydrolyzed carbohydrates in lignocellulosic biomass can react with amino acids from the hydrolysis of proteins in the microalgae to generate nitrogenous compounds in terms of pyrroles or pyridines.23,24 The cross-linking reactions among the substrates are extremely complex. A detailed reaction based on model compounds may help us determine the synergistic interactions, which are recommended for further study. In this study, the optimal biochar yield was achieved at an RRH of 0.6, and this ratio was selectively used for modification of the biochar. The pristine biochar derived from hydrothermal carbonization of rice husk was used as the control.
3.2. Characteristics of the pristine and modified biochar
The chemical compositions of the pristine and modified biochar are shown in Table 1. The N content of the modified biochar was as high as 7.37%, which is significantly higher than that of the pristine biochar (1.28%). The increase in the N content may be caused by the anchoring of nitrogen-containing functional groups on the surface of the pristine biochar, which mainly originates from amino acids in proteins of the microalgae. The hydrophilicity of the biochar is partially reflected by the O/C molar ratio.25 Compared to the pristine biochar, the decline in the O/C molar ratio after modification indicates that the modified biochar is more hydrophobic than the pristine biochar, suggesting that the affinity for water molecules decreased for the modified biochar. The polar groups (e.g. C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) on the biochar surfaces generally act as water-binding centers and assist the formation of water clusters.26 The polarity index of (O + N)/C of the modified biochar was lower than that of the pristine biochar, suggesting that the number of surface polar functional groups was decreased after modification.
O) on the biochar surfaces generally act as water-binding centers and assist the formation of water clusters.26 The polarity index of (O + N)/C of the modified biochar was lower than that of the pristine biochar, suggesting that the number of surface polar functional groups was decreased after modification.
| Parameters | Units | Rice husk | C. pyrenoidosa | Pristine biochar | Modified biochar |
|---|---|---|---|---|---|
| Carbon | % | 41.6 | 51.2 | 45.25 | 49.03 |
| Hydrogen | % | 5.2 | 6.8 | 3.58 | 6.82 |
| Nitrogen | % | 4.7 | 11.3 | 1.28 | 7.37 |
| Oxygen | % | 48.5 | 30.7 | 49.89 | 36.78 |
| H/C | 0.125 | 0.1328 | 0.079 | 0.1391 | |
| O/C | 1.1659 | 0.5996 | 1.1025 | 0.7501 | |
| (O + N)/C | 1.2788 | 0.8203 | 1.1308 | 0.9005 |
Thermal/hydrothermal treatment is known to change the porous structure of the biochar derived from biomass. The textural properties of the pristine and modified biochar were analyzed by BET (see Table 2). No significant difference was observed in the biochar before and after the modification, suggesting that the difference in chemical properties caused by the modification plays a more important role in affecting the adsorption capacity than the physical structure of the biochar. Besides, the precursory biomass greatly affects the porous structure of the biochar, in addition to the preparation conditions. In this study, the BET surface areas for the pristine and modified biochar were 10.42 m2 g−1 and 8.13 m2 g−1, respectively. These values are one to three orders of magnitude lower than that of biochars derived from woody biomass27,28 due to the lower lignin content.
| Parameters | Units | Pristine biochar | Modified biochar |
|---|---|---|---|
| BET surface area | m2 g−1 | 10.42 | 8.13 |
| Meso- and macropore volume | cm3 g−1 | 0.0299 | 0.0269 |
| Micropore volume | cm3 g−1 | 0.0027 | 0.0015 |
| Average pore size | nm | 11.65 | 13.45 |
The surface morphology of the pristine and modified biochar before and after copper ion sorption were compared by SEM and are shown in Fig. 2(B). The surfaces of the pristine and modified biochar particles before adsorption were quite rough and highly heterogeneous. Micrometer-sized spherically shaped particle dispersions were observed in the modified biochar. Cellulose, hemicelluloses and lignin are three major components of lignocellulosic biomass. Cellulose is a linear and syndiotactic polymer of β-D-glucose. The surface structure of the modified biochar has a similar morphology to the hydrothermal carbons obtained from glucose.1 According to Baccile et al.,29 lignin is barely transformed into biochar by hydrothermal processing under mild temperatures below 250 °C. Therefore, the spherically shaped particles observed in the modified biochar should mainly originate from hydrothermal processing of carbohydrates in rice husk, especially for cellulose. The modified biochar after copper ion sorption was relatively smooth structured and some splendent crystals appeared on the surface, suggesting that the porous structure had collapsed and that copper ions were adsorbed on it.
