Optical-electric properties of poly(amic acid) composite films with a low content of thermotropic liquid crystals†
Ling Dinga,
Yihe Zhang*a,
Leipeng Liua,
Jianshe Hu*b and
Fengzhu Lv*a
aBeijing Key Laboratory of Materials Utilization of Nonmetallic Minerals and Solid Wastes, National Laboratory of Mineral Materials, School of Materials Science and Technology, China University of Geosciences, No. 29 Xueyuan Road, Haidian District, Beijing, 100083, China. E-mail: lfz619@cugb.edu.cn; zyh@cugb.edu.cn; Fax: +86-10-82322345; Tel: +86-10-82322759
bCenter for Molecular Science and Engineering, College of Science, Northeastern University, Shenyang 110004, People's Republic of China. E-mail: hujs@mail.neu.edu.cn
First published on 6th June 2016
Abstract
Poly(amic acid) composite films with the thermotropic liquid crystal, 10-cholesteroxy-10-oxocaproic acid (COOA) were developed. The composites could still show liquid crystal behavior at low COOA loading (as low as 3 wt%) due to the phase separation under the induction of temperature. The synergistic reaction of the interaction of PAA with COOA and the arrangement of COOA endowed the films with strong fluorescence and a higher temperature shifted liquid crystal phase. Even more, the dielectric constant of the composites was enhanced. The composites were multifunctional optical-electric materials.
Introduction
It is well known that liquid crystalline materials are widely used in the optical-electronic field as their excellent optical properties are derived from their dielectric anisotropy.1 To stabilize the properties of liquid crystals (LCs), polymer-dispersed liquid crystal (PDLC) films have been developed.PDLCs are functional composite materials consisting of micron-sized droplets of liquid crystals dispersed in polymer binders.2–4 PDLC films have acquired greater importance since these materials are used in electro-optical devices ranging from light shutters, high-resolution displays, projection light valves to switching gratings. The properties of liquid crystals that cause the director responding to an applied electric field are the basis for most of the applications. Various methods, such as solvent induced phase separation (SIPS), polymerization induced phase separation (PIPS), thermal induced phase separation (TIPS) and encapsulation, are being used to prepare PDLC composite films, among which SIPS are widely used.5 Parab et al. have used the SIPS method by dissolving LC (E44) and poly(methyl methacrylate) in a common solvent to create a single phase. As the solvent evaporation takes place, the LC phase starts to generate in the form of droplets and is embedded between polymer walls.6 As the optical-electronic properties of LC are from their dielectric anisotropy, so the dielectric properties of PDLC are discussed with their liquid crystal properties generally. For example, Prasad N. et al. performed the first high-pressure dielectric measurements on a nano-colloidal system comprising a weakly polar nematic liquid crystal and its composites with aerosil particles in the soft gel regime.7 Manohar et al. reported the dielectric properties of poly-butyl methacrylate (PBMA) and LC, cholesterylnominate, as a function of frequency and temperature.8
But in the reports, the above properties are expressed by PDLC films with a high concentration of lyotropic liquid crystal, generally higher than 20 wt%. In the present work, composite films with a lower content of thermotropic liquid crystal (lower than 10 wt%) were developed to study the optical and dielectric properties. As the content of LC is 3 wt%, phase separation between poly(amic acid)s (PAAs) and LC takes place and the optical anisotropicity is observed, in which PAA is the polymer matrix. Different from the reports, phase separation was driven by temperature in the present work. One more interesting result was that strong emission fluorescence was obtained due to the arrangement of LC molecules. Evenmore, the composites showed enhanced dielectric constant compared to its matrix. The composites are multifunctional composite films and have potential applications in optical-electric fields.
