N-Doped biochar derived from co-hydrothermal carbonization of rice husk and Chlorella pyrenoidosa for enhancing copper ion adsorption
Abstract
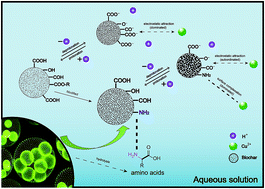
Biochar derived from rice husk was modified by microalgae Chlorella pyrenoidosa as a natural nitrogen-rich precursor in a hydrothermal environment for copper ion (Cu(II)) adsorption. Pristine biochar derived from hydrothermal carbonization of individual rice husks was included as a control. FTIR, SEM and BET analyses indicated that the modified biochar is more hydrophobic and basic than the pristine biochar due to the anchoring of surface nitrogenous functionalities. The adsorption of copper ions onto the pristine and modified biochar was investigated with respect to pH, adsorbent dosage, contact time, temperature, kinetics and isotherms. The results showed that modification of the biochar by nitrogen significantly increased the copper adsorption capacity from 13.12 mg g−1 for the pristine biochar to 29.11 mg g−1 for the modified biochar. Adsorption of copper ions by the modified biochar was dominated by surface complexation rather than through the electrostatic attractions that dominated adsorption for the pristine biochar.

 Please wait while we load your content...
Please wait while we load your content...