The chemical properties of the biochar affect the adsorption behavior, which is closely related to the functional groups. FTIR spectra for the pristine and modified biochar before and after Cu(II) sorption are presented in Fig. 2(C). The band between 3000 and 2800 cm−1 is ascribed to aliphatic carbon –CHx stretching vibrations, including asymmetric (2925 cm−1) and symmetric (2850 cm−1) –C–H stretching of methylene groups. The adsorption peak around 1515 cm−1 is mainly attributed to the asymmetric stretching of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxylic groups. The band at 1455 cm−1 is associated with –C
O in carboxylic groups. The band at 1455 cm−1 is associated with –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in aromatic ring carbons. This indicates that aromatization occurred during the hydrothermal treatments. Unlike the pristine biochar, a broad adsorption peak around 3400 cm−1 was observed in the modified biochar, which is assigned to N–H stretching. This revealed the formation of amino groups on the surface of the pristine biochar after modification, which should originate from the amino acids from hydrolysis of proteins in the microalgae. Fig. 2(C) also illustrates that, for the modified biochar, the N–H stretching vibration shifted marginally from 3407 to 3381 cm−1 after Cu(II) sorption, indicating that amino groups on the surface of the modified biochar were involved in the sorption of copper ions during the adsorption process.
C stretching in aromatic ring carbons. This indicates that aromatization occurred during the hydrothermal treatments. Unlike the pristine biochar, a broad adsorption peak around 3400 cm−1 was observed in the modified biochar, which is assigned to N–H stretching. This revealed the formation of amino groups on the surface of the pristine biochar after modification, which should originate from the amino acids from hydrolysis of proteins in the microalgae. Fig. 2(C) also illustrates that, for the modified biochar, the N–H stretching vibration shifted marginally from 3407 to 3381 cm−1 after Cu(II) sorption, indicating that amino groups on the surface of the modified biochar were involved in the sorption of copper ions during the adsorption process.
3.3. Effect of initial solution pH on copper removal
Copper removal from wastewater is greatly affected by the solution pH because pH has a profound effect on the surface chemistry of the adsorbents in terms of the speciation of metal ions, the surface charge of the adsorbent and the degree of ionization of the adsorbate.30 Therefore, the effect of the initial solution pH on copper adsorption capacities was firstly studied within the pH range 3.0–6.0. Fig. 3(A) demonstrates the effects of the initial solution pH (3.0–6.0) on copper adsorption at a temperature of 303 K, an adsorbent dosage of 1 g L−1, an initial copper concentration of 20 mg L−1 and a contact time of 180 min. The adsorbed copper per unit weight of adsorbent (qe) for the two biochars both increased in the pH range 3–5, whereas a slight decline emerged in the pH range 5–6.The point of zero charge (pHpzc) is the pH at which the net charge on the surface of the adsorbent is zero. In this study, the pHpzc of the pristine and modified biochar was 5.2 and 5.6, respectively (see Table 3). Detailed information about pHpzc is presented in Fig. 2(A). When the solution pH is lower than pHpzc, the surface of the biochar is positively charged due to a protonation reaction. The uptake capacity of Cu2+ was inhibited at low pH values. This is possibly because, at low pH values, the linkages of H+ on acidic oxygen-containing functional groups exclude the Cu2+ in the solutions, which decreases the sorption of copper ions onto the biochar. Another reason for the low adsorption capacity may be particle attrition at low pH.31 As the pH increases, an electrostatic attraction between the copper ions and the positively charged surface is favored. The competition of copper ions and H+ for active sites becomes less influential, leading to an increase in the adsorption capacity of Cu2+. Besides, the deprotonation of functional groups in the modified biochar is further promoted due to the donation of electrons by nitrogen atoms, resulting in a higher rate of copper removal compared to the pristine biochar. When the solution pH > pHpzc, the biochar surface is negatively charged, rendering a decrease in the Cu(II) adsorption efficiency due to the stronger electrostatic repulsion. In addition, in the pH range 5–6, the slight decrease in the adsorption capacity of the modified biochar may be due to proteolysis by copper ions at certain pH values, which needs further investigation.