Experimental part
Materials
4,4′-Oxydianiline (ODA) and pyromellitic anhydride (PMDA) were bought from Mitsubishi Gas Chemical Company, INC, Japan. N,N-Dimethylacetamide (DMAc) was provided by Sinopharm Chemical Reagent Co. Ltd, China. The small molecular weight liquid crystal, 10-cholesteroxy-10-oxocaproic acid (COOA) the molecular weight of which is 542, was provided by Northeastern University.Preparation
COOA/PAA composite films were prepared by in situ polymerization of ODA and PMDA at the presence of COOA,9 followed by film fabrication. Generally, 1.0012 g of ODA was dissolved in 25 ml of DMAc at room temperature by ultrasonication. Then 1.1070 g of PMDA and COOA were added slowly to the suspension. The sol was stirred vigorously at 0 °C for 5 h to yield a viscous solution, which was cast on a clean glass slide to form a uniform film. Then the film was heated and preserved at a certain temperature for 2 h and then quenched to room temperature immediately. In the series of composites, the weight of COOA was 0.3, 1, 2, 3, and 5 wt% of the final films, and the heat preservation temperature was 60, 120, 145, and 200 °C, respectively. Therefore, the final films were assigned as xCOOA/PAA-T, where x and T indicated the weight content of COOA and the heat preservation temperature for the film fabrication. Correspondingly, pure PAA films fabricated at different temperature were recorded as PAA-T where T still indicated the heat preservation temperature for the film fabrication.Characterization
X-ray diffraction (XRD) patterns were obtained from 10° to 70° at a scanning rate of 8° min−1 by a Rigaku D/Max-r A rotating anode X-ray diffractometer using a Cu Kα tube (λ = 1.5405 Å) and a Ni filter. Scanning electron microscope (SEM, HitachiS-450, Japan) was used to test the fracture surface morphology of COOA/PAA films. Fourier transform infrared (FT-IR) spectroscopy over the range of 2500–600 cm−1 was conducted with an Excalibur 3100 instrument. Differential scanning calorimetry (DSC) characterization of COOA was performed at a heating/cooling rate of 10 °C min−1 between 25 °C and 180 °C under a nitrogen atmosphere by a Q2000 V 24.10 instrument. The textures of the films were determined with an OLYMPUS BX51 Polarized light microscopy. Confocal Raman spectrometer (LabRAM HR) was used for observation of the compositional change of the composites. The fluorescence of the film range from 380 nm to 700 nm was conducted at room temperature by F7000 with a photomultiplier tube operating at 400 V and a 150 W Xe lamp and an excitation of 320 nm. The dielectric properties of the films were studied as a function of frequency in the range of 100 Hz–5 MHz using an impedance gain-phase analyzer (Agilent 4294A) instrument at room temperature. The data was the average of three times of measurement.Results and discussion
Composition of the composite films
The composition of the composite films was characterized first by FTIR (Fig. 1). In the FTIR spectrum (Fig. 1a) of PAA-60, the band at 819 cm−1 arises from a 1,4-disubstituted benzene ring, and the characteristic peak for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in PAA can be seen at 1714 cm−1.10 The band at 1224 cm−1 is the typical stretching vibration of C–O connecting aromatic groups in PAA. The bands from 1086 to 1167 cm−1 are the vibrations of C–N of the tertiary amide.11 While, for PAA-200, the vibration at around 1774 cm−1 arising from the asymmetric carbonyl stretching vibration of the imide groups is observed.12 This vibration peak still appears in PAA-120 and PAA-145, but becomes more obvious with the increase of annealing temperature. These results indicate that in PAA-120, PAA-145, and PAA-200, some PAA are imidized and the imidization degree increases with temperature.12,13 The FTIR spectra of 3COOA/PAA-120 and 3COOA/PAA-200 are all similar to their corresponding PAA film.14 While in 3COOA/PAA-60 and 3COOA/PAA-145 the characteristic peaks at 1714 cm−1 are obviously widened showing relatively strong interaction (hydrogen bonds) in the films,15 which is further confirmed by the absorption above 3000 cm−1. Seen from Fig. 1b, there is an obvious infrared absorption at 3489 cm−1 (free O–H stretching) whose intensity increases with temperature. The reason is with the increase of temperature, hydrogen bonds are partially destroyed and free OH vibration becomes more and more obvious.16
O in PAA can be seen at 1714 cm−1.10 The band at 1224 cm−1 is the typical stretching vibration of C–O connecting aromatic groups in PAA. The bands from 1086 to 1167 cm−1 are the vibrations of C–N of the tertiary amide.11 While, for PAA-200, the vibration at around 1774 cm−1 arising from the asymmetric carbonyl stretching vibration of the imide groups is observed.12 This vibration peak still appears in PAA-120 and PAA-145, but becomes more obvious with the increase of annealing temperature. These results indicate that in PAA-120, PAA-145, and PAA-200, some PAA are imidized and the imidization degree increases with temperature.12,13 The FTIR spectra of 3COOA/PAA-120 and 3COOA/PAA-200 are all similar to their corresponding PAA film.14 While in 3COOA/PAA-60 and 3COOA/PAA-145 the characteristic peaks at 1714 cm−1 are obviously widened showing relatively strong interaction (hydrogen bonds) in the films,15 which is further confirmed by the absorption above 3000 cm−1. Seen from Fig. 1b, there is an obvious infrared absorption at 3489 cm−1 (free O–H stretching) whose intensity increases with temperature. The reason is with the increase of temperature, hydrogen bonds are partially destroyed and free OH vibration becomes more and more obvious.16
In order to further study the interactions in the composites, Raman spectrum was performed. The peak at 737 cm−1 from N–CH3 stretching mode is considered as the characteristic peak of DMAc and the typical peak of PAA appears at 1608 cm−1 (C–X stretching mode of –C6H2–) (Fig. 2).17 In 3COOA/PAA-120, 3COOA/PAA-145, and 3COOA/PAA-200 obvious peaks at 1108 cm−1 assigned to PI (transverse stretching mode of C–N–C) appears showing the partial imidization of PAA. In the films, the content of residue solvent can be calculated as below:
Optical properties of the composite films
One interesting thing needed to be pointed out is that the Raman spectrum of 3COOA/PAA-145 can only be obtained after addition of fluorescence quenching agent, so the fluorescence of the 3COOA/PAA-145 was measured and shown in Fig. 3a. It is clear that 3COOA/PAA-145 shows two strong fluorescent peaks under the excitation of 320 nm. But COOA itself does not emit obvious fluorescence at the state of solid, which emits fluorescence in H2O and relatively high fluorescence in THF (Fig. 3b). Considering the structure of COOA which has long hydrophobic chain, it is preferred that COOA with –COOH groups aggregating together in H2O or THF, hydrogen bonds which can strengthen the rigid of chains form between COOA molecules, limiting its rotation, which increase the π–π stacking and cause the fluorescence emission.19 So the fluorescent peak centered at 420 nm in 3COOA/PAA-145 is from the emission of –COOH groups in COOA. The peak at 580 nm comes from the arrangement of COOA molecules which is in liquid crystalline phase. At 145 °C, owing to the steric effect of the polymers, rotation of COOA is further inhibited. The aggregation of the COOA molecules increase the dipole fields around them, so strong emission at 580 nm appears showing apparent AIEE (aggregation induced enhanced emission).20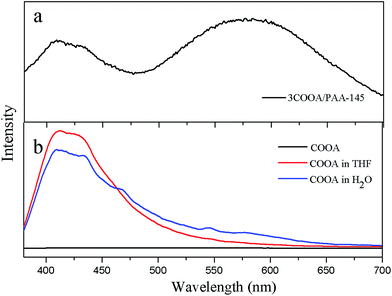 | ||
| Fig. 3 Fluorescence of (a) COOA/PAA-145 under the excitation of 320 nm, (b) COOA and COOA dispersed in different solvent. | ||
In order to investigate if COOA exists as a liquid phase in the composite films, POM measurement was carried out at room temperature. Under POM, pristine PAA, 3COOA/PAA-60 (Fig. 4a), 3COOA/PAA-120 (Fig. 4b) and 3COOA/PAA-200 (Fig. 4d), don't have obvious color texture. But obvious 4-brushes due to birefraction are observed for 3COOA/PAA-145 (Fig. 4c), which may derive from nematic phase21 or anticline smectic C.22 In the images, the brighter phase is COOA domain while the darker phase represents the isotropic polymer matrix.