| Parameters | Units | Pristine biochar | Modified biochar |
|---|---|---|---|
| Carboxyl | mmol g−1 | 3.5 | 1.9 |
| Lactones | mmol g−1 | 2.8 | 0.5 |
| Phenolic hydroxyl | mmol g−1 | 1.3 | 2.1 |
| Total acidic functional groups | mmol g−1 | 7.6 | 4.5 |
| pHpzc | 5.2 | 5.6 |
Variations in pH values before and after copper ion sorption were also measured. It was observed that the pH increased slightly after the adsorption and the increment ranges for both the pristine and modified biochar were decreased as the initial solution pH increased, indicating the release of H+ from the surface of the biochars. In this study, the optimum pH value for copper adsorption was selected as 5.0 for the given conditions and the initial pH of all solutions for subsequent experiments was kept at this value.
3.4. Effect of adsorbent dosage on copper removal
Fig. 3(B) illustrates the effect of adsorbent dosage (AD = 1–10 g L−1) on copper adsorption at a pH of 5.0, a temperature of 303 K, an initial copper concentration of 20 mg L−1 and a contact time of 180 min. It can be seen that the adsorbed copper per unit weight of adsorbent (qe) gradually decreased as the adsorbent dosage increased from 1 to 10 g L−1. Similarly, Amarasinghe et al.32 investigated the copper removal characteristics of tea waste, and it was observed that the qe value gradually decreased as the adsorbent dosage increased. However, it should be noted, as shown in Fig. 3(B), that the removal percentage of copper by the two biochars increased with the adsorbent dosage, which can be attributed to the increased active sites. Choy et al.31 studied the sorption of copper onto a bone char, and it was observed that the removal percentage of copper increased when the adsorbent dosage was increased from 6.5 to 10.5 g L−1. Taking into account the differences in the type of biochar and adsorption conditions, the results in the present study agreed well with results published in the literature. Additionally, the TOC values of the solutions after adsorption were also measured. It was found that in the range of the adsorbent dosage (AD = 1–10 g L−1), only slight changes were observed before and after adsorption, (the TOC values of the treated solutions were all below 2 mg L−1), suggesting that the adsorbent applied in this study will not cause secondary organic pollution.3.5. Effect of contact time on copper removal
Fig. 3(C) shows the effect of contact time (10–180 min) on copper adsorption at a solution pH of 5.0, a temperature of 303 K, an adsorbent dosage of 1 g L−1 and different initial copper concentrations ranging from 5 to 30 mg L−1. It can be seen that, at different copper concentrations, adsorption of copper mainly occurred within the initial 40 min, and then it tended to be a relatively slow process. This is because a large number of vacant surface active sites are available when adsorption occurs. The driving force, affected by the copper concentration gradient between the solid–liquid interface and the bulk solution, is high enough to overcome the resistance of external mass-transfer between the biochar and the solution. Surface active sites with higher affinities are occupied quickly, leading to rapid adsorption. A greater driving force due to the higher initial copper concentration promotes faster adsorption. Afterwards, the driving force is decreased due to the reduction of the copper concentration in solution. The remaining adsorption sites with lower affinities are occupied slowly, and thus the diffusion of copper into the interior of the biochar is limited, decreasing the overall adsorption rate. According to Wang et al.,33 the time taken for heavy metals to establish adsorption equilibrium was 60 min. Similar results were also reported for copper adsorption by other biochars.4,323.6. Adsorption kinetics of copper adsorption onto biochars
Adsorption kinetics can determine the adsorption rate of adsorbates and is expected to help the design of adsorption equipment. According to the Ritchie equation, the two most commonly used models,7,34 the pseudo-first-order model (PF-order model), and the pseudo-second-order model (PS-order model) were applied in this study.The pseudo-first-order model is expressed as eqn (5) and (6):
 | (5) |
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (6) |
The pseudo-second-order model is expressed as eqn (7) and (8):
 | (7) |
 | (8) |
The kinetic parameters for the two models were deduced by linear fitting and are summarized in Table 4. The results indicated that the PF-order model could not adequately describe the copper adsorption onto the two biochars because the determination coefficients (R2) for the two biochars were both lower than 0.9, and the qe values calculated were far lower than the experimental data. The PS-order model was observed to better describe the copper adsorption due to the higher R2 values, and plots of the PS-order model for the two biochars are shown in Fig. 4(A). This indicated that, in this study, the mechanism of copper sorption onto the biochars from HTC was chemisorption.
| Biochar | Concentration (mg L−1) | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k2 | R2 | ||
| Pristine | 5 | 1.65 | 0.061 | 0.887 | 2.46 | 0.059 | 0.994 |
| 10 | 3.27 | 0.079 | 0.891 | 4.81 | 0.048 | 0.998 | |
| 20 | 5.08 | 0.103 | 0.857 | 7.41 | 0.036 | 0.999 | |
| 30 | 5.97 | 0.136 | 0.815 | 8.62 | 0.028 | 0.999 | |
| Modified | 5 | 2.17 | 0.084 | 0.896 | 3.72 | 0.071 | 0.998 |
| 10 | 4.19 | 0.081 | 0.887 | 7.21 | 0.030 | 0.998 | |
| 20 | 8.34 | 0.089 | 0.809 | 14.5 | 0.018 | 0.999 | |
| 30 | 10.9 | 0.127 | 0.898 | 18.5 | 0.016 | 0.999 | |
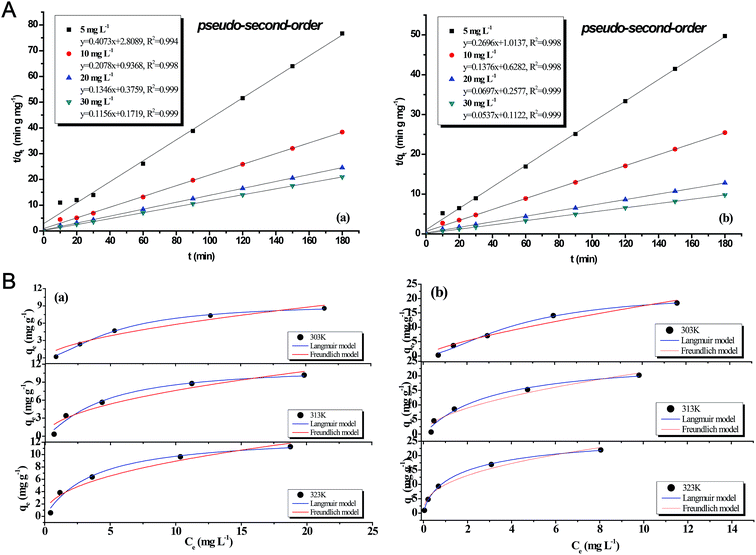 | ||
| Fig. 4 (A) Pseudo-second-order plots of copper sorption onto (a) pristine and (b) modified biochar. (B) Copper sorption isotherms of (a) pristine and (b) modified biochar. | ||
3.7. Adsorption isotherm model
The Langmuir and Freundlich models are the two most common equilibrium models evaluating the maximum adsorption capacity,30 which were applied in the present study. The Langmuir model is expressed as:
 | (9) |
The Freundlich model is expressed as:
 | (10) |
The copper adsorption isotherms were investigated at different temperatures ranging from 303 to 323 K. The experimental results for the pristine and modified biochars were fitted to the Langmuir and Freundlich models (Fig. 4(B)). The determination coefficient (R2) and corresponding isotherm constants for the two models are summarized in Table 5. The adsorption capacities for the two biochars both increased with temperature, indicating that the sorption of copper on biochars was an endothermic process. A high temperature promoted the diffusion rate of copper on the biochar's surface and porous structure. The experimental study is consistent with previous studies. For example, Meng et al.35 carried out a thermodynamic analysis of copper adsorption by biochar derived from swine manure, concluding that the reaction was endothermic. Vilar et al.36 studied the sorption of copper onto an industrial algal waste and reported that the equilibrium adsorption capacity gradually increased as the temperature increased from 293 to 308 K.