For clearly uncover the state of COOA in the films, XRD measurement was carried out. For the pristine PAA-120 (ESI S1†), an obvious broad peak centered at 2θ = 17.58 indicates that the polymers are amorphous. While for COOA (Fig. S1†), it is a crystalline powder at room temperature. In 3COOA/PAA-T, no sharp peaks are observed between 10 and 70° and in small angle region (1–10°, ESI, Fig. S2†) indicating COOA exist in 3COOA/PAA-145 as nematic phase while amorphous molecules in 3COOA/PAA-60, 3COOA/PAA-120, and 3COOA/PAA-200.
But the DSC curves (ESI S3†) indicate that COOA shows liquid crystal phase between 101 °C and 132 °C in increasing temperature procedure while shows mesomorphic phase between 137 °C and 116 °C in decreasing temperature process. That is in the composite, COOA shows liquid crystal phase beyond its mesomorphic phase formed by pure COOA. The high-temperature shifted liquid crystal phase may be caused by the strong interaction between COOA and PAA which limits the arrangement of COOA molecules at lower temperature. As the content of COOA in PAA is further increased, the 4-brushes texture becomes more obvious.
Optical performance origin of the composite films
In the reports, no matter mesogenic units as part of the composition of polymers or as fillers of polymers their lowest content to form liquid crystal phase in the system is generally higher than 20 wt%.23 But in the present system, COOA shows liquid crystal phase at the low content (3 wt%). So non-uniform dispersion of COOA or aggregation of COOA in some region may occur, where COOA are ordered. In order to verify whether phase separation occurred, SEM was carried out to observe the fracture surface of the films. As the content of COOA is 3%, no matter the annealing temperature is 60, 120, 145 or 200 °C, pores are observed (Fig. 5) representing the phase separation between COOA and PAA as many two-component systems.24,25 With the increase of annealing temperature, the pore diameter becomes larger. Meanwhile, the numbers of pores in 3COOA/PAA-120 and 3COOA/PAA-145 in fixed area are more than the other two. | ||
| Fig. 5 SEM of fracture surface of (a) 3COOA/PAA-60, (b) 3COOA/PAA-120, (c) 3COOA/PAA-145, and (d) 3COOA/PAA-200. | ||
Just as shown in Fig. 6, COOA contains one long alkyl chain which is more hydrophobic than PAA chains and drives phase separation.26 At the beginning of the film fabrication process, homogenous films are generated. But at the heating preservation period, with the evaporation of the solvent, phase separation is occurring due to the mobility of COOA under the driven of hydrophilic difference between COOA and PAA. Finally, equilibrium states form. At 60 and 120 °C, COOA is in an amorphous state. At 145 °C, liquid crystal state is formed by COOA. While at 200 °C, COOA is in a fluid state. In the cooling stage in the film fabrication, as the solvent has been evaporated away and the diffusion rate of COOA is slow compared to the cooling rate, so phase separation, as well as the ordered arrangement of the COOA molecules are preserved.5 The pore diameter change of the samples is drivened by the movement of the liquid crystals in the film fabrication process. COOA used is thermotropic liquid crystals confirmed by the DSC curve. Therefore, higher temperature indicated higher mobility for this liquid COOA.27 The PAA and COOA films are low critical solution temperature system which means obvious phase separation takes place at higher temperature28,29 and large pores form.
Fig. 7 is the photographs of the fracture surface of the composites with different COOA content. As the preparation temperature is 60 °C and the concentration of COOA is 2 wt%, obvious phase separation is observed, but the porous structure is not so obvious (Fig. 7a). As the concentration increased to 3 wt% (Fig. 7b), lots of little holes with a diameter smaller than 1 μm scattered on the PAA matrix. While as the concentration of COOA is further increased to 5 wt%, large holes filled with small spherical particles appear (Fig. 7c). Just as shown in Fig. 6 aggregates with double COOA layers form.
In summery, due to the interaction of PAA and COOA and their hydrophilic difference as well as their synergistic reaction, phase separation occurs which result in mesophase formation at low COOA concentration and strong fluorescent emission, which has great potential in laser adjustment. In the reports, the fluorescence can only be obtained by addition of a fluorescent dopant, while in the present work, COOA and PAA themselves do not possess obvious fluorescence, but their arrangement cause the formation of fluorescence. The formed films possess specific optical properties.
Dielectric properties of the composite films
Fig. 8 shows the dielectric properties of the composite films with frequency. The dielectric constant of all the samples decreases with frequency, but the decreasing degree of the composites annealed at lower temperature is higher than that fabricated at a higher temperature. As interface polarization mainly takes place at low frequency and contributes more to the increase of dielectric constant at low frequency. So the relatively high decreasing degree of the dielectric constant of the films annealed at lower temperature indicates the influence of interface from phase separation is more at low temperature. But the decrease of the dielectric constant of xCOOA/PAA-60 with frequency is not very large indicating macrosize phase separation has little influence on the dielectric properties. The dielectric loss of the composites decrease with annealing temperature and is all lower than 0.2. | ||
| Fig. 8 Variation in dielectric properties of (a and b) xCOOA/PAA-60, (c and d) xCOOA/PAA-120, (e and f) xCOOA/PAA-145, and (g and h) xCOOA/PAA-200. | ||
The dielectric constant and dielectric loss change with COOA content at 104 is summarized in Fig. 9. The dielectric constant change as an anti “s” type. As COOA is increased, the dielectric constant of the film is increased to a high value where the COOA is 1 wt%. Then the dielectric constant decreases to a lower dielectric constant at 3 wt% of COOA and then increases further. As COOA is a high dielectric constant filler and possesses a positive effect on the dielectric constant of the composites. As the content of COOA is more than 1 wt%, obvious phase separation occurs, the air in the pores, which has the lowest dielectric constant (ε = 1), decreases the dielectric constant of the films, the systems become three component systems. As the content of COOA reaches 3 wt%, the influence of air reaches highest. Then at even higher COOA content, the positive effect of COOA is higher than the negative effect of air so the dielectric constant increase. But the dielectric loss of the films changes little with content.
 | ||
| Fig. 9 Content dependence of (a) dielectric constant and (b) loss of xCOOA/PAA-T composite films at 104 Hz. | ||
At 104 Hz, the dielectric constant of the film at fixed COOA content but lower than 3 wt% was in the order of COOA/PAA-120 > COOA/PAA-60 > COOA/PAA-145 > COOA/PAA-200. At higher temperature, the imidization degree of PAA is increased, PI has a relatively lower dielectric constant than PAA, as PAA possess more polar groups (–COOH) than PI. So the film fabricated at a higher temperature has a lower dielectric constant. The dielectric constant of COOA/PAA-60 lower than COOA/PAA-120 is because some DMAc which has higher dielectric constant29 is in the film due to the lower fabrication temperature. The dielectric loss of the films at fixed COOA content is in the order of as dielectric constant. As the content of COOA is 5 wt%, the dielectric constant of 5COOA/PAA-145 is becomes the highest maybe due to the serious phase separation, by which ordered COOA phase forms and free COOA with polar COOH groups and large size volume are produced which is good for dielectric constant improvement.
Conclusions
Composite films composing of PAA and COOA liquid crystal are prepared by condensation the monomers of PAA at the presence of COOA. The composites can still show liquid crystal behaviors at low COOA loading due to the phase separation. The liquid crystal phase is higher temperature shifted due to the interaction of PAA with COOA which also endows the films strong fluorescence from the π–π stacking of COOH in COOA and PAA. Even more, the dielectric constant of the composites is increased after introduction. The addition of COOA endows the composite films multi-functions and the formed composite has potential as optical-electric materials.References
- S. E. San, M. Okutan, O. Koysal, H. Ono and N. Kawatsuki, Opt. Commun., 2004, 238, 79 CrossRef CAS.
- P. S. Drzaic, Liquid Crystal Dispersions, World Scientific, Singapore, 1, 1995 Search PubMed.
- P. Drzaic and P. S. Drzaic, Liq. Cryst., 2006, 33, 1281 CrossRef CAS.
- Y. J. Jeon, B. Yin, J. T. Rhee, D. L. Cheung and M. Jamil, Macromol. Theory Simul., 2007, 16, 643 CrossRef CAS.
- E. R. Soule, N. M. Abukhdeir and A. D. Rey, Macromolecules, 2009, 42, 9486 CrossRef CAS.
- S. S. Parab, M. K. Malik and R. R. Deshmukh, J. Non-Cryst. Solids, 2012, 358, 2713 CrossRef CAS.