| Biochar | T (K) | Langmuir model | Freundlich model | ||||
|---|---|---|---|---|---|---|---|
| KL | qmax | R2 | KF | 1/n | R2 | ||
| Pristine | 303 | 0.066 | 9.354 | 0.995 | 1.462 | 0.597 | 0.927 |
| 313 | 0.168 | 11.59 | 0.961 | 2.371 | 0.507 | 0.914 | |
| 323 | 0.262 | 13.12 | 0.966 | 3.169 | 0.451 | 0.927 | |
| Modified | 303 | 0.086 | 22.09 | 0.988 | 3.178 | 0.738 | 0.937 |
| 313 | 0.321 | 25.61 | 0.956 | 6.144 | 0.537 | 0.939 | |
| 323 | 0.613 | 29.11 | 0.997 | 9.941 | 0.398 | 0.969 | |
These results suggested that the Langmuir model was observed to better describe copper adsorption on biochars before and after modification due to a higher fitting accuracy, compared to the Freundlich model. The maximum adsorption capacity of modified biochar (29.11 mg g−1 at 323 K) was higher than that of the pristine biochar (13.12 mg g−1 at 323 K). The Langmuir adsorption constant (KL) for the modified biochar (0.613 L mg−1 at 323 K) was higher than that of the pristine biochar (0.262 L mg−1 at 323 K), verifying that the affinity for copper was increased by the modification. Datta et al.37 modified montmorillonite as an adsorbent for copper-ion removal by the amine-based solvent, trioctylamine. It was also concluded that the Langmuir equation could better predict the adsorption capacity of the modified adsorbent for copper ions.
3.8. Adsorption mechanism
Under identical adsorption conditions, the nitrogen-rich modified biochar adsorbed more copper ions than the pristine biochar. Considering the slight differences in textural properties between the pristine and modified biochar, the adsorption of heavy metal ions in aqueous solutions is more closely related to the types of functional groups on the biochar surface. Generally, electrostatic attraction and dispersion in terms of π–π interactions, hydrogen bonding and electron donor–acceptor interactions are primary adsorption mechanisms. The adsorption of heavy metal ions on acidic oxygen functional groups is related to electrostatic attraction, while nitrogen-containing functionalities are more associated with coordination mechanisms.38Acidic oxygen-containing functional groups in the pristine and modified biochar were quantified by Boehm titration (shown in Table 3). Compared with the pristine biochar, the total number of acidic oxygen-containing functionalities decreased after modification. This is consistent with the chemical composition analysis results (see Table 1), showing that the modified biochar is less hydrophilic, because acidic oxygen-containing functional groups are hydrophilic. Hydrogen bonds can be formed between water molecules and the hydrophilic groups on the biochars, which will produce clusters that block the channels for copper ions in the micropores. Therefore, competition occurs between the protons and Cu2+ ions for adsorption sites in the modified biochar. The pristine biochar is more hydrophilic than the modified biochar, so it absorbs more water than copper ions. As the initial solution pH increased, the copper adsorption capacity of the pristine and modified biochar both increased as the H+ ion concentration decreased, and the adsorption capacity of the pristine biochar was more affected by the initial solution pH compared to the modified biochar. This implies that adsorption of copper ions by the pristine biochar is dominated by ion exchange, due to the presence of acidic oxygen-containing functionalities.
The basicity of the biochar is increased after modification due to the anchoring of basic amino groups. The high hydrophobicity of the modified biochar will decrease the competition between the copper ions and protons for adsorption sites in the modified biochar. According to Shen et al.,14 the nitrogenation of carbonaceous materials is beneficial for the adsorption of heavy metals due to their coordination to nitrogen-containing functionalities. In this study, modification of biochar by nitrogen promotes a high binding affinity to metal ions because nitrogen atoms can share electrons with copper ions during complexation. Yang et al.4 studied amino-modified biochar for copper adsorption by nitration and reduction, and a significant change in the N 1s spectra before and after copper sorption was observed by XPS analysis, verifying the formation of strong complexes of surface amino groups with copper ions. Overall, adsorption of copper ions by the modified biochar would be dominated by surface complexation rather than through electrostatic attraction for the pristine biochar. Nitrogen-containing amino functional groups on biochar surfaces enhanced copper adsorption. Table 6 shows a comparison between the modified biochar in this study and other reported adsorbents, regarding the maximum copper adsorption capacity. The modified biochar is at least comparable or even better than some absorbents reported in the literature. This confirms that biochar as a by-product from HTC, in the presence of nitrogen-rich microalgae, can be used as an effective adsorbent for the removal of heavy metals from wastewater.
| Type of biochars | Thermochemical conversion | Adsorption temperature (°C) | Adsorption pH | Maximum adsorption capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|
| Modified biochar | Hydrothermal | 30 | 5.0 | 29.11 | This study |
| Pristine biochar | Hydrothermal | 30 | 5.0 | 13.12 | This study |
| Sawdust | Pyrolysis | 30 | 5.0 | 16.11 | 4 |
| Cow manure | Pyrolysis | 20 | 5.0 | 6.34 | 39 |
| Switch grass | Hydrothermal | 23 | 5.0 | 31 | 7 |
| Hardwood | Pyrolysis | 22 | 5.0 | 6.79 | 40 |
| Corn straw | Pyrolysis | 22 | 5.0 | 12.52 | 40 |
| Digested manure | Pyrolysis | 25 | 6.0 | 21.12 | 41 |
4. Conclusions
Hydrothermal carbon derived from rice husk was modified using nitrogen-rich microalgae. Nitrogen was successfully doped into the biochar. The maximum adsorption capacity of the modified biochar (29.11 mg g−1) was twice that of the pristine biochar (13.12 mg g−1). The enhanced uptake capacity of copper ions was mainly attributed to the low hydrophilicity and high basicity originating from the anchoring of nitrogenous functionalities on the surface of hydrothermal biochar. The adsorption behavior of the pristine biochar is governed by electrostatic attractions, while surface complexation is the main adsorption mechanism of the modified biochar.Acknowledgements
The authors gratefully acknowledge the financial support from the “100 Talents” Program of the Chinese Academy of Sciences to Zhengang Liu, the Beijing Natural Science Foundation (Project No. 8164064), the Opening Project of the Key Laboratory for Solid Waste Management and Environment Safety in Tsinghua University (Project No. SWMES 2015-13), and the Key Laboratory of Solid Waste Treatment and Resource Recycle in Southwest University of Science and Technology (Project No. 15zxgk01).References
- M. M. Titirici and M. Antonietti, Chem. Soc. Rev., 2010, 39, 103–116 RSC.
- K. Kadirvelu, K. Thamaraiselvi and C. Namasivayam, Bioresour. Technol., 2001, 76, 64–65 CrossRef.
- J. P. Chen, S. Wu and K. Chong, Carbon, 2003, 41, 1979–1986 CrossRef CAS.
- G. X. Yang and H. Jiang, Water Res., 2014, 48, 396–405 CrossRef CAS PubMed.
- Y. Liu, J. Chen, M. Chen, B. Zhang, D. Wu and Q. Cheng, RSC Adv., 2015, 5, 76160–76169 RSC.
- A. P. Puga, C. A. Abreu, L. C. A. Melo and L. Beesley, J. Environ. Manage., 2015, 159, 86–93 CrossRef CAS PubMed.
- P. Regmi, J. L. G. Moscoso, S. Kumar, X. Cao, J. Mao and G. Schafran, J. Environ. Manage., 2012, 109, 61–69 CrossRef CAS PubMed.
- E. Agrafioti, D. Kalderis and E. Diamadopoulos, J. Environ. Manage., 2007, 146, 444–450 CrossRef PubMed.
- Z. Liu and F. Zhang, J. Hazard. Mater., 2009, 167, 933–939 CrossRef CAS PubMed.
- M. Carrier, A. G. Hardie, U. Uras, J. Görgens and J. Knoetze, J. Anal. Appl. Pyrolysis, 2012, 6, 24–32 CrossRef.
- X. Huang, Y. Liu, S. Liu, X. Tan, Y. Ding, G. Zeng, Y. Zhou, M. Zhang, S. Wang and B. Zheng, RSC Adv., 2016, 6, 94–104 RSC.
- G. Yang, Z. Wang, Q. Xian, F. Shen, C. Sun, Y. Zhang and J. Wu, RSC Adv., 2015, 5, 40117–40125 RSC.
- J. Mumme, M. M. Titirici, A. Pfeiffer, U. Lüder and M. Toufiq Reza, ACS Sustainable Chem. Eng., 2015, 3, 2967–2974 CrossRef CAS.
- W. Shen and W. Fan, J. Mater. Chem. A, 2013, 1, 999–1013 CAS.
- L. Cao, G. Luo, S. Zhang and J. Chen, RSC Adv., 2016, 6, 15260–15270 RSC.
- M. D. Huff, S. Kumar and J. W. Lee, J. Environ. Manage., 2014, 146, 303–308 CrossRef CAS PubMed.
- M. Uchimiya, S. Chang and K. Thomas Klasson, J. Hazard. Mater., 2011, 190, 432–441 CrossRef CAS PubMed.