- P. N. Bapat, D. S. S. Rao, S. K. Prasad and C. V. Yelamaggad, J. Phys. Chem. B, 2010, 114, 12825 CrossRef CAS PubMed.
- R. Manohar, G. Tripathi, A. K. Singh, A. K. Srivastava, J. P. Shukla and A. K. Prajapati, J. Phys. Chem. Solids, 2006, 67, 2300 CrossRef CAS.
- F. Lv, L. Xu, Z. Xu, L. Fu and Y. Zhang, J. Appl. Polym. Sci., 2015, 132, 41224 Search PubMed.
- M. Zhu, X. Huang, K. Yang, X. Zhai, J. Zhang, J. He and P. Jiang, ACS Appl. Mater. Interfaces, 2014, 6, 19644 CAS.
- Y. Pan, Y. Zhao and F. Zhang, Mod. Instrum., 2000, 1, 1 Search PubMed.
- D. Chao, J. Zhang, X. Liu, X. Lu, C. Wang, W. Zhang and Y. Wei, Polymer, 2010, 51, 4518 CrossRef CAS.
- S. Cheng, D. Shen, X. Zhu, X. Tian, D. Zhou and L. Fan, Eur. Polym. J., 2009, 45, 2767 CrossRef CAS.
- A. M. Alam, Y. Liu, M. Park, S. J. Park and H. Y. Kim, Polymer, 2015, 59, 35 CrossRef CAS.
- G. M. Chen, Y. M. Ma, X. J. Zheng, G. Y. Xu, J. Liu, J. Q. Fan, D. Y. Shen and Z. N. Qi, J. Polym. Sci., Polym. Phys., 2007, 45, 654 CrossRef CAS.
- G. M. Chen, D. Y. Shen, M. Feng and M. S. Yang, Rapid Commun., 2004, 25, 1121 CrossRef CAS.
- B. W. Jo, K. H. Ahn and S. J. Lee, Polymer, 2015, 68, 74 CrossRef CAS.
- G. Qian, Z. Zhong, M. Luo, D. Yu, Z. Zhang, Z. Wang and D. Ma, Adv. Mater., 2008, 18, 111 Search PubMed.
- Z. Xu, G. Y. Zhong, X. M. Ding, E. Obbard, H. J. Ding, X. J. Wang, Y. Q. Zhan, S. T. Zhang and J. M. Zhao, Appl. Phys. A, 2005, 80, 1753 CrossRef CAS.
- A. Belaissaoui, S. J. Cowling and J. W. Goodby, Liq. Cryst., 2013, 40, 421 CrossRef CAS.
- F. C. Yu and L. J. Yu, Chem. Mater., 2006, 18, 5410 CrossRef CAS.
- D. Wang, L. Zhang, Y. Xing, H. Gao, K. Wang, Z. Yang, M. Hai, H. Cao, W. He and H. Yang, Liq. Cryst., 2015, 42, 1689 CrossRef CAS.
- J. C. Jansen, S. Darvishmanesh, F. Tasselli, F. Bazzarelli, P. Bernardo, E. Tocci, K. Friess, A. Randova, E. Drioli and B. V. Bruggen, J. Membr. Sci., 2013, 447, 107 CrossRef CAS.
- M. Padaki, A. M. Isloor, A. F. Ismail and M. S. Abdullah, Desalination, 2012, 295, 35 CrossRef CAS.
- Y. Jin, J. Tang, J. Hu, X. Han, Y. Shang and H. Liu, Colloids Surf., A, 2011, 392, 178–186 CrossRef CAS.
- M. Iqbal, Z. Man, H. Mukhtar and B. K. Dutta, J. Membr. Sci., 2008, 318, 167 CrossRef CAS.
- B. Wenclawiok and N. Aus, Der Chemie, 2007, 55, 1111 Search PubMed.
- M. Liu, Y. Wei, Z. Xu, R. Guo and L. Zhao, J. Membrane. Sci., 2013, 437, 169 CrossRef CAS.
- C. J. Luo, E. Stride and M. Edirisinghe, Macromolecules, 2012, 45, 4669 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10244a |
| This journal is © The Royal Society of Chemistry 2016 |