- D. A. Buttry, J. C. M. Peng, J. Donnet and S. Rebouilat, Carbon, 1999, 37, 1929–1940 CrossRef CAS.
- M. M. Titirici, R. J. White, C. Falco and M. Sevilla, Energy Environ. Sci., 2012, 5, 6796–6822 Search PubMed.
- C. Gai, Z. Liu, G. Han, N. Peng and A. Fan, Bioresour. Technol., 2015, 181, 148–154 CrossRef CAS PubMed.
- B. Xiao and K. M. Thomas, Langmuir, 2005, 21, 3892–3902 CrossRef CAS PubMed.
- C. Gai, Y. Li, N. Peng, A. Fan and Z. Liu, Bioresour. Technol., 2015, 185, 240–245 CrossRef CAS PubMed.
- D. Virág, A. Kiss, P. Forgó, C. Csutorás and S. Molnár, Microchem. J., 2013, 107, 172–177 CrossRef.
- C. Gai, Y. Zhang, W. Chen, P. Zhang and Y. Dong, Energy Convers. Manage., 2015, 96, 330–339 CrossRef CAS.
- Y. Chun, G. Sheng, C. T. Chiou and B. Xing, Environ. Sci. Technol., 2004, 38, 4649–4655 CrossRef CAS PubMed.
- Q. Fang, B. Chen, Y. Lin and Y. Guan, Environ. Sci. Technol., 2014, 48, 279–288 CrossRef CAS PubMed.
- F. N. D. Mukme, X. Zhang, L. C. R. Silva, J. Six and S. J. Parikh, J. Agric. Food Chem., 2013, 61, 2196–2204 CrossRef PubMed.
- M. Teixido, J. J. Pignatello, J. L. Beltran, M. Granados and J. Peccia, Environ. Sci. Technol., 2011, 45, 10020–10027 CrossRef CAS PubMed.
- N. Baccile, C. Falco and M. M. Titirici, Green Chem., 2014, 16, 4839–4869 RSC.
- X. Tan, Y. Liu, G. Zeng, X. Wang, X. Hu, Y. Gu and Z. Yang, Chemosphere, 2015, 125, 70–85 CrossRef CAS PubMed.
- K. K. H. Choy and G. McKay, Environ. Int., 2005, 31, 845–854 CrossRef CAS PubMed.
- B. M. W. P. K. Amarasinghe and R. A. Williams, Chem. Eng. J., 2007, 132, 299–309 CrossRef CAS.
- D. Wang, W. Zhang, X. Hao and D. Zhou, Environ. Sci. Technol., 2013, 47, 821–828 CrossRef CAS PubMed.
- A. G. Ritchie, J. Chem. Soc., Faraday Trans., 1977, 73, 1650–1653 RSC.
- J. Meng, X. Feng, Z. Dai, X. Liu, J. Wu and J. Xu, Environ. Sci. Pollut. Res., 2014, 21, 7035–7046 CrossRef CAS PubMed.
- V. J. P. Vilar, C. M. S. Botelho and R. A. R. Boaventura, Bioresour. Technol., 2008, 99, 750–762 CrossRef CAS PubMed.
- D. Datta, H. Uslu and S. Kumar, J. Chem. Eng. Data, 2015, 60, 3193–3200 CrossRef CAS.
- Y. Li, J. Shao, X. Wang, Y. Deng, H. Yang and H. Chen, Energy Fuels, 2014, 28, 5119–5127 CrossRef CAS.
- D. Kolodyńska, R. Wnetrzak, J. J. Leahy, M. H. B. Hayes, W. Kwapiński and Z. Hubicki, Chem. Eng. J., 2012, 197, 295–305 CrossRef.
- X. Chen, G. Chen, L. Chen, Y. Chen, J. Lehmann, M. B. McBride and A. G. Hay, Bioresour. Technol., 2011, 102, 8877–8884 CrossRef CAS PubMed.
- H. Jin, M. U. Hanif, S. Capareda, Z. Chang, H. Huang and Y. Ai, J. Environ. Chem. Eng., 2016, 4, 365–372 CrossRef CAS.
Footnote |
| † Present address: 18 Shuangqing Road, Beijing 100085, PR China. |
| This journal is © The Royal Society of Chemistry 2016 |